Abstract
Sarcandra glabra (Thunb.) Nakai is a perennial evergreen herb categorised within the Sarcandra Gardner genus under the Chloranthaceae family. Indigenous to tropical and subtropical regions of East Asia and India, this species is extensively distributed across China, particularly in the southern regions (Sichuan, Yunnan, and Jiangxi). In addition to its high ornamental value, S. glabra has a rich history of use in traditional Chinese medicine, evident through its empirical prescriptions for various ailments like pneumonia, dysentery, fractures, bruises, numbness, amenorrhea, rheumatism, and other diseases. Besides, modern pharmacological studies have revealed various biological activities, such as antitumour, anti-bacterial, anti-viral anti-inflammatory and immunomodulatory effects. The diverse chemical constituents of S. glabra have fascinated natural product researchers since the 1900s. To date, over 400 compounds including terpenoids, coumarins, lignans, flavonoids, sterols, anthraquinones, organic acids, and organic esters have been isolated and characterised, some featuring unprecedented structures. This review comprehensively examines the current understanding of S. glabra’s phytochemistry and pharmacology, with emphasis on the chemistry and biosynthesis of its unique chemotaxonomic marker, the lindenane-type sesquiterpenoids.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sarcandra (Sarcandra Gardner) is a genus under the family of Chloranthaceae. The genus name, Sarcandra, is integrated from ‘Sarkos’ and ‘andrus’, which means fleshy anthers in Greek, while the epithet ‘glabra’ translates to ‘hairless’ from Latin [1]. Sarcandra glabra (Thunb.) Nakai (syn. Chloranthus glaber (Thunb.) Makino) or Sarcandra glabra in short, represents an extensively researched species of the genus Sarcandra. The plant is an evergreen shrub that grows up to 2 m tall and has glossy green leaves with a distinctive aroma. The plant is occasionally planted for ornamental purposes, otherwise used to prepare medicinal tea.
Also referred to as 草珊瑚 (CaoShanHu) in Chinese, S. glabra is valued in Traditional Chinese Medicine for its immunomodulatory [2], anti-inflammatory [3], and anti-tumour properties [4], and used to treat a variety of health conditions, including arthritis, bronchitis, and cancer [5]. The distribution of the subshrub ranges from temperate East Asia to Southeast China, specifically in provinces such as Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Zhejiang, and Sichuan [1]. Ecologically, S. glabra thrives in geographic locations that are approximately 2000 m above sea level and can be found plentiful in forests, thickets, valleys, ravines, trail sides, grasslands, and swamps [1].
Within the S. glabra species, two subspecies are officially accepted, namely Sarcandra glabra subsp. glabra and Sarcandra glabra subsp. brachystachys [6]. S. glabra subsp. glabra, known as 原亚种 (YuanYaZhong) in Chinese, is a subspecies indigenous to continental East Asia, including North and Central China, Korea, Japan and the Ryukyu Islands. Sarcandra chloranthoides Gardner is treated as a synonym of this taxon and its distribution is centred in India and Sri Lanka [1].
The second subspecies, S. glabra subsp. brachystachys (Blume) has a widespread distribution in Northeast India, Northern Vietnam, Southern China and throughout the Malesian region. Sarcandra hainanensis (Pei) Swamy & Bailey (海南草珊) and Chloranthus brachystachys Blume are two synonyms of this taxon and are sometimes used interchangeably [1]. This subspecies differs from subsp. glabra by the length of its anther being almost as equal as the whole male structure, while in subsp. glabra the anther is much shorter and the non-antheriferous part is well-developed [7, 8]. Anatomically, the ventral vein in subsp. hainanensis is single, as opposed to the paired strands in subsp. glabra [9]. Sarcandra glabra var. melanocarpa (Ridl.) Verdc. or Chloranthus brachystachys var. melanocarpus Ridl. is a lesser-known variation of S. glabra subsp. brachystachys. It is an endemic plant found in the montane rainforests of North Sumatra and Malesia and is characterised by its unique black fruits [6].
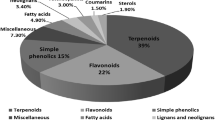
Since the mid-twentieth century, the diverse chemical constituents of S. glabra have piqued the scientific curiosity of various researchers. The observed diversity in the chemical constituents of S. glabra may be attributed to its proliferative nature and location-specific environmental factors influencing the plant’s growth and metabolism. To date, studies have reported nearly 400 compounds from this species, including terpenoids, coumarins, lignans, flavonoids, sterols, anthraquinones, organic acids, and organic esters, many of which have been found to possess interesting structures and/or significant pharmacological activities. These findings underscore the potential of S. glabra as a vast resource for drug discovery and its development.
While several published review papers have covered the phytochemistry of S. glabra [5, 10, 11], there remains a gap in individual and comprehensive reviews that specifically address its chemical constituents and the associated biogenetic pathways. As more recent work surfaced, this review aims to provide a categorical progress update (up to August 2023) on the isolation and structural elucidation of chemical constituents of S. glabra, along with the proposed biosynthetic pathways of specific dimeric and oligomeric sesquiterpenoids, and the biogenetic relationship among these terpenoid skeletons. Furthermore, this review covers an overview of the pharmacological and clinical exploration of crude extracts, medicinal preparations, and the bioactive compounds of S. glabra.
2 Chemical constituents
2.1 Isolation of terpenoids from S. glabra
As the most widely occurring and extensively studied family in natural products, terpenoids are generally distinguished by the number of isoprene (C5) units constituting their carbon skeleton [12]. The term terpenoids refers to modified terpenes that have been chemically altered through oxidation or rearrangement, and is occasionally used interchangeably with terpenes [12]. More than 200 terpenoids have been reported from S. glabra, including triterpenoids (C30), diterpenoids (C20), sesquiterpenoids (C15), monoterpenoids (C10), as well as meroterpenoids.
2.1.1 Sesquiterpenoids
Among the isolates from S. glabra, sesquiterpenoids constitute the largest proportion with more than 180 members. The structures of sesquiterpenoids from S. glabra are rather diverse and accompanied by complex stereochemistry. The structures of these sesquiterpenoids can be divided into eight main skeletal types, namely, eudesmane, lindenane, germacrane, eremophilane, aromadendrane, elemane, guaiane, and cadinene. Herein, the structures of all sesquiterpenoids isolated up to August 2023 are presented; however, only those that were first isolated from S. glabra are highlighted.
2.1.1.1 Eudesmane and eudesmane dimers
Known as selinanes in the early literature, the basic skeleton of eudesmane features an isopropyl-bicyclodecane with four asymmetric centres at C-4, C-5, C-7, and C-10 [13]. The formation of a lactone moiety involving C-7, C-8, C-11, and C-12 gives rise to eudesmane-type sesquiterpene lactones known as eudesmanolides, which account for the majority of the eudesmane-type sesquiterpenoids obtained from S. glabra. Currently, a total of 30 eudesmane compounds (1–30) have been reported (Table 1, Fig. 1), the majority of which were monomers isolated from the whole plants and leaves of S. glabra (1–27).
Sarcaglabosides A and B (2 and 3) were the first examples of hepatoprotective compounds reported from the whole plants of S. glabra [14]. Zhu et al. [15] reported compound 5 as the 9α-hydroxy eudesmanolide derivative of 4, while Hu et al. [16] reported compound 6 as a new eudesmanolide glycoside from the whole plant of S. glabra. Sancandralactone B (9) was reported as a new compound from S. glabra by He et al. [17] but it was originally characterised as serralactone A from Chloranthus serratus [18]. Glabranol B (12) is a new eudesmane-type sesquiterpenoid separated from the aerial parts of a Vietnamese S. glabra specimen [19]. Wang et al. [20] reported the trihydroxyeudesmanolide derivative 13, while Hu et al. [21] reported the 4,15-glycol derivative 14 from the whole plant of S. glabra. Designated as the aglycone of sarcaglaboside B (3), sarcandralactone E (15) was obtained from the whole plant of S. glabra of Guangxi origin [22]. The presence of three eudesmane compounds (16–18) was detected in the seeds of S. glabra [23], with 16 obtained as a racemic mixture and 17 characterised as the C-8 epimer of 14. The S. glabra (whole plant) collected from Jiangxi gave four eudesmane-type sesquiterpenoids (19–22) [24], among which compounds 19 and 20 represent eudesmanolides incorporating a rare 1,4-epoxy bridge. The same plant also yielded compounds 28 and 29, which were the first heterodimer representatives isolated from a Sarcandra plant that incorporate eudesmane and eremophilane sesquiterpenoid halves, along with compound 30, which is a symmetric homodimer featuring a different dimerisation pattern from the former dimeric compounds [24].
A chemical investigation of the leaves of S. glabra from Guangxi province led to the discovery of compounds 23–27, with compounds 23 and 24 being unusual γ-lactam-containing eudesmane-type sesquiterpenoids [25]. Unfortunately, while compounds 28–30, 19 and 20 were given the trivial names sarglanoids A-E, respectively, compounds 23–27 were coincidentally given the same names as both sets of compounds were published around the same time.
2.1.1.2 Lindenane and lindenane oligomers
Despite the limited distribution of lindenane-type sesquiterpenoids in natural sources, their presence is exceptionally prominent in S. glabra. The occurrences of lindenane-type sesquiterpenoids as oligomers, including homodimers, heterodimers, and trimers in S. glabra have gained much research interest due to their intriguing structures. Among them, sarcanolides, sarcandralactones, sarglabolides, sarglalactones, sarcaglabrins and sarcaglabosides could serve as the characteristic components and important chemotaxonomic markers of S. glabra due to their species-wide exclusivity [5].
In monomeric form, lindenane sesquiterpenoids possess a common skeleton comprising a unique linear 3/5/6 tricyclic ring. The system is embedded with a chiral carbon (C-10), an atypical trans-5/6 junction, and a sterically congested cyclopentane with an angular C-14 methyl group [28]. The structural variation of lindenane oligomers is attributed to the combination of monomeric units that make up the backbone, which could be assembled by different linkage patterns. Generally, most dimeric lindenanes from S. glabra were proposed to be constructed from endo-Diels–Alder reactions [29].
At present, 30 lindenane-type sesquiterpenoids (31–60) from S. glabra were reported, among which lindenane-type sesquiterpene lactones (lindenanolides) account for a significant proportion (Table 2, Fig. 2). The first lindenane-type sesquiterpenes (31–32) were isolated by Uchida et al. [30] from Chloranthus glaber (synonym of S. glabra). Subsequently, the presence of several known lindenane compounds (33–39) was reported from the same plant [14, 15, 26, 31, 32]. The whole plants of S. glabra from Xiushui, Jiangxi yielded two new lindenane glycosides, sarcaglabosides F and G (41 and 42), together with a known compound (40) [16]. In search for cytotoxic sesquiterpenoids from a Hainanese S. glabra plant, He et al. [17] reported sarcandralactone A (43), which is a C-5 hydroxylated analogue of shizukanolide A (34). The butanolic extract of S. glabra (whole plant) provided a new trihydoxylindenanolide, glabranol A (44) [19]. Compounds 47 and 48 were reported as new compounds from S. glabra by Ni et al. [22], and were named sarcandralactones C and D, respectively. A phytochemical investigation of the anti-inflammatory constituents from S. glabra led to the discovery of sarglabolide L (52), representing a rare lindenane derivative possessing an 18-membered macrocyclic triester, and shizukanolide 8-O-β-D glucopyranoside (53) [23]. From the dichloromethane and petroleum ether fractions of S. glabra, Chi et al. [33] reported the isolation of sarglalactones I-M (54–58), a group of unique 8,9-secolindenane derivatives featuring an opened lactone ring.
Lindenane-type sesquiterpenoid oligomers and their derivatives are recognised as the characteristic taxonomic symbol of S. glabra. Despite having complex structures and functionalities, 86 lindenane-type sesquiterpenoid oligomers (61–146) were successfully characterised from S. glabra (Table 3, Fig. 3).
Although most of the dimeric lindenane sesquiterpenoids from S. glabra were presumed to be biosynthesised via Diels–Alder, several compounds displayed variations in the linkage between two constitutional units. Chloranthalactone F (61), later renamed as chloranthalactone A photodimer, is a representative dimeric sesquiterpenoid formed by a [2 + 2] cycloaddition [26]. Being the only [6 + 6] lindenane cycloadduct isolated from S. glabra, cycloshizukaol A (67) has an interesting C-2-symmetrical structure that incorporates a cyclododecatetraene ring [17]. He et al. [17] isolated a group of structurally related lindenane dimers known as sarcandrolides A-E (69–73) from the whole plant of S. glabra. The same group also isolated two new dimers, sarcanolides A and B (74 and 75), featuring an unprecedented nonacyclic scaffold from S. hainanensis (subspecies of S. glabra).
Ni et al. [22] reported five new dimeric lindenanes, sarandrolides F-J (81–85), together with five known compounds (76–80) in the course of cytotoxicity screening of S. glabra sesquiterpenoids. Sarcandrolide F (81) represents the first example of a lindenane-type dimer with a hydroperoxy group at C-5 [22]. Wang et al. [37] investigated the seeds of S. glabra, which led to the isolation of 11 new lindenane dimers, sarglabolide A-K (87–97). Among the isolates, compound 87 has a notable 17-membered macrocyclic ester ring that differs from the usual 18-membered system featured in other lindenane dimers. The continued endeavour of Wang’s group [38] resulted in the discovery of two uncommon heterodimers, sarglaperoxides A and B (98 and 99), featuring a lindenane and a normonoterpene unit assembled via a 1,2-dioxane ring, from the seeds of S. glabra. With the guidance of MS/MS molecular networking, Wang et al. [39] reported the occurrence of four additional isomeric heterodimers, sarcaglarols A-D (117–120), whose skeletal structures resemble those of 98 and 99, from the leaves of S. glabra. Another structurally related heterodimer sarcaglarone A (141) was isolated by Sun et al. [40] from the seeds of S. glabra, which incorporates a distinctive lactone in the monoterpene fragment.
An investigation by Chi et al. [33] on S. glabra from Guangxi afforded a series of unprecedented 8,9-secolindenane-type sesquiterpenoid oligomers, including three trimers, sarglalactones A-C (101–103), and five dimers, sarglalactones D-H (104–108). S. glabra specimens from the Guangxi region were also found to contain a rare lindenane-monoterpene heterodimer sarcaglabrin A (109), two new lindenane dimers sarcaglabrins B and C (110–111), and five known compounds (112–116) [4]. Sun et al. [41] reported the isolation of five norlindenane dimers, sarglaromatics A-E (123–127), bearing a naphthalene or a dihydronaphthalene core from the roots of S. glabra, whereas Xiao et al. [42] reported the addition of three new sarcanolides (128–130) from the roots of S. glabra. Compared to their congeners (74 and 75), sarcanolide C (128) bears a supplemental orthoformate ring at C-4 and C-5, whereas sarcanolide D (129) was elucidated as the 4-O-acetyl derivative of sarcanolide A (74). From the leaves of S. glabra, Wang et al. [43] encountered an unprecedented [4 + 2]-type lindenane dimer, sarglafuran A (131), which represents the first dimer to contain a furan moiety in its lindenane monomer unit instead of an α,β-unsaturated lactone or an opened-ring lactone. Wang’s group [43] also reported the isolation of sarglalactones N and O (132 and 133), a pair of C-4 epimers that also possess a [2 + 2]-type dimeric skeleton and were identified as dihydroxy derivatives of 61.
Zhou et al. [44] revealed the presence of seven new dimeric lindenanes (135–140) with significant antimalarial activities and a known compound (134) from the roots of S. glabra subsp. brachystachys. 15′-O-4-Hydroxytigloylfortunoid C (134) and 13′-O-methyl succinyl-15′-O-tigloylfortunoid C (135) share a common heterodimeric core comprising a lindenane and a eudesmane unit, whereas 4″-hydroxysarcandrolide A (137) and 13′-O-acetylsarcandrolide B (138) were identified as structural analogues of 70. 6α-Hydroxysarglaperoxide A (142) and 7′-oxyisosarcaglabrin A (143) are two new heterodimers isolated from the seeds of S. glabra, and were determined as the derivatives of 98 and 109, respectively [40]. Guided by a single-node-based molecular networking approach, Cui et al. [45] discovered the first tetrahydrofuran-linked-lindenane-normonoterpene heterodimer sarglaoxolane A (144) from the leaves of S. glabra, together with a pair of pseudonatural epimers sarglaoxolanes B and C (145–146), which were spontaneously formed from 144.
2.1.1.3 Germacranes
Germacranes are an elemental class of sesquiterpenoids containing a characteristic cyclodecane ring formed from the ring closure of C-1 and C-10 of farnesane [51]. The carbon skeleton features an isopropyl group at C-7 and two methyl groups at C-4 and C-10 [52]. Five germacrane-type sesquiterpenoids (147–151) have been isolated from the essential oils and ethanolic extract of S. glabra (Table 4, Fig. 4), with sarcaglaboside E (148) being identified as a new glucoside derivative [14].
2.1.1.4 Eremophilanes
Eremophilanes belong to a family of sesquiterpenoids featuring a bicyclodecane skeleton similar to that of eudesmane-type sesquiterpenoids. The carbon skeleton of eremophilane is naturally derived from the rearrangement of eudesmane derivatives, exemplified by the migration of the methyl group from position C-10 to C-5 [51, 54]. The number of eremophilane-type sesquiterpenoids isolated from S. glabra amounts to five so far (Table 5, Fig. 5), four of which were first isolated from other plants, i.e., 152–155. Sarglanoid F (156), the 8-ethoxy derivative of 153, is the only eremophilane that was first reported from S. glabra [25].
2.1.1.5 Aromadendranes
The scaffold of aromadendranes usually incorporates a gem-dimethylcyclopropane ring that is fused to a hydroazulene skeleton [57]. The skeleton bears a strong resemblance to that of guaiane-type sesquiterpenoids, differing only in having a cyclopropane ring formed by the bond linking C-6 and C-11 in aromadendranes. Hence, aromadendrane-type sesquiterpenoids are also known as 6,11-cycloguaianes [51]. At present, eight aromadendrane-type sesquiterpenoids (157–164) from S. glabra were documented in the literature (Table 6, Fig. 6). Sarglanoid G (164) was reported for the first time from the roots of S. glabra [58].
2.1.1.6 Elemanes
Elemanes constitute a small group of sesquiterpenoids in S. glabra and are olefinic compounds with cyclohexane as their core [60]. A total of eight sesquiterpenes of this class (165–172) have been reported from S. glabra (Table 7, Fig. 7), including two new elemanolide aglycons, sarcaglaboside C and D (165 and 166)[14], five known compounds (167–171), and a new furan-bearing elemane-type sesquiterpenoid sarglanoid H (172) [58].
2.1.1.7 Guaiane, cadinane, and other sesquiterpenoids
Compound 173 is the only guaiane-type sesquiterpenoid isolated from the whole plant of S. glabra featuring a distinctive epoxy bridge at C-4 and C-6, whereas compound 174, a component of cade oil [62], is the only cadinane-type sesquiterpenoid isolated from the essential oil of S. glabra (Table 8, Fig. 8).
Apart from the aforementioned sesquiterpenoid subtypes, there are more than a dozen other sesquiterpenoids isolated from S. glabra (Table 8, Fig. 8). These include aliphatic farnesenes (175, 179–181), cyclic farnesenes (177 and 178), megastigmane-type sesquiterpenoids (182 and 187), and their glucosides (176, 183, 186, 188). Li et al. [63] reported the isolation of two new compounds, sarcabosides A and B (184 and 185), from S. glabra specimens collected from Sichuan province. Both compounds possess interesting, highly conjugated skeletons.
2.1.2 Monoterpenoids
Monoterpenoids are a terpenoid class consisting of two isoprene units with the general molecular formula C10H16 [65]. They are derived from the putative precursor, geranyl diphosphate, and may exist in linear forms (acyclic) or comprise ring structures (cyclic). Acyclic monoterpenoids are formed by the head-to-tail polymerisation of isoprene monomers, whereas bicyclic monoterpenoids are formed from additional cyclisation and rearrangement via monoterpene synthases [66]. To date, 16 monoterpenoids (189–204) have been reported from the essential oils, whole plants, and seeds of S. glabra (Table 9, Fig. 9). Among them, seven are acyclic (192, 197–199, 201–202, 204), six are monocyclic (193–196, 200, 203), and three have bicyclic structures (189–191). 6-Hydroxy-2,6-dimethylhepta-2,4-dienal (204) is a nine-carbon normonoterpene newly isolated from S. glabra [38].
2.1.3 Diterpenoids and triterpenoids
The molecular formula of diterpenoids (C20H32) is indicative of their 20-carbon skeleton derived from the condensation of four isoprene units [65]. All diterpenoids originate from a common substrate, geranylgeranyl diphosphate, of which cyclisation into different scaffolds by diterpene synthase gives rise to their structural diversity [67]. All four reported diterpenoids (205–208) from S. glabra are labdane-type diterpenoids, three of which (206–208) were isolated as diastereomers (Table 10, Fig. 10).
Composed of six isoprene units (C30H48), triterpenoids have a common acyclic biosynthetic precursor, squalene [68]. Overall, their skeletons may be categorised based on the number of rings present in the structure. Pentacyclic triterpenoids are mostly prevalent in the whole plants of S. glabra (Table 10, Fig. 10). Seven of these triterpenoid compounds (209–215), including two new triterpenoid saponins, sarcandrosides A and B (210 and 211), were isolated from the aerial parts and whole plants of S. glabra.
2.1.4 Meroterpenoids
The term meroterpenoid was initially coined by Cornforth [71] and is used to define natural products of mixed biosynthetic origin that are partially derived from terpenoid pathways. Presently, a total of 13 meroterpenoids (216–228) have been isolated from S. glabra (Table 11, Fig. 11). Yang et al. [72] reported the discovery of three novel meroterpenoids, namely a chalcone-coupled monoterpenoid, glabralide A (216), and two geranylated meroterpenoids, glabralides B and C (217 and 218), from the whole plants of S. glabra. Compound 216 contains a bicyclo[2.2.2]octene core, while compound 218 displays an unprecedented linearly fused 6/6/6 ring system. Subsequently, a study on a S. glabra species from Anhui province [49] led to the isolation of five new glabralide meroterpenoids, glabralides D-H (219–223), possessing structures resembling that of 218. Sarglamides A-E (224–228) represent five unprecedented indolidinoid-monoterpenoid hybrids derived from toussaintine C and α-phellandrene isolated from the whole plant of S. glabra subp. brachystachys [73].
2.2 Isolation of phenylpropanoids from S. glabra
Phenylpropanoids are a vast and structurally diverse group of natural products in the plant kingdom. They play vital roles in plant development through their interaction with the environment and other living organisms. Phenylpropanoids are derived from the aromatic amino acids, phenylalanine and tyrosine, via the shikimate pathway [74]. 4-Coumaroyl-CoA represents a crucial enzyme in the metabolic network that leads to the biosynthesis of phenylpropanoid compounds such as coumarins, lignins, flavonoids, and phenolic acids [75]. Diversification of the subsets of phenylpropanoids is achieved by an array of successive enzymatic transformations such as acylation, condensation, cyclisation, glycosylation, hydroxylation, methylation, and prenylation [76].
2.2.1 Coumarins
Coumarins represent a class of aromatic lactones derived from the ortho-hydroxylated cis-hydroxycinnamic acid in the phenylpropanoid pathway [77]. Due to the versatility of the coumarin scaffold, various pharmacophores and functionalised coumarins could be generated via a series of transformations and substitutions, which explains the exceptional pharmacological activities displayed by this class of compounds [78,79,80]. Currently, the identification of 26 coumarins (229–254) has been reported in various literature of S. glabra (Table 12, Fig. 12), which mostly consist of known compounds, particularly isofraxidin (230) and its dimers (233 and 238), as well as its substituted analogues at C-6, C-7 and C-8 (231–232, 234–237, 239–241, 243, 245–246, 254). This designates isofraxidin (230) as a representative compound of this class and establishes its inclusion as a quality control marker for medicinal preparations of S. glabra in the Chinese Pharmacopoeia [81]. Feng et al. [78] discovered a new coumarin, sarcandracoumarin (244), from the whole plant of S. glabra bearing a 1-phenylethyl substituent at C-3. Unfortunately, the absolute configuration of this compound remains unclear. Wang et al. [82] reported a new coumarin, 3,5-dihydroxycoumarin-7-O-α-L-rhamnopyranosyl-2H-chromen-2-one (247), possessing anti-inflammatory properties, from S. glabra. From the stems of S. glabra, Du et al. [83] uncovered the presence of five coumarins, namely, a pair of coumarin-phenylpropanoid enantiomers (7S,8S) and (7R,8R)-sarcacoumarin (250 and 251) with promising acetylcholinesterase inhibitory activities, and three known compounds (252–254).
2.2.2 Lignans and neolignans
Lignans and neolignans are dimeric structures commonly derived from the oxidative coupling of two lignol units. They may differ in the degree of oxidation of the three-carbon sidechain and the substitution on the aromatic ring [91]. Up to the present, only two lignans (255 and 256) and an array of neolignans (257–263) were isolated from the whole plants of S. glabra (Table 13, Fig. 13). The isolation of a pair of dihydrobenzofuran neolignan enantiomers, (+) and (−)-sarcanan A (262 and 263), from the aerial parts of S. glabra was recently reported [79].
2.2.3 Flavonoids
Flavonoids, a diversified group of phytochemicals found in many natural sources, are the second most predominant secondary metabolites in S. glabra. Despite having a common structure, flavonoids in S. glabra have intrigued the interest of numerous scientists due to their extensive pharmacological and biological activities that hold significant pharmaceutical importance [93, 94].
Chemically, the general structure of flavonoids consists of a C6-C3-C6 diphenylpropane backbone [95]. Flavonoids can be divided into a myriad of subgroups in terms of ring arrangements and functionalisation of the ring systems. Flavonoids in which ring B is attached to the C-3 position of ring C are called isoflavonoids [96], while those in which ring B is linked to C-2 of ring C are further categorised into different subtypes depending on the hydroxylation pattern and variations in the chromane ring or ring C [97]. Some relevant examples of these subtypes include flavans, flavones, flavonols, flavanones, flavanols, flavanonols, and anthocyanins. Chalcone otherwise known as open-chain flavonoids are distinguished from other flavonoids by their two aromatic rings (A and B), which are linked by an α,β-unsaturated carbonyl chain.
2.2.3.1 Chalcones
Following the isolation of a series of dihydrochalcones (264–269) from the aerial parts of S. glabra, subsequent studies have reported 21 more chalcones (270–272, 276–293) from S. glabra and an additional three (272–275) from its subspecies S. hainanensis (Table 14, Fig. 14). Interestingly, Liu et al. [98] isolated a rare class of uncommon monoterpene-chalcone conjugates with potential autophagy-inducing activities from the aerial parts of S. glabra, some of which are connected via a dihydrofuran ring or an ether bridge. The compounds include four monoterpene-conjugated chalcones, glabratins A–D (279–282), seven monoterpene-conjugated dihydrochalcones, glabratins E-K (283–289), and four known analogues (290–293).
2.2.3.2 Anthocyanidins
Anthocyanidins are a class of flavonoids known for their roles as natural pigments that bestow fruits and flowers different colours. Their distinctive skeletal backbone is based on a flavylium cation that lacks the ketone group at C-4 [99] So far, only three anthocyanidin-type compounds (294–296) were characterised from S. glabra (Table 14, Fig. 15). Ishikura [100] was the first researcher to report the occurrence of two glycosides of anthocyanidins (294 and 295) from the fruits of C. glaber (syn. S. glabra), whereas Li et al. [89] isolated a leucoanthocyanidin compound (296) from the whole plant of S. glabra.
2.2.3.3 Flavones and flavonols
The backbones of flavones and their hydroxylated derivatives (flavonols) are distinguished by the presence of a double bond between C-2 and C-3, which extends the π-conjugation onto the carbonyl group in the pyranone ring [105]. Collective studies on S. glabra have reported a total of 15 flavone and flavonol-type compounds (297–311), including a number of quercetin and kaempferol derivatives (Table 15, Fig. 16).
In a recent study, Qin et al. [106] revealed the presence of two new methylenedioxyflavones (297 and 298) and two known derivatives (299 and 300) from S. glabra, among which compounds 297, 299 and 300 showed pronounced cytotoxic effects. Ubiquitous in various natural sources, the structure of quercetin (301) comprises a pentahydroxyflavone, which is oftentimes conjugated with residual sugars to form quercetin glycosides [107]. Overall, all reported quercetin-type flavonoids from S. glabra were obtained from the whole plants and are derivatives substituted with glycoside groups at C-3, such as glucuronide (302–304), rhamnoside (305), rutinoside (306), galactose (307) and glucoside (308). Kaempferol (309) is a tetrahydroxyflavone in which the four hydroxyl groups are at C-3, C-5, C-7 and C-4′ [108]. So far, only three compounds of this class (309–311) have been isolated from the whole plants of S. glabra and S. hainanensis (subsp. brachystachys).
2.2.3.4 Flavanones and flavanols
Derived from flavones, flavanones are important precursors and key intermediates in the flavonoid biosynthetic pathway. Flavanones and their hydroxylated derivatives (flavanols) differ from flavones and flavonols by two features: the presence of a C-2 chiral centre and the absence of a double bond between C-2 and C-3 [112]. Up to now, a total of 29 flavanone and flavanol-type compounds (312–339), including naringenin and catechin derivatives, were reported from S. glabra (Table 16, Fig. 17).
Naringenin is a pertinent example of a flavanone aglycone having three hydroxyl groups at C-5, C-7, and C-4' [113]. Most of the compounds belonging to this class occur as methoxy and glycosidic derivatives (312–314) and were isolated from the whole plants of S. glabra and S. hainanensis (subsp.). Interestingly, some of the naringenin-type compounds isolated from S. glabra are epimeric at C-2, as exemplified by 315–318 [89, 114].
Catechins are secondary metabolites that contribute to the antioxidative activities in plants [115]. They exist as minor constituents in S. glabra and are flavan-3-ols characterised by the absence of a ketone group at the C-4 position. To date, only six catechin-type flavanols (319–323) were documented in the literature. Li [59] reported the isolation of a rhamnopyranoside derivative (319) and two new epimeric phenylpropanoid-substituted catechin glycosides, glabraosides A and B (320 and 321), from the whole plant of S. glabra. Subsequently, Wang et al. [20] expanded the family by reporting two new catechin glycosides, glabraosides C and D (322 and 323), with their phenylpropanoid fragments fused to ring A at C-5/C-6 and C-7/C-8, respectively. Liu et al. [98] reported glabratins L-N (337–339) as three monoterpene-flavanone conjugates from the aerial parts of S. glabra. Compounds 337 and 338 were obtained as a pair of C-2 epimers, whereas the flavanone core of 339 is linked to the monoterpene unit via an ether bridge instead of a C–C bond.
2.2.3.5 Dimeric flavonoids
The occurrence of dimeric flavonoids was found to be exclusive to the S. hainanensis subspecies, as evidenced by the isolation of four compounds (340–343) from the dichloromethane extract of the whole plant (Table 16, Fig. 18). Sarcandrones A and B (340 and 341) represent new hybrid flavan-chalcones that are linked from C-4 of the flavan moiety to C-3′ of the chalcone moiety [116]. On the other hand, sarcandrones C and D (342 and 343) were characterised as analogues that are linked from the flavan moiety to the flavanone moiety at C-4 to C-8′ and C-4 to C-6′, respectively [111].
2.3 Isolation of anthraquinones, organic acids, and organic esters from S. glabra
Anthraquinones, also referred to as anthracenediones or dioxoanthracenes, are associated with the quinone class under the polyketide family. A characteristic feature of anthraquinones is their polycyclic aromatic structure wherein two keto groups are located on the central ring [117]. Current literature on S. glabra has cumulatively reported five anthraquinone-type compounds (344–348), which mainly consist of hydroxy, methoxy or glycoside derivatives (Table 17, Fig. 19).
As shown in Table 17 and Fig. 20, S. glabra plants are rich sources of organic acids. At present, more than a dozen known organic acids have been isolated from S. glabra (349, 352–355, 357–367), while three (350–351, 356) were obtained from the subspecies S. hainanensis. These include dicarboxylic acids (349 and 364), long-chained fatty acids (350, 351, 354, 356, 361–363), and multi-substituted phenolic acids (352–353, 355, 357–360, 364–367).
Organic esters (368–391) were particularly abundant in S. glabra. These compounds are distributed in different plant parts and their structures could be divided into several categories (Table 17, Fig. 21). For instance, compounds 386, 387, and 391 were classified as caffeic acid derivatives, while compounds 381, 383, and 384 are generally known as caffeoylshikimic acids. Esters in which their quinic acid core is acylated with one or more caffeoyl groups are known as caffeoylquinic acids or chlorogenic acids. Eight compounds of this group (372, 377–380, 385, 388–389) were isolated from the polar partitions of S. glabra. From the whole plant of S. glabra, Wu et al. [79] reported a new glycoside compound (390), benzyl 2-β-glucopyranosyloxybenzoate. Among all organic esters, rosmarinic acid (369) is specifically recognised for its pronounced pharmacological activities and its high content within S. glabra [3, 118]. For this reason, rosmarinic acid is established as one of the key quality control markers in the Chinese Pharmacopoeia [81].
Interestingly, the distribution of organic esters differs between the plant parts of S. glabra. This hypothesis was validated by Zhou et al. [114] who concluded that chlorogenic acids, caffeic acids, and 4-O-glucopyranosyl rosmarinic acid were mostly concentrated in the stems, whereas chlorogenic acids were dominant in the leaves. On the other hand, rosmarinic acids were found to be distributed in the aerial parts (stems and leaves) of S. glabra.
2.4 Isolation of alcohols and sterols from S. glabra
A total of ten alcoholic compounds (392–401) were reported from the whole plants of S. glabra, including a saturated fatty alcohol (393), a sugar alcohol (394), and eight phenol derivatives (392, 395–401) (Table 18, Fig. 22).
Sterols are a subgroup of steroids distinguished by their hydroxyl group at C-3 of ring A [127]. All four stigmastane-types phytosterols (402–405) reported in the literature were isolated from the whole plants of S. glabra, except for compound 402, whose occurrence was also reported from the subspecies S. hainanensis.
2.5 Isolation of other compounds from S. glabra
Apart from the mentioned classes of secondary metabolites, the rare presence of a ketone (406), monosaccharide (407), alkaloids (408, 412–413), alkane (410), and lactones (409 and 411) have also been reported during the isolation process (Table 18, Fig. 23).
3 Biogenetic pathways of oligomeric sesquiterpenoids and meroterpenoids from S. glabra
The biosynthesis of oligomeric sesquiterpenoids and meroterpenoids from S. glabra is a complex process involving a series of reactions. Generally, the majority of lindenane-type oligomers in S. glabra were assembled via Diels–Alder reaction, facilitated by the presence of diene and dienophile elements within the monomeric units. Occasionally, the dimerisation or oligomerisation process also involves the integration of direct cyclisation, oxidative coupling, esterification, acetalisation, aldol reaction, and Michael-type reaction using various linkers [129]. Herein, the proposed biogenetic routes for a selection of oligomeric sesquiterpenoids and meroterpenoids isolated from S. glabra are summarised. A biogenetic relationship that connects the various key terpenoid skeletons obtained from S. glabra is also presented.
3.1 Biosynthesis of shizukaol A and lindenatriene as a key biogenetic building block
The first dimeric lindenene sesquiterpene, shizukaol A, was reported by Kawabata et al. [130]. Since then, numerous other heterodimeric sesquiterpenes have been reported with a carbon skeleton similar to that of shizukaol A. Shizukaol A was hypothesised to be biogenetically derived from a Diels–Alder reaction (Scheme 1) based on a sealed tube pyrolysis experiment of shizukaol A, yielding lindenatriene and chloranthalactone A as the retro-Diels–Alder adducts [130]. However, since lindenatriene was highly unstable and rapidly decomposed in situ, it was only obtained in trace amounts and partially characterised by 1H NMR.
Pyrolysis of shizukaol A and the biosynthetic hypothesis of dimeric lindenanes [130]
The structure proposed for lindenatriene by Kawabata’s group was finally confirmed almost two decades later through a synthesis by Eagan et al. [131]. They showed that lindenatriene was unstable and readily isomerised to its more thermodynamically stable tautomer, iso-lindenatriene, under mildly basic conditions (Scheme 2). Due to the inherent instability of the triene moiety, Yuan et al. [132] encountered a setback in obtaining the anticipated Diels–Alder adduct in their model biomimetic reaction, in which they employed the equally unstable des-hydroxy derivative of lindenatriene. Despite synthesising lindenatriene, the isolated and characterised compound was its tautomer, iso-lindenatriene. Consequently, the discrepancies between the 1H NMR chemical shifts reported by Yuan et al. and those reported by Kawabata et al. raised questions regarding the validity of the proposed biogenetic mechanism. Finally, Martinez et al. [133] re-assessed the literature and NMR data, affirming lindenatriene’s continued relevance as a building block in Kawabata’s original biogenetic hypothesis.
3.2 Biosynthesis of sarglanoids A-C (28–30)
Li et al. [24] proposed that sarglanoids A-C (28–30) originate from a common precursor farnesyl diphosphate (FDP). A cascade of spontaneous cyclisation, oxidation, lactonisation, and double-bond migration was hypothesised to result in the formation of the eudesmane (i) and eremophilane (ii, iii) monomers (Scheme 3). Subsequently, the lactone moieties of the monomeric units are directly connected by a C–C bond via free-radical coupling reactions to form sarglanoids A-C.
3.3 Biosynthesis of sarcanolides A-D (74, 75, 128 and 129)
As depicted in Scheme 4, He et al. [47] proposed a biosynthetic route for sarcanolides A (74), B (75), C (128) and D (129), which involves a Diels–Alder reaction between lindenatriene and chloranthalactone A. The resulting cycloadduct then undergoes an acid-catalysed intramolecular cyclisation connecting C-11 to C-7′, generating a β-oriented lactone. Sequential oxidation and acylation of the nonacyclic intermediate would ultimately form sarcanolide A (74), which could then be transformed into sarcanolides B, C and D (75, 128 and 129) by dehydration, ortho-ester formation, and acetylation, respectively.
3.4 Biosynthesis of sarglaperoxides A and B (98 and 99), sarcaglarols A-D (117–120), sarcaglarone A (141), and 6α-hydroxysarglaperoxide A (142)
Wang et al. [39] proposed a common biosynthetic pathway for compounds 98–99, 117–120, 141 and 142 as shown in Scheme 5. It was inferred that geraniol and chloranthalactone A, two abundant components of S. glabra, serve as biogenetic precursors for the formation of these dimers. The fundamental core of these dimers was hypothesised to be formed through a photocatalytic aerobic [2 + 2 + 2] cycloaddition step. Firstly, intermolecular cycloaddition between geraniol and chloranthalactone A in the presence of O2 generates an intermediate that yields sarcaglarols A-D (117–120) via a series of oxidative reactions. The sarcaglarols undergo successive oxidative cleavage at the C-2′,C-3′-diol fragment, leading to the formation of sarglaperoxide A (98) and its hydroxylated analogues, 99 and 142. Finally, the formation of the lactone functionality in sarcaglarone A (141) involves oxidation of the sarcaglarols, followed by a free-radical-mediated intramolecular cyclisation that links C-1′ and C-5′.
3.5 Biosynthesis of sarglalactones A-H (101–108)
Based on the postulation by Chi et al. [33], the oligomers sarglalactones A-H (101–108) could originate from chloranthalactone A, whose highly conjugated lactone undergoes oxidative cleavage at the Δ8,9 double bond to yield two presumptive precursors chloranerectuslactone and 8,9-secolindenane (Scheme 6). The skeleton of the trimers 101–103 arises from the condensation between a chloranerectuslactone core and two units of chloranthalactone E through acetalisation and transesterification, while the dimers 106–108 are assembled in the same manner, except that they lack a second unit of chloranthalactone E. On the other hand, epimers 104 and 105 are linked by the monomers, 8,9-secolindenane and chloranthalactone E, through a cyclic acetal moiety.
3.6 Biosynthesis of sarcaglabrin A (109) and 7′-oxysarcaglabrin A (143)
Sarcaglabrin A (109) was presumed to be the Diels–Alder adduct of chloranthalactone A and a naturally occurring geranyl diphosphate (GDP)-derived monoterpene, β-E-ocimene (C10) [4]. On the other hand, the hydroxylated analogue 143 isolated by Sun et al. [40] was deduced to adopt a similar biosynthetic route (Scheme 7).
3.7 Biosynthesis of sarglaromatics A-E (123–127)
According to Sun et al. [41], the unprecedented skeletons of sarglaromatics A-E (123–127) were presumed to derive from chlorahololide D (80) and sarcandrolide A (69), two abundant [4 + 2] lindenane sesquiterpenoid dimers from S. glabra (Scheme 8). The removal of H-6 of the precursors consequently generates a C-11 free radical that performs an intramolecular attack onto C-11′ of the lactone ring, thereby forming the key radical intermediate (iv). Subsequently, radical termination could occur in two possible ways. The first pathway (A) involves a decarboxylation process that gives rise to a dimer bearing an aromatic framework, which eventually transforms into sarglaromatics A-C (123–125) by way of a carbocation intermediate (v). Alternatively, the opening of the cyclopropane ring in the carbocation intermediate leads to the formation of sarglaromatic D (126). In the second pathway (B), the free radical at C-11′ is terminated with the formation of an exocyclic bond. By way of a carbocation formation at C-4 (vi), the corresponding dimer, sarglaromatic E (127), is formed.
3.8 Biosynthesis of sarglafuran A (131)
According to Wang et al. [43], sarglafuran A (131) was proposed to be biosynthesised through the hydroxylation of the C-13 methyl group in sarglabolide C (89), followed by an intramolecular nucleophilic addition to form a hemiketal intermediate. Finally, dehydration at C-8 furnishes the characteristic furan moiety in 131 (Scheme 9).
3.9 Biosynthesis of sarglaoxolanes A-C (144–146)
A possible biogenetic and transformational pathway of sarglaoxolanes A-C (144–146) is presented in Scheme 10 [45]. The biosynthesis commences with the epoxidation and oxidation of the precursors chloranthalactone A and geraniol, which results in a carbocation intermediate and a tertiary alcohol, respectively. Subsequently, the skeleton of heterodimer 144 is constructed through two consecutive nucleophilic additions. Meanwhile, epimers 145 and 146 could be spontaneously formed from 144 by nucleophilic addition of the tertiary alcohol to the carbocation C-7, followed by tautomerisation.
3.10 Biosynthesis of glabralides A-F (216–221)
Yang et al. [72] proposed that the scaffold of glabralide A (216) is constructed via an enzyme-catalysed Diels–Alder reaction between α-phellandrene and a chalcone derivative (Scheme 11). On the other hand, glabralides B-F (217–221) were postulated to adopt a divergent route featuring a geranylated phenylacetic intermediate (Scheme 12). Oxidation of the phenylacetic intermediate forms a conjugated ketone moiety, which then undergoes radical lactonisation to give a lactone radical. Successive radical addition involving 2,2,3-trimethyloxirane, followed by an acetaldehyde elimination step eventually gives glabralide B (217). From the same phenylacetic intermediate, an alternative radical intermediate is formed through methyl esterification and oxidation, which, by radical addition and oxidation produces a tertiary carbocation intermediate. Subsequent cationic cyclisation and oxidation then give rise to a fused 6/6/6 ring system exhibited by glabralide C (218). Ultimately, hydroxylation of the ring system or the geranyl moiety would result in the formation of glabralides D-F (219–221).
3.11 Biosynthesis of sarglamides A-E (224–228)
The indolidinoid-monoterpene conjugates, sarglamides A-E (224–228), were proposed to derive from the precursors (S)-α-phellandrene and toussaintine C (Scheme 13) [73]. The biosynthesis of sarglamides A and B (224 and 225) was assumed to involve a head-to-head endo-Diels Alder between (S)-α-phelladrene and (+)-toussaintine C. The two regioisomers differ in the approach direction of the (S)-α-phellandrene dieneophile during the reaction, with the isopropyl group assuming an α orientation for 224 and a β orientation for 225. Conversely, sarglamide C (226) was postulated to form via an endo-Diels Alder between (S)-α-phellandrene and (-)-toussaintine C in a head-to-tail manner. Sarglamide D (227) could be derived from 226 via acetalisation at the C-7 carbonyl, followed by an electrophilic addition that links the C-7 oxygen to C-1″. On the other hand, an acid-catalysed electrophilic addition of the C-4 hydroxyl to the Δ1′′,2′′ alkene in 226 would give rise to 228.
3.12 Biogenetic relationship among various terpenoid skeletons of S. glabra
A biogenetic pathway linking the various terpenoid skeletons of S. glabra is depicted in Scheme 14. The terpenoid constituents of S. glabra are produced via a common mevalonate pathway. Within this pathway, geranyl diphosphate (GDP) and farnesyl diphosphate (FDP) serve as pivotal intermediates, directing the synthesis of monoterpenoids and sesquiterpenoids, respectively. Cyclisation of GDP yields the monoterpene α-phellandrene, a precursor of meroterpenoids from S. glabra, e.g., glabralides A-F (216–221) and sarglamides A-E (224–228).
On the other hand, the addition of an isopentenyl diphosphate (IDP) unit to GDP forms FDP, an acyclic precursor of different classes of cyclic sesquiterpenes. The process involves the formation of the highly reactive farnesyl carbocation intermediate, which undergoes cyclisation and rearrangements to construct the diverse ring systems exhibited by germacrane, elemane, guaiane, eudesmane, eremophilane and cadinene-type sesquiterpenoids [134].
Alternatively, lindenane-type sesquiterpenoids may be synthesised from FDP through isofuranodiene, a naturally occurring constituent discovered in plants of the Chloranthaceae family [135]. Intramolecular cyclopropanation of isofuranodiene would generate lindenene, the biosynthetic precursor from which shizukanolide A and lindenatriene are derived. Through Michael addition and Diels–Alder reaction, lindenatriene could oligomerise into the trimer trishizukaol A (122), whereas modifications and functionalisation of shizukanolide A (34) at C-4, C-8, C-9, and C-15 enables its conversion into various lindenane-type sesquiterpenoids, including chloranthalactone A (31).
Chloranthalactone A (31) is a lindenane-type sesquiterpenoid with a highly unsaturated skeleton that exhibits versatility in undergoing dimerisation or oligomerisation via diverse pathways. The resulting array of dimeric and oligomeric sesquiterpenoids varies based on the number and positions of double bonds engaged in the reaction. For example, [2 + 2] and [6 + 6] cycloadditions between two units of chloranthalactone A (31) lead to the formation of the homodimers chloranthalactone F (61) and cycloshizukaol A (67), respectively.
Additionally, chloranthalactone A assumes a pivotal role as a building block in the biosynthesis of the [4 + 2]-type dimeric lindenanes, the chemotaxonomic constituents of S. glabra. The basic skeleton of this class of compounds is depicted by shizukaol A (100), a cycloadduct of chloranthalactone A and lindenatriene. Through subsequent transformations such as acetylation, esterification, oxidation, glycosylation, epoxidation, and lactonisation, shizukaol A can be transformed into various [4 + 2]-type congeners. Chloranthalactone A is also the putative precursor of a series of sesquiterpene-normonoterpene heterodimers, exemplified by compounds 98, 99, 141, 142, 117–120, and 144–146. The biosynthetic pathway occurs through [2 + 2 + 2] cycloaddition or nucleophilic addition reactions with geraniol. On the other hand, the conjugation of chloranthalactone A with a different monoterpene moiety, β-E-ocimene, would yield compounds 109 and 143. The conjugation of chloranthalactone A derivatives with chloranthalactone E through an atypical acetalisation and esterification process would result in the formation of 8,9-secolindenane-derived dimers and oligomers (101–108).
4 Biological activities
The multifarious biological activities of S. glabra revealed by modern pharmacological studies include antioxidative, antibacterial, antifungal, antiviral, antimalarial, anti-thrombocytopenic, antitumour, anti-inflammatory, immunomodulatory, hepatoprotective effects, etc. A compilation of the reported bioactivities exhibited by plant extracts, medicinal formulations, and isolates from S. glabra can be found in Table 19.
4.1 Antioxidative
The antioxidative properties of S. glabra extract (SGE) have been widely investigated in several animal models. Preliminary and in vivo studies reported that SGE can ameliorate radiation-induced reactive oxygen species (ROS) injury and aid post-radiation recovery in guinea pig and rat models [136,137,138], besides moderating stress-attenuated immune response in restrained mice [139]. In miniature pigs, S. glabra powder exhibited prominent scavenging activities against radiation-induced ROS in the parotid gland [140].
The antioxidant properties of SGE exhibited concentration-dependent behaviour, as demonstrated by in vitro free radical scavenging assays [141, 142]. The most pronounced antioxidant efficacy was observed in the 75% ethanolic stem extract and 95% ethanolic leaf extract as determined by a comprehensive assessment employing 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and ferric reducing antioxidant power (FRAP) assays [143]. The antioxidative potential of S. glabra could be ascribable to the presence of phytochemical constituents such as phenolic acids, flavonoids, and polysaccharides, which possess intrinsic redox properties that help stabilise ROS due to their structural conformations [125, 144,145,146]. For example, the primary polyphenols and flavonoids of S. glabra, namely rosmarinic acid (369) and astilbin (335), possess the ability to scavenge ROS via hydrogen/electron transfer or Fe2+ chelation [147]. Rosmarinic acid (369) was also found to attenuate ROS by regulating PGC1-a/NOX4 signalling [118].
It was also established that the phenolic-rich ethyl acetate fraction of SGE manifested a strong antioxidative activity (IC50 = 6.84 ± 0.45 µg/mL) and displayed neuroprotective effects by enhancing cholinergic signalling in cognitive deficit mice [142]. In a bioactive structural basis study, it was unveiled that the antioxidant efficacy of proteoglycans from S. glabra was attributed to the presence of monosaccharides, namely xylose, glucosamine, and glucuronic acid [148].
4.2 Antibacterial, antifungal and antiviral
Preliminary studies on SGE have shown promising results regarding its antimicrobial activity, among which ethyl acetate and n-butanol fractions in particular exhibited excellent inhibition against pathogenic bacterial strains in the disk diffusion test [87, 149]. The n-butanol extract of S. glabra was also recently reported to show minimum inhibitory concentrations of 15 mg/mL and 10 mg/mL against Staphylococcus aureus and Escherichia coli, respectively [150]. In another study, sarglaperoxide A (98), an isolate from the seeds of S. glabra, displayed 64.5% inhibition against S. aureus at a concentration of 25 μg/mL [38]. In addition to demonstrating bacteriostatic effects against Propionibacterium acnes [151], the ethanolic extract of S. glabra displayed notable antifungal activities against Phomopsis mangiferae at 2.5 mg/mL. Over a period of seven days post-treatment, the maximum inhibition rate reached a notable 50.29% [152].
The antiviral properties of SGE have also been well-characterised and extensively studied, particularly on influenza viruses [80, 153,154,155,156,157]. Rosmarinic acid 4-O-β-D-glucoside (382) was reported to mitigate pulmonary oedema and post-influenza infection by impeding virus proliferation [154], while eleutheroside B1 (232) displayed broad-spectrum antiviral activities in vitro, with IC50 ranging from 64 to 125 μg/mL [155]. Subsequent investigations into the structure–activity relationship of eleutheroside B1 (232) confirmed the significance of its aglycone moiety in mediating the observed viral nucleic acid suppression. Jin et al. [80] reported that isofraxidin (230) effectively inhibited platelet aggregation through the PI3K/AKT and mitogen‑activated protein kinase (MAPK) pathways, thereby alleviating lung inflammation induced by influenza A. In the work by Pan et al. [158], SGE was found to inhibit HIV-1 protease and cathepsin L with IC50 values ranging from 0.003 to 0.07 mg/mL and 0.11 to 0.26 mg/mL, respectively. Notably, chlorogenic acid (377), a prominent constituent of the extract, displayed the most potent inhibitory activity against the two viral proteases. The unique dual-inhibitory function of SGE and its active components against viral proteases could be useful in discovering lead compounds for developing new antiviral agents.
4.3 Antimalarial
An assessment of antimalarial properties has been conducted on several compounds derived from S. glabra [44]. Among the compounds, 13′-O-methylsuccinylshizukaol C (136) exhibited remarkable efficacy against chloroquine-resistant Plasmodium falciparum, with an EC50 value of 4.3 pM. This potency surpassed that of artemisinin by approximately 1,000-fold. Furthermore, the compound demonstrated exceptional selectivity against the malaria parasite, as confirmed through a toxicity evaluation (IC50 = 39.0 ± 1.3 µM) conducted on a mammalian embryonic cell line.
4.4 Anti-thrombocytopenic
The anti-thrombocytopenic properties of SGE have been extensively studied on mice models [80, 93, 94, 159,160,161,162,163]. It was unveiled that SGE promoted platelet activation and prevented platelet apoptosis via the mitochondrial pathway. Its effective constituents, specifically flavonoids, played a role in regulating mitochondrial transmembrane potential, externalisation of phosphatidylserine, and expressions of pro-apoptotic markers on circulating thrombocytes [93, 161]. At 63 mg/kg and 94.5 mg/kg, S. glabra flavonoids significantly increased the number of peripheral platelets and the polyploid ratio of megakaryocytes (p < 0.01) in thrombocytopenic mice [162]. The mechanism was associated with the elevation of thrombopoietin levels, which triggered megakaryocyte differentiation via the TPO-C-mpl pathway [162, 163].
4.5 Antitumour
S. glabra has gained recognition for its significant cytotoxic effects. Extensive in vitro and in vivo investigations have been conducted over the past decades on various cell lines, focusing on plant extracts [164,165,166,167,168,169], medicinal formulations [170,171,172,173], and chemical constituents. The main cytotoxic chemical components identified from S. glabra include sesquiterpenoids [4, 17, 22, 33], coumarins [78, 79, 85, 174], flavonoids [175,176,177], and polysaccharides [146, 148, 178,179,180,181,182].
A number of isolates were reported to exhibit selective and potent activities against a panel of cell lines. For example, sarcandrolides A-C (69–71) significantly inhibited HL-60 leukocyte cell line with IC50 < 10 μM [17], whereas sarcandracoumarin (244) demonstrated moderate activity against human cervical carcinoma (HeLa) with an IC50 value of 49.3 μg/mL [78]. In a combination therapy, sarglalactones A-H (101–108) and doxorubicin exhibited exceptional synergistic cytotoxic effects on human osteosarcoma epithelial cells (U2OS) [33].
Caffeic acid 3,4-dihydroxyphenethylester (CADPE), a natural polyphenol derived from the aqueous extract of S. glabra, exhibits potent anticancer attributes. Its proposed mode of action involves the initiation of tumour senescence via the Twist1-mediated signalling pathway [178]. In a pharmacokinetic investigation, CADPE rapidly underwent hydrolysis into its anticancer metabolites, hydroxytyrosol and caffeic acid [179]. Additionally, CADPE regulated glycogen synthase kinase-3β (GSK3β), prompting the ubiquitin-dependent degradation of the proto-oncogene c-Myc [182]. Consequently, this modulates cell cycle regulators and anti-apoptotic proteins, resulting in tumour cell cycle arrest and apoptosis.
Malignant tumours frequently exhibit increased expression of eukaryotic initiation factor 4F (eIF4F), a protein primarily modulated by mitogen-activated protein kinase (MAPK). An acidic polysaccharide derived from S. glabra (SGP-2) employs this mechanism to trigger a MAPK-mediated intrinsic apoptosis pathway, effectively impeding tumour growth in both human and murine models [180]. Furthermore, the polysaccharide demonstrates excellent anti-proliferative effects on human osteosarcoma MG-63 cells, achieved through regulation of apoptotic cell population and activation of caspase-3 [181].
Validated through an in vivo xenograft assay, a preliminary study by Zheng et al. [175] validated the antitumour effects of uvangoletin (272) on HL60 cells. The proposed mechanism involves the interaction between mitochondria-mediated apoptotic proteins, leading to cytochrome C release into the cytosol and subsequent activation of apoptosis-executing caspases. Building upon this finding, Shen et al. [177] reported the promising cytotoxic activities of uvangoletin (272) on hepatocellular carcinoma cells (HepG2). This was evidenced by detected autophagy and apoptosis both in vivo and in vitro, coupled with metastasis suppression. The likely underlying mechanism involves the modulation of MAPK, AKT/mTOR, and TGF-β/smad2 signalling pathways.
4.6 Anti-inflammatory
S. glabra is renowned for its remarkable anti-inflammatory activities, substantiated by multiple studies reporting its bioactivities in vitro [23,24,25, 37, 38, 42, 48,49,50, 73, 82, 183,184,185,186] and in vivo [3, 187,188,189,190,191,192,193,194]. The anti-inflammatory properties were found to be imparted by the presence of polysaccharides [183, 193, 194], phenolics [3, 185], coumarins [3, 82, 188, 189], and flavonoids [192].
Among the compounds identified from S. glabra, lindenane-type sesquiterpenoids exhibit prominent potential as anti-inflammatory agents, with extensive studies conducted on their effectiveness against lipopolysaccharide (LPS)-induced RAW264.7 macrophage [23,24,25, 37, 38, 42, 48,49,50, 73, 184, 186]. Three newly characterised dimeric lindenanes, namely sarcanolides C-E (128–130), showed greater inhibition of LPS-induced nitric oxide (NO) production compared to the positive control (L-NMMA) at a concentration of 25 μM. The observed IC50 values for these dimers ranged from 13.4 to 17.2 μM [42]. Additionally, sarglanoid C (25) was recently revealed to display anti-inflammatory effects on LPS-induced RAW 264.7 cells. The IC50 value recorded was 20.00 ± 1.30 μM, indicating a twofold potency compared to L-NMMA (IC50 = 41.40 ± 2.30 μM) [25].
In another study, Li et al. [24] reported the promising anti-inflammatory properties of linderaggredin D (21) and sarglanoid C (30). Their IC50 values were 25.7 ± 0.2 μM and 11.5 ± 0.3 μM, respectively, which were comparable to that of the positive control, dexamethasone (IC50 = 9.3 ± 0.2 μM). Bioinformatics and transcription factor analysis revealed that the anti-inflammatory activity of linderaggredin D (21) was associated with multiple pathways related to transcription factor NF-κB, a key component in inflammation progression.
A neuroinflammatory assay utilising the Griess reaction was employed to evaluate the anti-neuroinflammatory potential of meroterpenoids and sesquiterpenoid dimers derived from S. glabra [49]. These compounds exhibited significant inhibitory effects comparable to dexamethasone at concentrations < 5 µM. Utilising protein–protein interaction (PPI) network analysis and molecular docking, the anti-inflammatory mechanisms of the meroterpenoids glabralides G and H (222 and 223) were predicted. The PPI analysis indicated a prominent interaction with Hsp90AA1, a heat shock protein associated with neuroinflammation.
Regarding mechanistic action, Wei et al. [48] proposed that shizukaol D (78) elicits its anti-inflammatory effects by activating the AKT/Nrf2/HO-1 signalling cascade, which subsequently enhances the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-px). Additionally, shizukaol A’s potent inhibitory effect on NO (IC50 = 13.79 ± 1.11 μM) was attributed to its capacity to trigger antioxidant genes and regulate oxidative stress via the HMGB1/Nrf2/HO-1 pathway [186].
The anti-inflammatory activities of a S. glabra polysaccharide, SERP-30, were reported for the first time by Feng et al. [193]. Corroborated by western blotting results, the postulated mechanism of SERP 30 involves the inhibition of LPS-induced phosphorylation of p38 and p65 via NF-κB and MAPK signalling pathways, leading to the protection of endothelial glycocalyx in LPS induced-acute respiratory distress syndrome (ARDS) in mice. In addition, the polysaccharides sourced from S. glabra exhibited the capacity to mitigate muscular injury in rats subjected to prolonged high-intensity exercise. This effect was achieved by elevating the levels of recuperative enzymes and cytokines such as creatine kinase (CK), lactate dehydrogenase (LDH), and tumour necrosis factor (TNF-α) [194].
The anti-neuroinflammatory activities of three indolidinoid-monoterpene compounds isolated from S. glabra, sarglamides C-E (226–228), along with toussaintine C were recently reported [73]. These compounds were evaluated for their impact on NO production in BV-2 microglial cells induced by LPS. Notably, sarglamides C-E (226–228) exhibited considerable inhibitory activities (> 30%) at concentrations < 20 µM, while showing no cytotoxicity on BV-2 cells (cell viability > 80%). On the other hand, the enantiomers of toussaintine C, namely (+)-toussaintine C (412) and (-)-toussaintine C (413), displayed similar effects at 5 µM.
4.7 Immunomodulatory
As evidenced by multiple studies [2, 139], SGE was found to enhance immunity by mediating immune response and balancing the proportion of lymphocytes in restraint mice models. Comparable immunoprotective effects were also observed in S. glabra-derived polysaccharides, wherein increased expression of cell surface molecules and immune factors (IL-1β, IL-10, and iNOS) was observed in RAW 264.7 cells [195]. An acidic polysaccharide purified from S. glabra (p-SGP) was found to stimulate anti-tumour immune responses, making it an ideal candidate as a tumour vaccine adjuvant. The mechanism involves the upregulation of delta-like ligand 4 (DLL4) gene, which in turn activates the differentiation and maturation of T-helper cells and dendritic cells [196].
4.8 Hepatoprotective
A group of sesquiterpene glycosides, namely sarcaglabosides A-E (2, 3, 165, 166 and 148), together with chloranoside A (35) exhibited notable inhibitory activities in vitro at a concentration of 10–4 M [14]. Among them, sarcaglaboside B (3) exhibited the most pronounced hepatoprotective effect, displaying an inhibition rate of 65.9%. Furthermore, Li et al. [197] reported that SGE exerted hepatoprotective effects on mice models by inhibiting the activities of alanine aminotransferase level (ALT) and leukotriene B. The inhibitory rates were 78.5%, 70.3%, and 55.1% at concentrations of 500, 250, and 125 mg/kg, respectively.
4.9 Gastroprotective
Based on a histopathological examination and conjoint metabolomics and network analysis, SGE was also found to associate with multiple signalling pathways related to gastric cell inflammation, metabolism, apoptosis, and differentiation [198]. The extract exerted profound protective effects on the gastric mucosa of rat models by alleviating oxidative stress, promoting antioxidant activity, and inhibiting the expression of inflammatory factors.
4.10 Hypoglycaemic and hypolipidemic
It was reported that SGP-2 and SERP 1, two proteoglycans isolated from S. glabra, demonstrated remarkable α-glucosidase inhibitory activities, with IC50 values of 87.06 ± 11.76 μg/mL and 49.01 μg/mL, respectively [199, 200]. These polysaccharides mitigated insulin resistance, improved lipid metabolism, and attenuated oxidative stress under hyperglycaemic conditions through α-glucosidase inhibition. Similarly, CMSERP, a carboxymethylated polysaccharide from S. glabra, displayed substantial hypoglycaemic effects, achieving a maximum inhibition of 83.38% ± 2.30% at 1000 μg/mL concentration [201].
In addition to flavonoids [202], sesquiterpene dimers from S. glabra were discovered to possess notable hypolipidemic effects. Sarcaglarol A (117) reduced lipid droplets in L02 cells as indicated by oil red O staining, while sarglaromatics A and B (123 and 124) effectively mitigated lipid accumulation in L02 cells exposed to free fatty acids [39, 41]. The inhibitory potential of the dimers was postulated to be amplified by the free hydroxyl groups present in their structures, suggesting potential application in treating non-alcoholic steatohepatitis.
4.11 Other biological activities
Apart from the mentioned biological activities, S. glabra and its chemical constituents were evaluated for their anti-multidrug resistance, neuroprotective, and autophagy-inducing activities. Chi et al. [33] described the anti-multidrug resistance against MCG-7/DOX cells displayed by several sesquiterpenoids isolated from the leaves of S. glabra, whose reversal fold values ranged from 11.8 to 129.2.
Du et al. [83] recently disclosed the acetylcholinesterase (AchE) inhibiting activity of coumarins from S. glabra. Notably, 5-methoxy-6,7-methylenedioxycoumarin (252) exhibited significant AchE inhibitory efficacy (IC50 = 1.982 ± 0.003 μM), surpassing donepezil (IC50 = 3.118 ± 0.006 μM). Molecular docking results showed that the main interaction involved hydrogen bonding with two target amino acids, Phe-288 and Arg-289. Additionally, Liu et al. [98] introduced the first report on the autophagy-inducing effects of S. glabra compounds, including glabratin A (279), glabratins D-F (282–284), glabratins I-N (287–289 and 337–339), and adunctin E (293). The compounds demonstrated enhanced conversion of the protein light chain LC3-II to its non-lipidated form (LC3-I) in vitro.
5 Conclusions
This paper presents a comprehensive review on the compound isolation, biosynthesis, and pharmacological attributes of S. glabra. In essence, S. glabra is a highly prolific producer of secondary metabolites, including terpenoids, coumarins, lignans, flavonoids, sterols, anthraquinones, organic acids, and organic esters, several of which hold substantial research value due to their unique chemical structures and extensive range of biological effects. The taxonomical markers of this plant include lindenane-type sesquiterpenoids, characterised by a distinct linear 3/5/6 polycyclic ring and typically formed through a [4 + 2] Diels–Alder cycloaddition.
Through an analysis of existing literature, several gaps in research have been discerned. In the domains of phytochemistry and pharmacology, certain subspecies of S. glabra are underexplored. Continued research on these lesser-known variants holds the potential to unveil therapeutic properties or reveal hitherto unprecedented compounds. Despite the wide distribution of S. glabra across Asia, the majority of investigations have focused on specimens collected exclusively from China. Exploring specimens from diverse geographical origins would be of interest, facilitating a comprehensive understanding of common and distinct secondary metabolites among these plants.
From a biological activity viewpoint, S. glabra holds promise for further exploration of its pharmacological potential. Future studies are expected to analyse the mechanism of action of the active principles and evaluate the possible synergistic action within SGE. Besides, the establishment of precise analytical methods is crucial to standardise the secondary metabolites present in medicinal preparations like ZhongJieFeng, an herbal extract of S. glabra with excellent antitumour and anti-inflammatory activities. Such scientific validation is imperative to ensure the effectiveness and safety of this traditional Chinese medicine, thereby optimising its medicinal utility.
Abbreviations
- DCM:
-
Dichloromethane
- PE:
-
Petroleum ether
References
Forest Research Institute Malaysia (FRIM). Flora of Malaysia i-Newsletter Part 3. 2013:1969–1969. https://www.mybis.gov.my/art/1985. Accessed 21 Dec 2022.
He RR, Wang M, Li YF, Dai Y, Duan YH, Yao XS, et al. Effects of Sarcandra glabra extract on immune activity in restraint stress mice. Zhongguo Zhongyao Zazhi. 2009;34:100–3.
Liu CP, Liu JX, Gu JY, Liu F, Li JH, Yang B, et al. Combination effect of three main constituents from Sarcandra glabra inhibits oxidative stress in the mice following acute lung injury: a role of MAPK-NF-κB pathway. Front Pharmacol. 2021;11:2082. https://doi.org/10.3389/fphar.2020.580064.
Yang XR, Tanaka N, Tsuji D, Lu FL, Yan XJ, Itoh K, et al. Sarcaglabrin A, a conjugate of C15 and C10 terpenes from the aerial parts of Sarcandra glabra. Tetrahedron Lett. 2020;61: 151916. https://doi.org/10.1016/j.tetlet.2020.151916.
Zeng YL, Liu JY, Zhang Q, Qin XH, Li ZL, Sun GJ, et al. The traditional uses, phytochemistry and pharmacology of Sarcandra glabra (Thunb.) Nakai, a Chinese herb with potential for development: review. Front Pharmacol. 2021;12:652926. https://doi.org/10.3389/fphar.2021.652926.
Chloranthaceae VB. Flora Malesiana—series 1. Spermatophyta. 1984;10:123–44.
Verdcourt B. Notes on Malesian Chloranthaceae. Kew Bull. 1985;40:213–24. https://doi.org/10.2307/4108497.
Vossen HAM, Wessel M, editors. Plant resources of South-East Asia, No 16, Stimulants. Leiden: Backhuys Publishers; 1999. https://doi.org/10.2307/4118801.
Swamy BGL, Bailey IW. Sarcandra, A vesselless genus of the Chloranthaceae. J Arnold Arbor. 1950;31:128.
Zhou B, Liu KY, Chang J, Cheng CQ. Advances on chemical constituents and pharmacological activities of Sarcandra glabra. Chin J Mod Appl Pharm. 2009;6:982–6.
Zhang H, Zhao P, Zhang YY, Huang XX, Song SJ. Chemical constituents and pharmacological properties of Sarcandra glabra (Thunb.) Nakai. Asian J Trad Med. 2019;14:237–46.
Ludwiczuk A, Skalicka-Woźniak K, Georgiev MI. Terpenoids. In: Badal S, Delgoda RBT, editors. Pharmacognosy: fundamentals. Applications and Strategy, Boston: Academic Press; 2017. p. 233–66. https://doi.org/10.1016/B978-0-12-802104-0.00011-1.
Cocker W, McMurry TBH. Stereochemical relationships in the eudesmane (selinane) group of sesquiterpenes. Tetrahedron. 1960;8:181–204. https://doi.org/10.1016/0040-4020(60)80028-2.
Li Y, Zhang DM, Li JB, Yu SS, Li Y, Luo YM. Hepatoprotective sesquiterpene glycosides from Sarcandra glabra. J Nat Prod. 2006;69:616–20. https://doi.org/10.1021/np050480d.
Zhu LP, Li Y, Yang JZ, Zuo L, Zhang DM. Two new sesquiterpene lactones from Sarcandra glabra. J Asian Nat Prod Res. 2008;10:541–5. https://doi.org/10.1080/10286020801966773.
Hu XR, Yang JS, Xu XD. Three novel sesquiterpene glycosides of Sarcandra glabra. Chem Pharm Bull. 2009;57:418–20. https://doi.org/10.1248/cpb.57.418.
He XF, Yin S, Ji YC, Su ZS, Geng MY, Yue JM. Sesquiterpenes and dimeric sesquiterpenoids from Sarcandra glabra. J Nat Prod. 2010;73:45–50. https://doi.org/10.1021/np9006469.
Teng F, Zhong HM, Chen CX, Liu HY. Four new eudesmane sesquiterpenoid lactones from Chloranthus serratus. Helv Chim Acta. 2009;92:1298–303. https://doi.org/10.1002/hlca.200800450.
Do TO, Pham TK, Hang NTB, Pham HY, Tran HH, Nguyen XC, et al. Two new sesquiterpenes from Sarcandra glabra. Nat Prod Commun. 2010;5:1717–20.
Wang C, Li Y, Li CJ, Yu SS, Zhang DM. Three new compounds from Sarcandra glabra. Chinese Chem Lett. 2012;23:823–6. https://doi.org/10.1016/j.cclet.2012.05.007.
Hu XR, Wu HF, Zhang XP, Yang JS, Dai Z, Lin RC, et al. A new sesquiterpene lactone from Sarcandra glabra. Nat Prod Res. 2013;27:1197–201. https://doi.org/10.1080/14786419.2012.722084.
Ni G, Zhang H, Liu HC, Yang SP, Geng MY, Yue JM. Cytotoxic sesquiterpenoids from Sarcandra glabra. Tetrahedron. 2013;69:564–9. https://doi.org/10.1016/j.tet.2012.11.023.
Yaermaimaiti S, Wang P, Luo J, Li RJ, Kong LY. Sesquiterpenoids from the seeds of Sarcandra glabra and the potential anti-inflammatory effects. Fitoterapia. 2016;111:7–11. https://doi.org/10.1016/j.fitote.2016.03.020.
Li YT, Li SF, Lei C, You JQ, Huang JC, Hou AJ. Dimeric sesquiterpenoids and anti-inflammatory constituents of Sarcandra glabra. Bioorg Chem. 2022;124: 105821. https://doi.org/10.1016/j.bioorg.2022.105821.
Wang YY, Li QR, Chi J, Li JX, Kong LY, Luo J. Sesquiterpenoids from the leaves of Sarcandra glabra. Chin J Nat Med. 2022;20:215–20. https://doi.org/10.1016/S1875-5364(21)60102-4.
Takeda Y, Yamashita H, Matsumoto T, Terao H, Chloranthalactone F. A sesquiterpenoid from the leaves of Chloranthus glaber. Phytochemistry. 1993;33:713–5. https://doi.org/10.1016/0031-9422(93)85480-F.
Wong KC, Tan MS, Ali DMH, Teoh SG, Osman H, Tan SK. Essential oil of the leaves of Sarcandra glabra (Thunb.) Nakai. J Essent Oil Res. 2009;21:71–3. https://doi.org/10.1080/10412905.2009.9700114.
Yue GZ, Yang L, Yuan CC, Du B, Liu B. Progress in total syntheses of lindenane-type sesquiterpenoids and their dimers. Chin J Org Chem. 2013;33:90–100. https://doi.org/10.6023/cjoc201207003.
Liao SG, Yue JM. Dimeric sesquiterpenoids. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, editors. Prog Chem Org Nat Prod, vol. 101. Cham: Springer International Publishing; 2016. p. 1–112. https://doi.org/10.1007/978-3-319-22692-7_1.
Uchida M, Kusano G, Kondo Y, Takemoto T, Nozoe S. Two new sesquiterpenoids from Chloranthus glaber Makino. Heterocycles. 1978;9:139–44. https://doi.org/10.3987/r-1978-02-0139.
Okamura H, Nakashima N, Iwagawa T, Nakayama N, Nakatani M. The structures of two lindenane sesquiterpene glucosides from Chloranthus glaber. Chem Lett. 1994;23:1541–2. https://doi.org/10.1246/cl.1994.1541.
Tsui WY, Brown GD. Cycloeudesmanolides from Sarcandra glabra. Phytochemistry. 1996;43:819–21. https://doi.org/10.1016/0031-9422(96)00352-4.
Chi J, Wei SS, Gao HL, Xu DQ, Zhang LN, Yang L, et al. Diverse chemosensitizing 8,9-secolindenane-type sesquiterpenoid oligomers and monomers from Sarcandra glabra. J Org Chem. 2019;84:9117–26. https://doi.org/10.1021/acs.joc.9b00986.
Wang C, Zhu LP, Yang JZ, Li CJ, Zhang DM. Chemical constituents from Sarcandra glabra. China J Chin Mater Medicaedica. 2010;35:714–7. https://doi.org/10.4268/cjcmm20100612.
Li X, Zhang YF, Yang L, Feng Y, Liu YM, Zeng X. Sesquiterpenoids from the whole plant of Sarcandra glabra. Yaoxue Xuebao. 2011;46:1349–51.
Zheng XF, Liu HY, Zhong HM. Chemical constituents from Sarcandra glabra. Nat Prod Res Dev. 2014;26:1221–4.
Wang P, Luo J, Zhang YM, Kong LY. Sesquiterpene dimers esterified with diverse small organic acids from the seeds of Sarcandra glabra. Tetrahedron. 2015;71:5362–70. https://doi.org/10.1016/j.tet.2015.05.112.
Wang P, Li RJ, Liu RH, Jian KL, Yang MH, Yang L, et al. Sarglaperoxides A and B, sesquiterpene-normonoterpene conjugates with a peroxide bridge from the seeds of Sarcandra glabra. Org Lett. 2016;18:832–5. https://doi.org/10.1021/acs.orglett.6b00112.
Wang YY, Cui ZR, Chi J, Tang PF, Zhang MH, Li JX, et al. Sarcaglarols A–D, lindenane−monoterpene heterodimers from Sarcandra glabra based on molecular networks. Chin J Chem. 2021;39:129–36. https://doi.org/10.1002/cjoc.202000456.
Sun YP, Wang YY, Li YQ, Wang SY, Zhang DY, Kong LY, et al. Sarcaglarone A, a lindenane-monoterpene heterodimer from the seeds of Sarcandra glabra. Org Biomol Chem. 2022;20:9222–7. https://doi.org/10.1039/d2ob01830f.
Sun YP, Chi J, Zhang LJ, Wang SY, Chen ZH, Zhang H, et al. Sarglaromatics A-E: a class of naphthalene-like architecture fused norlindenane sesquiterpene dimers from Sarcandra glabra. J Org Chem. 2022;87:4323–32. https://doi.org/10.1021/acs.joc.2c00014.
Xiao LG, Li P, Yan H, Ni W, He L, Liu HY. Sarcanolides C-E: Three new lindenane sesquiterpenoid dimers with anti-inflammatory activities from Sarcandra glabra. Org Biomol Chem. 2022;20:1320–6. https://doi.org/10.1039/d1ob02417e.
Wang YY, Chen ZH, Li QR, Cui LT, Chi J, Li JX, et al. Sarglafuran A, a lindenane-type sesquiterpene dimers with unique furan ring from the leaves of Sarcandra glabra. Tetrahedron Lett. 2022;98: 153834. https://doi.org/10.1016/j.tetlet.2022.153834.
Zhou B, Zimbres FM, Butler JH, Xu CH, Haney RS, Wu Y, et al. Picomolar antimalarial agent from a Chinese medicinal plant. Sci China Chem. 2022;65:82–6. https://doi.org/10.1007/s11426-021-1124-x.
Cui ZR, Wang YY, Li JX, Chi J, Zhang PP, Kong LY, et al. Natural and pseudonatural lindenane heterodimers from Sarcandra glabra by molecular networking. Org Lett. 2022;24:9107–11. https://doi.org/10.1021/acs.orglett.2c03769.
Luo YM. Study on Jiangxi characteristic Chinese medicinal materials Sarcandra glabra and Cinnamomum Camphora. China Academy of Chinese Medical Sciences, 2004.
He XF, Zhang S, Zhu RX, Yang SP, Yuan T, Yue JM. Sarcanolides A and B: two sesquiterpenoid dimers with a nonacyclic scaffold from Sarcandra hainanensis. Tetrahedron. 2011;67:3170–4. https://doi.org/10.1016/j.tet.2011.03.021.
Wei SS, Chi J, Zhou MM, Li RJ, Li YR, Luo J, et al. Anti-inflammatory lindenane sesquiterpeniods and dimers from Sarcandra glabra and its upregulating AKT/Nrf2/HO-1 signaling mechanism. Ind Crops Prod. 2019;137:367–76. https://doi.org/10.1016/j.indcrop.2019.05.041.
Bai M, Liu YY, Li YL, Shi WY, Li KX, Lu LW, et al. Meroterpenoids and sesquiterpene dimers from Sarcandra glabra with anti-neuroinflammatory activity. Ind Crops Prod. 2022;183: 114983. https://doi.org/10.1016/j.indcrop.2022.114983.
Tao R, Tang PF, Gao JJ, Li JX, Sun YP, Luo J, et al. The anti-inflammatory activity by suppressing the TRAF6/MAPKs pathway of trishizukaol A from Sarcandra glabra. Phytomedicine. 2022;98: 153952. https://doi.org/10.1016/j.phymed.2022.153952.
Banik BK, Tiwari A, Sahoo BM. Sesquiterpenes: a chemical synthesis and biological activity. Terpenoids, 2022, p. 487–516. https://doi.org/10.1201/9781003008682-15.
Tashkhodzhaev B, Abduazimov BK. Stereochemistry of sesquiterpenes of the germacrane type. Chem Nat Compd. 1997;33:382–8. https://doi.org/10.1007/BF02282357.
Luo YM, Liu AH, Yu BW, Kang LJ, Huang LQ. Studies on chemical constituents of Sarcandra glabra. Chin Pharm J. 2005;40:1296–8. https://doi.org/10.3321/j.issn:1001-2494.2005.17.005.
Harrowven DC, Pattenden G. Transannular electrophilic cyclizations. In: Trost BM, Fleming IBT, editors. Comprehensive organic synthesis. Oxford: Pergamon; 1991. p. 379–411. https://doi.org/10.1016/b978-0-08-052349-1.00067-6.
Wang AQ, Feng SC, He X, Xu RS. A new sesquiterpene lactone from Sarcandra glabra. Acta Pharm Sin. 1988;23:64–6.
Zeng AH, Luo YM, Liu N. Determination of istanbulin A in Sarcandra glabra. J Chinese Med Mater. 2006;29:443–4.
Durán-Peña MJ, Botubol Ares JM, Hanson JR, Collado IG, Hernández-Galán R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat Prod Rep. 2015;32:1236–48. https://doi.org/10.1039/c5np00024f.
Chen ZH, Sun YP, Wang YY, Kong LY, Luo J. Two new sesquiterpenoids from the roots of Sarcandra glabra. Nat Prod Res. 2022. https://doi.org/10.1080/14786419.2022.2089670.
Li Y. Studies on the chemical constituents and bioactivities of Sarcandra glabra, Cercis chinensis and Photinia parvifolia. Chinese Academy of Medical Science and Perking Union Medical College, 2006.
Karamenderes C, Bedir E, Pawar R, Baykan S, Khan IA. Elemanolide sesquiterpenes and eudesmane sesquiterpene glycosides from Centaurea hierapolitana. Phytochemistry. 2007;68:609–15. https://doi.org/10.1016/j.phytochem.2006.10.013.
Wu SX, Lv GY, Zhao LS, Chen SH, Zhou HK, Li QL. Determination of β-elemene in volatile oil of fresh Sarcandra glabra (Thunb.) Nakai by HPLC. Chin J Mod App Pharm. 2010;27:59–61.
Sela F, Karapandzova M, Stefkov G, Cvetkovikj I, Trajkovska-Dokikj E, Kaftandzieva A, et al. Chemical composition and antimicrobial activity of berry essential oil of Juniperus oxycedrus L. (Cupressaceae) grown wild in Republic of Macedonia. Maced Pharm Bull. 2013;59:41–8.
Li X, Zhang YF, Zeng X, Liu YM, Feng Y. Two new skeleton compounds from Sarcandra glabra. Helv Chim Acta. 2012;95:998–1002. https://doi.org/10.1002/hlca.201100352.
Wu HF, Hu XR, Zhang XP, Chen SL, Yang JS, Xu XD. Isolation and chemotaxonomic significance of megastigmane-type sesquiterpenoids from Sarcandra glabra. J Med Plants Res. 2012;6:4501–4. https://doi.org/10.5897/jmpr12.532.
Cox-Georgian D, Ramadoss N, Dona C, Basu C. Therapeutic and medicinal uses of terpenes. Med Plants from Farm to Pharm. 2019. https://doi.org/10.1007/978-3-030-31269-5_15.
Ninkuu V, Zhang L, Yan J, Fu Z, Yang T, Zeng H. Biochemistry of terpenes and recent advances in plant protection. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22115710.
Heskes AM, Sundram TCM, Boughton BA, Jensen NB, Hansen NL, Crocoll C, et al. Biosynthesis of bioactive diterpenoids in the medicinal plant Vitex agnus-castus. Plant J. 2018;93:943–58. https://doi.org/10.1111/tpj.13822.
Kushiro T, Ebizuka Y. Triterpenes. In: Liu HW, Mander LBT, editors. Comprehensive Natural Products II: Chemistry and Biology, vol. 1, Oxford: Elsevier; 2010, p. 673–708. https://doi.org/10.1016/b978-008045382-8.00007-1.
Luo YM, Liu AH, Zhang DM, Huang LQ. Two new triterpenoid saponins from Sarcandra glabra. J Asian Nat Prod Res. 2005;7:829–34. https://doi.org/10.1080/10286020410001721104.
Fu JQ, Liang JY. Studies on the chemical constituents of Sarcandra glabra. Strait Pharm J. 2013;25:46–50.
Cornforth JW. Terpenoid biosynthesis. Chem Br. 1968;4:102–6.
Yang WQ, Hai P, Xiao H, Gao Y, Tao YH, Miao DR, et al. Glabralides A–C, three novel meroterpenoids from Sarcandra glabra. Tetrahedron. 2018;74:341–7. https://doi.org/10.1016/j.tet.2017.12.001.
Zhou B, Gong Q, Fu Y, Zhou JS, Zhang HY, Yue JM. Sarglamides A-E, indolidinoid-monoterpenoid hybrids with anti-neuroinflammatory activity from a Sarcandra species. Org Lett. 2023;25:1464–9. https://doi.org/10.1021/acs.orglett.3c00196.
Noel JP, Austin MB, Bomati EK. Structure-function relationships in plant phenylpropanoid biosynthesis. Curr Opin Plant Biol. 2005;8:249–53. https://doi.org/10.1016/j.pbi.2005.03.013.
Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3:2–20. https://doi.org/10.1093/mp/ssp106.
Pedras MSC, Zheng Q. The chemistry of Arabidopsis thaliana. In: Liu HW, Mander LBT, editors. Comprehensive Natural Products II: Chemistry and Biology, vol. 3, Oxford: Elsevier; 2010, p. 1297–315. https://doi.org/10.1016/b978-008045382-8.00090-3.
Das AB, Goud VV, Das C. Phenolic compounds as functional ingredients in beverages. In: Grumezescu AM, Holban AM, editors. Value-Added Ingredients and Enrichments of Beverages: Volume 14: The Science of Beverages, Academic Press; 2019, p. 285–323. https://doi.org/10.1016/B978-0-12-816687-1.00009-6.
Feng SX, Xu LX, Wu M, Hao J, Qiu SX, Wei XY. A new coumarin from Sarcandra glabra. Fitoterapia. 2010;81:472–4. https://doi.org/10.1016/j.fitote.2009.12.009.
Wu HF, Hu XR, Zhang XP, Chen SL, Yang JS, Xu XD. Benzyl 2-β-glucopyranosyloxybenzoate, a new phenolic acid glycoside from Sarcandra glabra. Molecules. 2012;17:5212–8. https://doi.org/10.3390/molecules17055212.
Jin L, Ying ZH, Yu CH, Zhang HH, Yu WY, Wu XN. Isofraxidin ameliorated influenza viral inflammation in rodents via inhibiting platelet aggregation. Int Immunopharmacol. 2020;84: 106521. https://doi.org/10.1016/j.intimp.2020.106521.
China Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China. vol. 1. Beijing SE: China Medical Science Press Beijing; 2010. LK—https://worldcat.org/title/901666820.
Wang MJ, Zhao J, Zhao Y, Huang RY, Li G, Zeng X, et al. A new coumarin isolated from Sarcandra glabra as potential anti-inflammatory agent. Nat Prod Res. 2016;30:1796–801. https://doi.org/10.1080/14786419.2015.1079186.
Du NN, Bai M, Zhang X, Zhou L, Huang XX, Song SJ. Coumarins from Sarcandra glabra (Thunb.) Nakai and acetylcholinesterase inhibiting activity. Chem Biodivers. 2022. https://doi.org/10.1002/cbdv.202200558.
Huang MJ, Li YL, Zeng GY, Yuan W, Tan JB, Tan GS, et al. Chemical constituents of Sarcandra glabra. Cent South Pharm. 2007;5:459–61.
Wang F, Yuan ST, Zhu DN. Active components of antitumor fraction from Sarcandra glabra. Chin J Nat Med. 2007;5:174–8.
Xu XD, Hu XR, Yuan JQ, Yang JS. Studies on chemical constituents of Sarcandra glabra. Zhongguo Zhongyao Zazhi. 2008;33:900–2. https://doi.org/10.2991/icimm-15.2015.43.
Yuan K, Zhu JX, Si JP, Cai HK, Ding XD, Pan YJ. Studies on chemical constituents and antibacterial activity from n-butanol extract of Sarcandra glabra. Zhongguo Zhongyao Zazhi. 2008;33:1843–6.
Zhu L, Li Y, Yang J, Zuo L, Zhang D. Studies on chemical constituents of Sarcandra glabra. China J Chin Mater Medicaedica. 2008;33:155–7.
Li X, Zhang YF, Zeng X, Yang L, Deng YH. Chemical profiling of bioactive constituents in Sarcandra glabra and its preparations using ultra-high-pressure liquid chromatography coupled with LTQ orbitrap mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:2439–47. https://doi.org/10.1002/rcm.5123.
Luo YM, Lin FX, Du YQ, Li HT, Jiang CX, Liu H. Studies on the chemical constituents of Sarcandra glabra. Proceedings of the 5th international conference on information engineering for mechanics and materials, Advances in engineering research, Atlantis Press; 2015, p. 226–9. https://doi.org/10.2991/icimm-15.2015.43.
Zálešák F, Bon DJYD, Pospíšil J. Lignans and Neolignans: plant secondary metabolites as a reservoir of biologically active substances. Pharmacol Res. 2019;146: 104284. https://doi.org/10.1016/j.phrs.2019.104284.
Liu J, Wen QY, Tian HR, Zhan BJF, Zhu LP, Yang DP, et al. (±)-Sarcanan A, a pair of new enantiomeric dihydrobenzofuran neolignans from the aerial parts of Sarcandra glabra. Nat Prod Res. 2022;37:2480–5. https://doi.org/10.1080/14786419.2022.2050229.
Zhu XQ, Jiang YL, Zheng Q, Zhang AP, Shi L, Xia LM, et al. Apoptosis of platelets inhibited by Herba Sarcandrae extract through the mitochondria pathway. Evid-Based Complement Altern Med. 2018;2018:1956902. https://doi.org/10.1155/2018/1956902.
Lu XN, Sun HJ, Zhu J, Hu XY, Yan XJ, Shang GB. Effects of flavonoids from Sarcandrae glabra on differentiation and maturation of megakaryocytes in cell co-culture system. Trad Chin Med Clin Pharmacol. 2019;30:1277–83.
Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur J Pharm Sci. 2021;7:25. https://doi.org/10.1186/s43094-020-00161-8.
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5: e47. https://doi.org/10.1017/jns.2016.41.
Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–46. https://doi.org/10.3390/nu2121231.
Liu X, Yang J, Fu J, Xu PL, Xie TG, Bai LP, et al. Monoterpene-flavonoid conjugates from Sarcandra glabra and their autophagy modulating activities. Bioorg Chem. 2021;112: 104830. https://doi.org/10.1016/j.bioorg.2021.104830.
Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. https://doi.org/10.1080/16546628.2017.1361779.
Ishikura N. Pelargonidin glycosides in fruits. Experientia. 1971;27:1006. https://doi.org/10.1007/BF02138844.
Li Y, Zhang DM, Yu SS, Li JB, Luo YM. A novel phenylpropanoid-substituted catechin glycoside and a new dihydrochalcone from Sarcandra glabra. Chin Chem Lett. 2006;17:207–10.
Zou X, Gao H, Wu B, Wang Y, Wang S, Yang S. Study on the chemical constituents of Sarcandra glabra. Chin Trad Herb Drugs. 2007;38:354–6.
Cao CM, Xu LJ, Chen K, Peng Y, Xiao PG. Chemical study on petroleum ether portion of Sarcandra hainanensis. Zhongguo Zhongyao Zazhi. 2009;34:1009–10.
Zheng YB, Xu XP, Zou XW, Guan LS, Zhang DY, Liu J, et al. Chemical constituents with the antioxidant activity in Sarcandra glabra. J Fujian Norm Univ Nat Sci Ed. 2016;32:98–102.
Spiegel M, Andruniów T, Sroka Z. Flavones’ and flavonols’ antiradical structure–activity relationship—a quantum chemical study. Antioxidants. 2020. https://doi.org/10.3390/antiox9060461.
Qin YQ, Liu W, Yin R, Xiao PT, Wang ZY, Huang TQ, et al. New 4’,5’-methylenedioxyflavone derivatives from the whole plant of Sarcandra glabra. Nat Prod Res. 2022. https://doi.org/10.1080/14786419.2022.2111562.
Li Y, Yao JY, Han CY, Yang JX, Chaudhry MT, Wang S, et al. Quercetin, inflammation and immunity. Nutrients. 2016. https://doi.org/10.3390/nu8030167.
Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem. 2011;11:298–344. https://doi.org/10.2174/138955711795305335.
Huang MJ, Zeng GY, Tan JB, Li YL, Tan GS, Zhou YJ. Studies on flavonoid glycosides of Sarcandra glabra. Zhongguo Zhongyao Zazhi. 2008;33:1700–2.
Tong SQ, Huang J, Wang LB. Studies on the chemical constituents of Sarcandra glabra. Zhongcaoyao. 2010;41:198–201.
Cao CM, Xu LJ, Peng Y, Shi QW, Xiao PG. Two new flavan-flavanones from Sarcandra hainanensis. Chem Pharm Bull. 2010;58:1395–8. https://doi.org/10.1248/cpb.58.1395.
Duodu KG, Awika JM. Phytochemical-related health-promoting attributes of sorghum and millets. In: Taylor JRN, Duodu K, editors. Sorghum and millets: chemistry technology, and nutritional attributes. AACC International Press; 2018. p. 225–58. https://doi.org/10.1016/B978-0-12-811527-5.00008-3.
Bodduluru LN, Kasala ER, Madhana RM, Barua CC, Hussain MI, Haloi P, et al. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int Immunopharmacol. 2016;30:102–10. https://doi.org/10.1016/j.intimp.2015.11.036.
Zhou H, Liang JL, Lv D, Hu YF, Zhu Y, Si J, et al. Characterization of phenolics of Sarcandra glabra by non-targeted high-performance liquid chromatography fingerprinting and following targeted electrospray ionisation tandem mass spectrometry/time-of-flight mass spectrometry analyses. Food Chem. 2013;138:2390–8. https://doi.org/10.1016/j.foodchem.2012.12.027.
Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018. https://doi.org/10.3390/molecules23040965.
Cao CM, Peng Y, Xu LJ, Wang YJ, Yang JS, Xiao PG. Two flavonoid dimers from Sarcandra hainanensis (Pei) Swamy et Bailey. Chem Pharm Bull. 2009;57:743–6. https://doi.org/10.1248/cpb.57.743.
Yadav AN, Kour D, Rana KL, Yadav N, Singh B, Chauhan VS, et al. Chapter 20—Metabolic engineering to synthetic biology of secondary metabolites production. In: Gupta VK, Pandey AB, editors, Amsterdam: Elsevier; 2019, p. 279–320. https://doi.org/10.1016/B978-0-444-63504-4.00020-7.
Zhang TT, Liu C, Ma SS, Gao YR, Wang RS. Protective effect and mechanism of action of rosmarinic acid onradiation-induced parotid gland injury in rats. Dose-Response. 2020;18:1559325820907782. https://doi.org/10.1177/1559325820907782.
Yu F, Fu J, Liang J. Chemical constituents of Sarcandra glabra. World J Biotechnol. 2012;5–6.
Wang AQ, Ma XR. Preliminary study on the active constituents of Sarcandra glabra. Chin Trad Herb Drugs. 1979:8–9.
You Y, Cheng G. Determination of fumaric acid in Sarcandra glabra (Thunb.) Nakai by HPLC. Chin J Chin Mater Medica. 1997;22.
Zeng AH, Luo YM. Chemical constituents of Sarcandra glabra. Chin J Med Mater. 2005;28:292–3. https://doi.org/10.3321/j.issn:1001-4454.2005.04.014.
Duan YH, Dai Y, Gao H, Ye WC, Yao XS. Chemical constituents from Sarcandra glabra. Chin Trad Herb Drugs. 2010;41:29–32. https://doi.org/10.3321/j.issn:0253-2670.2005.01.006.
Li XX, Huang MJ, Li YL, Zeng GR, Tan JB, Liang JN, et al. Study on antioxidant constituents of Sarcandra glabra (Thunb.) Nakai. Chin J Med Chem. 2010;20:57–60.
Li B, Huang MJ, Li YL, Zeng GY, Tan JB, Zhou YJ. Antioxidant constituents of Sarcandra glabra (Thunb.) Nakai. J Shenyang Pharm Univ. 2009;26:900–3.
Liu X, Zhang YF, Yang L, Feng Y, Liu YM, Zeng X. Studies on phenolic acid constituents from the whole plant of Sarcandra glabra. Trad Chinese Drug Res Clin Pharmacol. 2012;23:295–8.
Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev. 2011;111:6423–51. https://doi.org/10.1021/cr200021m.
Duan YH, Li C, Dai Y, Yao XS. A new phenylpropanediol from Sarcandra glabra (Chloranthaceae). Plant Divers Resour. 2012;34:208–10. https://doi.org/10.3724/sp.j.1143.2012.11162.
Zhao WY, Yan JJ, Liu TT, Gao J, Huang HL, Sun CP, et al. Natural sesquiterpenoid oligomers: a chemical perspective. Eur J Med Chem. 2020;203: 112622. https://doi.org/10.1016/j.ejmech.2020.112622.
Kawabata J, Fukushi Y, Tahara S, Mizutani J, Shizukaol A. a sesquiterpene dimer from Chloranthus japonicus. Phytochemistry. 1990;29:2332–4. https://doi.org/10.1016/0031-9422(90)83065-9.
Eagan JM, Kanyiva KS, Hori M, Snyder SA. Total synthesis, reactivity, and structural clarification of lindenatriene. Tetrahedron. 2019;75:3145–53. https://doi.org/10.1016/j.tet.2019.04.051.
Yuan C, Du B, Deng H, Man Y, Liu B. Total syntheses of sarcandrolide J and shizukaol D: lindenane sesquiterpenoid [4+2] dimers. Angew Chemie Int Ed. 2017;56:637–40. https://doi.org/10.1002/anie.201610484.
Martinez RM, Burdge HE, Shenvi RA. Reanalysis of lindenatriene, a building block for the synthesis of lindenane oligomers. Tetrahedron. 2019;75:3140–4. https://doi.org/10.1016/j.tet.2019.03.011.
Durairaj J, Di Girolamo A, Bouwmeester HJ, Ridder D, Beekwilder J, Dijk AD. An analysis of characterized plant sesquiterpene synthases. Phytochemistry. 2019;158:157–65. https://doi.org/10.1016/j.phytochem.2018.10.020.
Kawabata J, Tahara S, Mizutani J. Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae). Agric Biol Chem. 1981;45:1447–53. https://doi.org/10.1080/00021369.1981.10864706.
Qin J, Wang RS, Teng JA, Chen J, Hang R. Experimental study on the effect of Sarcandra glabra extracts on radiation-induced acute injury to parotid gland. Chin J Radiol Med Prot. 2008;28:351–3.
Qin J, Teng JA, Wang RS. Experiment of radioprotective early radiation damage of Sarcandra glabra on salivary gland: observe the tissue morphology following fractionated radiation on head-neck region in guinea-pig models. Chin J Radiol Health. 2008;17:269–71.
Yao YD, Zhang TT, Hu K, Wang RS. Protective effect of Sarcandra glabra on radiation-induced parotid injury in rats. Chin J Radiol Med Prot. 2020;12:11–8.
He RR, Yao XS, Li HY, Dai Y, Duan YH, Li YF, et al. The anti-stress effects of Sarcandra glabra extract on restraint-evoked immunocompromise. Biol Pharm Bull. 2009;32:247–52. https://doi.org/10.1248/bpb.32.247.
Zhang HD, Wang RS, Ma SS, Liang FF, Xiao S, Li GJ. Scavenging effect of Sarcandra glabra powder on radiation-induced reactive oxygen species in the parotid gland of miniature pigs. J South Med Univ. 2011;31:93–5.
Chan MFE, Geronimo AJO, Aspiras APF, Busaing EJW, Dato RJB, et al. Analysis of phytochemical, antimicrobial, and antioxidant properties of Sarcandra glabra (Thunb.) Nakai in relation to its ethnomedicinal relevance in Cordillera, Philippines. Indian J Trad Knowl. 2016;15:411–6.
Hai NT, Thu DK, Tung BT. Sarcandra glabra extract protects against scopolamine induced cognitive deficits by modulating neuroinflammation and the cholinergic System. Curr Enzym Inhib. 2018;14:210–6. https://doi.org/10.2174/1573408014666180907155741.
Yang YC, Wu WH, Tang SC, Wang Q, Xiao YF, Li DD, et al. Comparative study on antioxidant activities of different extracts of Sarcandra glabra. Mod Agric Sci Tech. 2022;17:190–5.
Jin L, Guan X, Liu W, Zhang X, Yan W, Yao WB, et al. Characterization and antioxidant activity of a polysaccharide extracted from Sarcandra glabra. Carbohydr Polym. 2012;90:524–32. https://doi.org/10.1016/j.carbpol.2012.05.074.
Li ZY, Li HY, Wang XL, Hao ZB. Absorption and separation of macroporous resins for total flavonoids of Sarcandra glaber (Thunb.) Nakai and antioxidant activities in vitro. J Guilin Univ Tech. 2011;1:110–4.
Zhang ZZ, Xiao BH, Chen Q, Lian XY. Synthesis and biological evaluation of caffeic acid 3,4-dihydroxyphenethyl ester. J Nat Prod. 2010;73:252–4. https://doi.org/10.1021/np900519d.
Liu JJ, Li XC, Lin J, Li YR, Wang TT, Jiang Q, et al. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: a bioevaluation and mechanistic chemistry. BMC Complement Altern Med. 2016;16:423. https://doi.org/10.1186/s12906-016-1383-7.
Sun XY, Zhao QQ, Si Y, Li KD, Zhu JY, Gao XD, et al. Bioactive structural basis of proteoglycans from Sarcandra glabra based on spectrum-effect relationship. J Ethnopharmacol. 2020;259: 112941. https://doi.org/10.1016/j.jep.2020.112941.
Meyanungsang K. Documentation and biological and phytochemical analysis of Chungtia medicinal plants of Nagaland, India. Macquire University, Sydney, 2015. https://doi.org/10.25949/19434758.v1.
Wu WH, Li KJ, Wang Q, Li DD, Wang HL, Li JL, et al. Study on antibacterial activity of different extracts from Sarcandra glabra (Thunb.) Nakai. Mod Agric Res. 2023;29:96–100.
Chen YY. Application of herbal Sarcandra in remediation of acne vulgaris. Chung Yuan Christian University, Taiwan, 2016. https://doi.org/10.6840/cycu201600042.
Tong Y, Xu X, Shi XQ, Li JK. Antioxidant activity of ethanol extract of Sarcandra glabra and its antifungal activity on main pathogens of postharvest mango. Storage Process. 2022;22:8.
He RR, Cao HJ, Tan RR, Hu YA, Li YF, Hiroshi K. Protective effect of Sarcandra glabra extract against influenza virus-induced pneumonia in restraint-stressed mice. International Conference on Pulmonary & Respiratory Medicine, vol. 2, Chicago-North Shore, USA: 2012, p. 36. https://doi.org/10.4172/2161-105X.S1.002.
Liu JX, Zhang Y, Hu QP, Li JQ, Liu YT, Wu QG, et al. Anti-inflammatory effects of rosmarinic acid-4-O-β-D-glucoside in reducing acute lung injury in mice infected with influenza virus. Antiviral Res. 2017;144:34–43. https://doi.org/10.1016/j.antiviral.2017.04.010.
Wang YT, Yan W, Chen QL, Huang WY, Yang ZF, Li X, et al. Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus. Biomed Pharmacother. 2017;87:583–8. https://doi.org/10.1016/j.biopha.2016.12.117.
Huo YH, Zhang Y, An M, Li X, Lai XP, Liu XH, et al. Effects of different parts of Sarcandra glabra extract on oxidative stress in mice with viral lung injury. J Chin Med Mater. 2020;43:2555–9.
Ma L, Mok CK, Chu JJH. Antiviral effectiveness of Sarcandra glabra and Fragaria vesca extracts against influenza A H1N1. Int J Infect Dis. 2020;101:253. https://doi.org/10.1016/j.ijid.2020.11.097.
Pan BW, Li SM, Xiao JW, Yang X, Xie SX, Zhou Y, et al. Dual inhibition of HIV-1 and cathepsin L proteases by Sarcandra glabra. Molecules. 2022. https://doi.org/10.3390/molecules27175552.
Zhong LY, Liu TH, Chen YX, Zhong XY, Du X, Lu ZS, et al. The study on effect of Sarcandra glabra on prevention and treatment of thrombocytopenia by chemotherapy. J Chinese Med Mater. 2005;28:35–8.
Zhang CL. Pharmacodynamic study of active fraction of Sarcandra glabra for mice with sequenced thrombocytopenic purpura. Inf Trad Chin Med. 2013. https://doi.org/10.3969/j.issn.1002-2406.2013.03.014.
Jiang YL, Zheng Q, Zhang AP, Le CL, Xia LM, Luo MH. Flavone from Zhongjiefeng (Herba Sarcandrae Glabrae ) inhibits platelet apoptosis in immune-induced bone marrow failure through mitochondrial pathway. J Trad Chin Med. 2017;37:643–9. https://doi.org/10.1016/s0254-6272(17)30318-7.
Lu XN, Zhang J, Peng W, Wu Q, Xu GL, Yan XJ. Effects of flavonoids Sarcandrae on bone marrow stromal cells and megakaryocytes of mice with cytarabine-induced thrombocytopenia. Pharmacol Clin Chin Mater Medica. 2018;34:32–5.
Lu XN, Zhang J, Yan XJ, Xu GL, Shang GB. Effects of flavonoids from Sarcandra Herba on expression of SDF-1 and CXCR-4 in the bone marrow of chemotherapy-induced thrombocytopenia model mice. Tradit Chin Drug Res Clin Pharmacol. 2018;29:433–7. https://doi.org/10.19378/j.issn.1003-9783.2018.04.010.
Li WY, Chiu LCM, Lam WS, Wong WY, Chan YT, Ho YP, et al. Ethyl acetate extract of Chinese medicinal herb Sarcandra glabra induces growth inhibition on human leukemic HL-60 cells, associated with cell cycle arrest and up-regulation of pro-apoptotic Bax/Bcl-2 ratio. Oncol Rep. 2007;17:425–31. https://doi.org/10.3892/or.17.2.425.
Lee MH. Studies on enhancing U937 cells phagocytosis from the leaves of Chloranthus glabra. Chia Nan University of Pharmacy and Science, 2007. https://hdl.handle.net/11296/kwx5kr.
Kang M, Tang AZ, Liang G, Yi X, Liu J. Study on the apoptosis of nasopharyngeal carcinoma cell line administrated with Sarcandra glabra extracts in vivo and its mechanism. J Chin Med Mater. 2008;31:1529–33.
Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Bartlett J, Smith PT, et al. Anti-proliferative activities of selected Chinese medicinal herbs against human cancer cell lines. Phytopharmacology. 2013;4:206–19.
Wang XX. Research on effects of herb of Glabrous Sarcandra on human prostate carcinoma DU-145 Cells PI3K / m TOR signal transduction pathway. Proceedings of the 6th International Conference on Mechatronics, Materials, Biotechnology and Environment, Advances in Engineering Research, Yinchuan, China: Atlantis Press; 2016, p. 688–91. https://doi.org/10.2991/icmmbe-16.2016.128.
Zheng YH, Pan XM, Li LT. Study on the anti-tumor activity of extracts from S. hainanensis. Contemp Med Symp. 2020;16:74–6.
Huang YM, Zhao Y, Yang YP, Xiao BH, Chen Q. Anti-tumor effect of Zhongjiefeng injection and its combination with adriamycin. Trad Chin Drug Res Clin Pharmacol. 2007;18:200–2.
Zhao Y, Sun YZ, Chen Q. Studies on the antitumor activity of ZhongJieFeng in vivo. Chin J Ethnomed Ethnopharmacy. 2008;17:8–9.
Zhao Y, Sun YZ, Chen Q. Inhibitory action of Sarcandra glabra injection on the growth of SGC-7901 transplantation tumor in nude mice and its induction on apoptosis. China Pharm. 2009;6:412–5.
Zhang SW, Zhou SY, Ding WJ. Influence of Sarcandra glabra on multiplication and apoptosis of human prostatic cancer DU-145 cells. Liaoning J Trad Chinese Med. 2012;1:172–5.
Wu JT, Lv SM, Lu CH, Gong J, An JB. Effect of 3,3′-biisofraxidin on apoptosis of human gastric cancer BGC-823 cells. Trop J Pharm Res. 2015;14:1803–11. https://doi.org/10.4314/tjpr.v14i10.10.
Zheng ZZ, Qiao ZH, Gong R, Wang YL, Zhang YQ, Ma YP, et al. Uvangoletin induces mitochondria-mediated apoptosis in HL-60 cells in vitro and in vivo without adverse reactions of myelosuppression, leucopenia and gastrointestinal tract disturbances. Oncol Rep. 2016;35:1213–21. https://doi.org/10.3892/or.2015.4443.
Sun HJ, Lu XN, Hu XY, Chen Z, Zhu J, Lu LJ, et al. Effect and mechanism of total flavonoids from Sarcandrae Herba on the apoptosis in human leukemia K562 cells. Pharmacol Clin Chinese Mater Medica. 2019;6:54–7. https://doi.org/10.13412/j.cnki.zyyl.2019.06.012.
Shen JY, Zhu XR, Wu ZR, Shi YJ, Wen TF. Uvangoletin, extracted from Sarcandra glabra, exerts anticancer activity by inducing autophagy and apoptosis and inhibiting invasion and migration on hepatocellular carcinoma cells. Phytomedicine. 2022;94: 153793. https://doi.org/10.1016/j.phymed.2021.153793.
Dong AL, Fang YZ, Zhang L, Xie J, Wu X, Zhang LP, et al. Caffeic acid 3,4-dihydroxy-phenethyl ester induces cancer cell senescence by suppressing twist expression. J Pharmacol Exp Ther. 2011;339:238–47. https://doi.org/10.1124/jpet.111.181081.
Guo X, Shen L, Tong YH, Zhang J, Wu G, He Q, et al. Antitumor activity of caffeic acid 3,4-dihydroxyphenethyl ester and its pharmacokinetic and metabolic properties. Phytomedicine. 2013;20:904–12. https://doi.org/10.1016/j.phymed.2013.04.002.
Zhang ZZ, Zheng Y, Zhu R, Zhu YQ, Yao WB, Liu W, et al. The ERK/eIF4F/Bcl-XL pathway mediates SGP-2 induced osteosarcoma cells apoptosis in vitro and in vivo. Cancer Lett. 2014;352:203–13. https://doi.org/10.1016/j.canlet.2014.06.015.
Zhang ZZ, Liu W, Zheng Y, Jin L, Yao WB, Gao XD. SGP-2, an acidic polysaccharide from Sarcandra glabra, inhibits proliferation and migration of human osteosarcoma cells. Food Funct. 2014;5:167–75. https://doi.org/10.1039/c3fo60378d.
Tang MM, Xie X, Shi MR, Xin WX, Zheng GW, Zhang YH, et al. Antileukemic effect of caffeic acid 3,4-dihydroxyphenetyl ester. Evidences for its mechanisms of action. Phytomedicine. 2021;80:153383. https://doi.org/10.1016/j.phymed.2020.153383.
Xie Y, Zeng JW, Zheng YF, Lin PL, Liang YC. Study on anti-inflammatory active sites of Sarcandra glabra (Thunb.) Nakai produced in Fujian province. J Fujian Univ TCM. 2010;20:35–8.
Li XQ, Shen J, Jiang YY, Shen T, You L, Sun XB, et al. Anti-inflammatory effects of chloranthalactone B in LPS-stimulated RAW264.7 cells. Int J Mol Sci. 2016. https://doi.org/10.3390/ijms17111938.
Tsai YC, Chen SH, Lin LC, Fu SL. Anti-inflammatory principles from Sarcandra glabra. J Agric Food Chem. 2017;65:6497–505. https://doi.org/10.1021/acs.jafc.6b05125.
Tang PF, Li QR, Liao ST, Wei SS, Cui LT, Xu W, et al. Shizukaol A exerts anti-inflammatory effect by regulating HMGB1/Nrf2/HO-1 pathway. Phytomedicine. 2021;82: 153472. https://doi.org/10.1016/j.phymed.2021.153472.
Cao HJ, Tan RR, He RR, Tang LP, Wang XL, Yao N, et al. Sarcandra glabra extract reduces the susceptibility and severity of influenza in restraint-stressed mice. Evid-Based Complement Altern Med. 2012;2012: 236539. https://doi.org/10.1155/2012/236539.
Liu L, Mu QL, Li WF, Xing W, Zhang HL, Fan T, et al. Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes. Immunobiology. 2015;220:406–13. https://doi.org/10.1016/j.imbio.2014.10.007.
Niu XF, Wang Y, Li WF, Mu QL, Li HN, Yao H, et al. Protective effects of isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2015;24:432–9. https://doi.org/10.1016/j.intimp.2014.12.041.
Hou BC, Zhan YP, Liu HY, Liu WC, Chen SB. Sarcandra glabra attenuates LPS-induced acute lung injury via inhibiting inflammation in rats. Int J Clin Exp Pathol. 2016;9:715–23.
Liu TY, Chen SB. Sarcandra glabra combined with lycopene protect rats from lipopolysaccharide induced acute lung injury via reducing inflammatory response. Biomed Pharmacother. 2016;84:34–41. https://doi.org/10.1016/j.biopha.2016.09.009.
Sun SB, Yan ZJ, Shui XL, Qi WH, Chen YL, Xu X, et al. Astilbin prevents osteoarthritis development through the TLR4/MD-2 pathway. J Cell Mol Med. 2020;24:13104–14. https://doi.org/10.1111/jcmm.15915.
Feng Q, Si Y, Zhu LL, Wang F, Fang JQ, Pan C, et al. Anti-inflammatory effects of a SERP 30 polysaccharide from the residue of Sarcandra glabra against lipopolysaccharide-induced acute respiratory distress syndrome in mice. J Ethnopharmacol. 2022;293: 115262. https://doi.org/10.1016/j.jep.2022.115262.
Wang Y, Hou GX, Liu YQ. Intervention effect and mechanism of polysaccharides of Sarcandra glabra on exercise-induced muscle damage. J Sanming Univ. 2022;2:1–9.
Jiang Z, Chen Z, Li X, Zhao J, Li S, Hu J, et al. Immunomodulatory effects of Sarcandra glabra polysaccharides on macrophage RAW264.7. Chin J Exp Trad Med Formulae. 2014;20:178–82.
Liu W, Gong XQ, Luo JH, Jiang LL, Lu WS, Pan C, et al. A purified acidic polysaccharide from Sarcandra glabra as vaccine adjuvant to enhance anti-tumor effect of cancer vaccine. Carbohydr Polym. 2021;263: 117967. https://doi.org/10.1016/j.carbpol.2021.117967.
Li HY, He RR, Liang T, Ye WC, Yao XS, Kurihara H. Effect of Sarcandra glabra (Thunb) Nakai extract on mice model of immunological hepatitis and acute inflammation. Chinese Pharmacol Bull. 2008;24:244–50.
Li C, Wen R, Liu DW, Yan LP, Gong QF, Yu H. Assessment of the potential of Sarcandra glabra (Thunb.) Nakai. in treating ethanol-induced gastric ulcer in rats based on metabolomics and network analysis. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.810344.
Liu W, Zheng Y, Zhang ZZ, Yao WB, Gao XD. Hypoglycemic, hypolipidemic and antioxidant effects of Sarcandra glabra polysaccharide in type 2 diabetic mice. Food Funct. 2014;5:2850–60. https://doi.org/10.1039/c4fo00430b.
Liu W, Lu WS, Chai Y, Liu YM, Yao WB, Gao XD. Preliminary structural characterization and hypoglycemic effects of an acidic polysaccharide SERP1 from the residue of Sarcandra glabra. Carbohydr Polym. 2017;176:140–51. https://doi.org/10.1016/j.carbpol.2017.08.071.
Liu W, Hu C, Liu YM, Dai SJ, Lu WS, Lv X, et al. Preparation, characterization, and α-glycosidase inhibition activity of a carboxymethylated polysaccharide from the residue of Sarcandra glabra (Thunb.) Nakai. Int J Biol Macromol. 2017;99:454–64. https://doi.org/10.1016/j.ijbiomac.2017.02.065.
Ji N. Effects of total flavonoids of Sarcandrae glabra on mouse blood-fat content. J Mt Agric Biol. 2012;31:268–70.
Acknowledgements
We thank REM Corporation Sdn. Bhd. (Dr Donald Chen) for inspiration and financial support.
Author information
Authors and Affiliations
Contributions
JNC wrote and prepared the manuscript. KHL and PK reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, JN., Krishnan, P. & Lim, KH. A comprehensive review on the chemical constituents, sesquiterpenoid biosynthesis and biological activities of Sarcandra glabra. Nat. Prod. Bioprospect. 13, 53 (2023). https://doi.org/10.1007/s13659-023-00418-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-023-00418-8