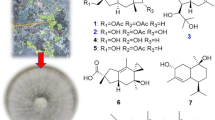
Abstract
One novel spirolactone, aquilarisinolide (1), three new sesquiterpenoids, (2R,4S,5R,7R)-2-hydroxyeremophila-9,11-dien-8-one (2), (1R,4S,5S,7R,11R)-13-hydroxyepidaphnauran-9-en-8-one (3), and (4R,5S,7R,8S,10S,13R)-8,13-dihydroxyrotunda-1,11-dien-3-one (4), together with 13 known compounds (5–17) were isolated from the resinous heartwood of Aquilaria sinensis (Thymelaeaceae). The structures of the new compounds were elucidated based on the analysis of NMR and MS data and theoretical calculations their ECD spectra. The isolated compounds were evaluated for their protective activities against PC12 cell injury induced by corticosterone (CORT) and 1-methyl-4-phenylpyridine ion (MPP+), as well as inhibitory activities against BACE1. Compound 4, 5,6-dihydroxy-2-(2-phenylethyl)chromone (5), daphnauranol B (7), 6-methoxy-2-[2-(3-methyoxyphenyl)ethyl]chromone (10), isoagarotetrol (14), and 1-hydroxy-1,5-diphenylpentan-3-one (16) showed significant protective effects on CORT-induced injury in PC12 cells at a concentration of 20 μM (P < 0.001). Isoagarotetrol (14) showed a significant protective effect on MPP+-induced injury in PC12 cells at a concentration of 20 μM (P < 0.001), while compound 4 showed a moderate activity (P < 0.01). The BACE1-inhibitory activities of all tested compounds were very weak with less than 30% inhibition at a concentration of 20 μM.
Graphic Abstract

Similar content being viewed by others
1 Introduction
The resinous heartwood of Aquilaria sinensis (Lour.) Spreng. (Thymelaeaceae) is known as agarwood (chen-xiang in Chinese). Chen-xiang, a traditional Chinese medicine, is used to treat thoraco-abdominal distension and pain (xiong-fu zhang-men teng-tong), vomiting and hiccups due to stomach cold (wei-han ou-tu e-ni), and asthma due to kidney deficiency (shen-xu qi-ni chuan-ji) [1]. The major chemical constituents from Aquilaria plants are sesquiterpenoids and chromones [2,3,4]. The fractions and components from agarwood and Aquilaria trees show various pharmacological activities, such as neural activity, gastrointestinal regulation, cytotoxicity, analgesic effects, and antibacterial, antifungal, anti-inflammatory, antiasthmatic, anti-diabetic, and antioxidant activities [4].
In a previous study, we reported several neuroprotective compounds from the resinous heartwood of A. sinensis with the origin in Guangdong, China. One hexahydrochromone and three sesquiterpenoids exert significant protective effects on rat adrenal pheochromocytoma (PC12) cell injury induced by corticosterone (CORT), while the hexahydrochromone and one sesquiterpenoid exhibit significant protective effects on 1-methyl-4-phenylpyridine ion (MPP+)-induced PC12 cell injury. All of these compounds from the plant are inactive against beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) [5]. In this paper, the isolation and structural elucidation of 17 compounds (1–17, Fig. 1), including four new compounds (1–4), from chen-xiang with the origin in Hainan, China, along with bioassay results in the models of CORT-induced and MPP+-induced PC12 cell damage and BACE1 inhibition, are reported.
2 Results and Discussion
2.1 Structural Elucidation
The molecular formula of aquilarisinolide (1) was determined to be C14H20O3 based on 13C NMR data (Table 1) and the positive ion at m/z 259.1307 [M + Na]+ (calcd for C14H20NaO3, 259.1310) in the HRESIMS. The NMR data (Table 1) indicated the presence of one carbonyl group (δC 209.0), one α,β-unsaturated γ-lactone [δH 5.79 (br s); δC 172.0, 169.0, 118.8, and 99.4] [6], three methyl groups [δH 2.07 (s), 1.04 (t, J = 7.5 Hz), and 0.84 (d, J = 7.2 Hz); δC 15.5, 15.3, and 8.0], four methylenes, and two methines. Based on the COSY correlations (Fig. 2), two connections, C-9-C-8-C-7-(C-15)-C-6-C-10 and C-12-C-13 were deduced. Through its HMBC spectrum, correlations from H3-14 to C-3, C-4, and C-5, from H-3 to C-2 and C-5, from H2-8 and H2-10 to C-5, from H-6 to C-11, and from H3-13 to C-11 (Fig. 2) were observed. Combining these 2D NMR correlations and the HRESIMS, the planar structure of 1, with a spiro ring system, was deduced to be 4,7-dimethyl-6-(2-oxobutyl)-1-oxaspiro[4.4]non-3-en-2-one. The relative configuration of 1 was deduced from its ROESY spectrum. H-6 was first assumed to be α-oriented; thus, the C-6-C-10 bond should be β-oriented. Based on the ROESY correlations of H3-14/H2-10 and H3-15/H2-10, the C-4-C-5 bond and 7-Me should be β-oriented, and thus, the C-5-O bond, H-6, and H-7 should be α-oriented.
According to 13C NMR data (Table 1) and HRESIMS, the molecular formula of compound 2 was deduced to be C15H22O2. Its NMR data indicated the presence of one terminal double bond [δH 4.90 (1H, m) and 4.71 (1H, m); δC145.1 and 114.1], one α,β-unsaturated ketone [δH 5.79 (1H, d, J = 1.2 Hz); δC 202.0, 170.1, and 125.2], three methyl groups [δH 1.75 (3H, br s), 1.12 (3H, s), and 0.98 (3H, d, J = 6.7 Hz)], three methylenes, three methines including one oxygenated group [δH 3.57 (1H, m); δC 72.3], and one quaternary carbon (δC 40.7). Two fragments of C-1-C-2-C-3-C-4-C-15 and C-6-C-7 were deduced by correlations (Fig. 2) from its COSY spectrum. Based on the key HMBC correlations from H-1 to C-9, from H-9 to C-1, from H3-14 and C-4, C-5, C-6, and C-10, from H2-6 to C-8 and C-10, and from H3-13 to C-7, C-11, and C-12, the planar structure of 2 was elucidated as 2-hydroxyeremophila-9,11-dien-8-one with an eremophilane skeleton. In order to deduce the relative configuration of 2, H-2 was assumed to be α-oriented. Because a large coupling constant between H-2 and H-1β (J1β,2 = 11.5 Hz) was observed, the orientations of the two protons of C-1 were determined. In the ROESY spectrum of 2, the correlations of H-1β/H3-14, H3-14/H3-15, and H-4/H-7 were observed. Thus, 4-Me and 5-Me should be β-oriented and H-7 should be α-oriented.
Compound 3 had the molecular formula C15H22O2 based on its 13C NMR data (Table 2) and the positive ion at m/z 257.1516 [M + Na]+ (calcd for C15H22NaO2, 257.1518) in the HRESIMS. The 1H NMR spectrum showed resonances for one trisubstituted double bond [δH 5.90 (br s)], as well as two methyl groups [δH 2.03 (d, J = 1.2 Hz) and 1.02 (d, J = 7.2 Hz)] (Table 2). The 13C NMR spectrum showed resonances for 15 carbon signals indicating the presence of one α,β-unsaturated ketone (δC 209.8, 172.5, and 130.0), two methyl groups (δC 22.5 and 17.3), five methylenes including one oxygenated group (δC 64.1), four methines, and one quaternary carbon atom (δC 53.5). By comparing its NMR data with those of daphnauranol A (6) and daphnauranol B (7) [7], compound 3 was deduced to be this type of sesquiterpenoid.
From the COSY correlations of 3 (Fig. 2), one moiety of C-2-C-3-C-4-(C-15)-C-5-C-6-C-7-C-11-(C-13)-C-12 was elucidated. According to the HMBC correlations (Fig. 2) from H-2 to C-10, from H-3 to C-1, from H3-15 to C-3 and C-5, from H2-6 to C-8, from H-11 to C-1 and C-8, from H2-12 to C-10, and from H3-14 to C-1, C-9, and C-10, the planar structure of 3 with a 5/6/7 ring system was elucidated as shown in Fig. 2. Its structure was very similar to that of daphnauranol A, except for the lack of a hydroxy group at C-7 in 3. In the ROESY spectrum of 3, correlations of H-2β/H-12β, H-3β/H-12β, H3-15/H-6β, H3-15/H-12β, H-6β/H2-13, and H-3α/H-5 were observed. The relative configuration of 3 was deduced to be 13-hydroxydaphnauran-9-en-8-one as shown in Fig. 2.
Compound 4 was assigned the molecular formula C15H20O3, as determined by 13C NMR data (Table 2) and the positive ion at m/z 271.1314 [M + Na]+ (calcd for C15H20NaO3, 271.1310) in the HRESIMS. The IR spectrum showed absorption bands for hydroxy groups (3424 cm−1), an α, β-unsaturated ketone (1687 cm−1), and an exocyclic double bond (3071 cm−1). The 1H and 13C NMR data in DMSO-d6 (Table 2) indicated the presence of one α,β-unsaturated ketone [δH 5.94 (d, J = 1.1 Hz); δC 210.7, 189.7, and 126.7], one exocyclic double bond [δH 4.97 (br s) and 4.95 (br s); δC 153.6 and 115.9], two methyl groups [δH 1.17 (s) and 0.89 (d, J = 7.4 Hz); δC 22.5 and 17.3], two methylenes, five methines including two oxygenated groups [δH 4.13 (m) and 3.58 (d, J = 4.0 Hz); δC 74.0 and 64.5), one quaternary carbon atom (δC 42.8), and two hydroxy groups [δH 5.11 (d, J = 4.0 Hz) and 4.84 (d, J = 3.0 Hz)]. According to the COSY correlations (Fig. 2), a fragment comprising of C-15-C-4-C-5-C-6-C-7-C-8-C-9 was deduced. By key HMBC correlations from H-2 to C-4 and C-5, from H3-15 to C-3 and C-5, from H2-6 to C-11, from H-12 to C-7 and C-13, and from H3-14 to C-1, C-9, C-10, and C-13, compound 4 was elucidated to be 8,13-dihydroxyrotunda-1,11-dien-3-one, with a very rare tricyclic rotundane skeleton. The relative configuration of 4 was deduced from its ROESY spectrum. The ROESY correlations (Fig. 2) of H3-15/H-6β and H-6β/H-13 indicated that 13-OH should be α-oriented; the ROESY correlations of 13-OH/H-8, 13-OH/H-9β, and H-5/H-9α indicated that 8-OH and H-5 should also be α-oriented.
The absolute configurations of 1–4 were determined to be 5S,6S,7S-1, 2R,4S,5R,7R-2, 1R,4S,5S,7R,11R-3, and 4R,5S,7R,8S,10S,13R-4 (Fig. 1), by comparison of the experimental electronic circular dichroism (ECD) spectra with the theoretical results (Fig. 3).
5,6-Dihydroxy-2-(2-phenylethyl)chromone (5) was recently reported with NMR data measured in CDCl3 [8]. Its NMR data in methanol-d4 are shown in Table 3. The structure of 6,7-dimethoxy-2-[2-(3-methoxyphenyl)ethyl]chromone (12) was found in the SciFinder database. However, no literature was provided in the database. The NMR data of 12 are presented in this paper (Table 3). The relative configuration of compound 15 has been reported [9]. Its absolute configuration was determined to be (5S,6S,7S,8R)-8-chloro-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydrochromone by ECD calculations (Supplementary Material, Fig. S39). Daphnauranol A (6) [7], daphnauranol B (7) [7], 2-(2-phenylethyl)chromone (8) [10], 6-methoxy-2-(2-phenylethyl)chromone (9) [11], 6-methoxy-2-[2-(3-methyoxyphenyl)ethyl]chromone (10) [11], 6,7-dimethoxy-2-(2-phenylethyl)chromone (11) [11], 6,7-dimethoxy-2-[2-(4-methoxyphenyl)ethyl]chromone (13) [12], isoagarotetrol (14) [13], 1-hydroxy-1,5-diphenylpentan-3-one(16) [14], and 3-hydroxy-1,5-diphenylpentan-1-one (17) [14] were determined by comparing their obtained spectroscopic data with those reported in the literature.
2.2 Neuroprotective Activities
CORT-induced PC12 cell damage is used as an in vitro experimental model for depression studies [15, 16], while MPP+ has been widely used as a neurotoxin to induce Parkinson’s disease (PD) symptoms in cells or rodents’ models [17, 18]. Additionally, BACE1 plays a critical role in Alzheimer’s disease (AD) pathophysiology and thus, BACE1 inhibition is considered to be a therapeutic approach for AD [19].
Compounds 1–12 and 14–17 were evaluated for their protective activities against PC12 cell injury induced by CORT and MPP+ and inhibitory activities against BACE1. Compound 13 was not evaluated for all activities due to an insufficient amount. The results are presented in Table 4. Compared with the negative control, compounds 4, 5, 7, 10, 14, and 16 showed significant protective effects on CORT-induced injury in PC12 cells at a concentration of 20 μM (P < 0.001), while compounds 3 and 11 showed weak protective activities (P < 0.05). Other tested compounds were inactive (P > 0.05). Compared with the negative control, compound 14 showed a significant protective effect on MPP+-induced injury in PC12 cells at a concentration of 20 μM (P < 0.001); compound 4 showed a moderate effect on MPP+-induced injury in PC12 cells at a concentration of 20 μM (P < 0.01); and compound 16 showed a weak protective activity (P < 0.05). Other tested compounds were inactive (P > 0.05). The BACE1-inhibitory activities of all tested compounds were very weak with inhibition less than 30% at a concentration of 20 μM, compared with the positive control LY2886721 with 75.39% inhibition at a concentration of 0.2 μM.
3 Experimental Section
3.1 General Experimental Procedures
Optical rotations were recorded using a JASCO P-1020 polarimeter (Jasco Corp., Tokyo, Japan). UV spectra were recorded on a Shimadzu UV-2401 PC spectrophotometer (Shimadzu, Kyoto, Japan). Electronic circular dichroism (ECD) spectra were recorded on a Chirascan CD spectrometer (Applied Photophysics Ltd., Leatherhead, UK). IR spectra were measured on a Bruker Tensor 27 FTIR Spectrometer (Bruker Corp., Ettlingen, Germany) with KBr disks. 1H and 13C NMR spectra were collected on Bruker DRX-500, Avance III-600, and Ascend™ 800 MHz NMR spectrometers (Bruker Corporation, Karlsruhe, Germany), with TMS as an internal standard. ESIMS and HRESIMS analyses were performed on an API QSTAR Pulsar 1 spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA). Silica gel G (80–100 and 300–400 mesh, Qingdao Meigao Chemical Co., Ltd., Qingdao, China), C18 silica gel (40–75 μm, Fuji Silysia Chemical Ltd., Aichi, Japan), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used for column chromatography. Thin-layer chromatography (TLC) spots were visualized under UV light at 254 nm and by dipping in 5% H2SO4 in alcohol followed by heating. Semipreparative high-performance liquid chromatography (HPLC) was performed on an Agilent 1200 series pump (Agilent Technologies, Santa Clara, USA) equipped with a diode array detector, a Welch Ultimate AQ-C18 column (5 μm, ϕ 7.8 × 250 mm, Welch Materials Inc., Shanghai, China), an Agilent Zorbax SB-C18 column (5.0 μm, ϕ 9.4 × 250 mm), and a chiral-phase CD-Ph column (5.0 μm, ϕ 4.6 × 250 mm; Shiseido, Japan). The absorbance from the MTS assay was measured by a Thermo Multiskan FC microplate reader (Waltham, MA, USA). The fluorescence values in the BACE1-inhibitory activity assay were read by a FlexStation 3 Multi-Mode microplate reader (Molecular Devices, San Jose, CA, USA). Desipramine was purchased from Beijing Pujing Kangli Technology Co., Ltd. (Beijing, China); corticosterone, penicillin, streptomycin, MTS, MPP+, and the BACE1 kit were purchased from Sigma; MTS was purchased from Promega; DMEM, FBS, and PBS were obtained from Biofluids Inc. (Rockville, MD, USA); and LY2886721 (CAS No. 1262036–50-9) was from Shanghai Lanmu Chemical Co., Ltd. (Shanghai, China).
3.2 Plant Material
The resinous heartwood of A. sinensis with the origin in Hainan, China was purchased from Xiamen Yanlaifu Pharmaceutical Co., Ltd., China (production lot number 140303), in December, 2017. The plant material was also identified by Prof. Shu-De Yang, at Yunnan University of Traditional Chinese Medicine, China. A voucher specimen (no. HN140303) was deposited at the Yunnan Key Laboratory for Wild Plant Resources, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3 Extraction and Isolation
The resinous heartwood of A. sinensis (0.5 kg) was ground into a powder and ultrasonically extracted with 95% EtOH at 60 ºC for half an hour. The extract was subjected to reduced pressure evaporation to yield a gum (53.8 g). The gum was dissolved in water and extracted successively with petroleum ether, EtOAc, and n-BuOH to yield fractions A (0.6 g), B (36.4 g), and C (14.7 g), respectively.
Fraction B was subjected to silica gel column chromatography (CC, petroleum ether-EtOAc, 50:1 → 0:1, v/v) to yield four main fractions (B1–B4). Fraction B1 was subjected to reversed-phase C18 (RP-C18) silica gel CC with MeOH-H2O (10% → 100%) to afford four main subfractions B1-1 to B1-4. Subfraction B1-1 (121.8 mg) was subjected to SephadexLH-20 CC (MeOH) to yield subfractions B1-1–1 and B1-1–2. Subfraction B1-1–1 (87.1 mg) was purified by silica gel CC (petroleum ether-EtOAc, 15:1) to yield 7 (17.6 mg). Subfraction B1-1–2 (22.9 mg) was purified by semipreparative HPLC (Agilent Zorbax SB-C18, CH3CN-H2O, 30:70, 2 mL/min) to yield 1 (1 mg, tR = 40.197 min). Subfraction B1-2 (113.7 mg) was separated by silica gel CC (petroleum ether-EtOAc, 50:1) and semipreparative HPLC (Agilent Zorbax SB-C18, MeOH-H2O, 60:40, 2 mL/min) to yield 16 (4.2 mg, tR = 33.431 min) and 17 (2.6 mg, tR = 39.728 min). Subfraction B1-3 (658.2 mg) was subjected to Sephadex LH-20 CC (MeOH) to yield 8 (192.6 mg). Subfraction B1-4 (1.1 g) was subjected to Sephadex LH-20 CC (MeOH) to yield 5 (6.0 mg) and a mixture (953.4 mg). The mixture was purified by silica gel CC (petroleum ether-EtOAc, 50:1 → 10:1) to yield 9 (25.2 mg) and 10 (44.0 mg).
Fraction B2 was subjected to RP-C18 silica gel CC with MeOH-H2O (10% → 100%) to afford 11 (15.6 mg) recrystallized from MeOH and two other subfractions, B2-1 and B2-2. Subfraction B2-1 (436.3 mg) was purified by Sephadex LH-20 CC (MeOH) and semipreparative HPLC (Agilent Zorbax SB-C18, CH3CN-H2O, 30:70, 2 mL/min) to yield 3 (1.5 mg, tR = 47.901 min), 2 (2.4 mg, tR = 40.414 min), and 6 (1.8 mg, tR = 27.272 min). Subfraction B2-2 (3.7 g) was separated by silica gel CC (CH2Cl2-acetone, 5:1) and semipreparative HPLC (Chiral CD-Ph, MeOH-H2O, 80:20, 1 mL/min) to obtain 12 (0.6 mg, tR = 43.207 min) and 13 (1.2 mg, tR = 35.603 min).
Fraction B3 was subjected to RP-C18 silica gel CC with MeOH-H2O (10% → 100%) to afford a main fraction (199.6 mg), which was purified by silica CC (petroleum ether-EtOAc, 2:1) and semipreparative HPLC (Welch Ultimate AQ-C18, MeOH-H2O, 17:83, 2 mL/min) to yield 4 (8.3 mg, tR = 50.308 min).
Fraction B4 was subjected to RP-C18 silica gel CC with MeOH-H2O (10% → 100%) to afford two main subfractions B4-1 and B4-2. Subfraction B4-1 (872.5 mg) was subjected to Sephadex LH-20 CC (MeOH) and was further purified by silica gel CC (CH2Cl2-acetone, 8:1) to yield 14 (8.3 mg). Subfraction B4-2 (696.4 mg) was subjected to Sephadex LH-20 CC (MeOH) and was further purified by silica gel CC (CH2Cl2-acetone, 8:1) and semipreparative HPLC (Welch Ultimate AQ-C18, MeOH-H2O, 45:55, 2 mL/min) to yield 15 (24.4 mg, tR = 27.000 min).
3.4 Spectroscopic Data of Compounds
3.4.1 Aquilarisinolide (1)
White solid; [α] 27D + 71 (c 0.08, MeOH); UV (MeOH) λmax (logε) 215 (4.10) nm; ECD (c 0.016, MeOH): ∆ε219 nm + 5.06; 1H and 13C NMR data, see Table 1; ESIMS m/z 259 [M + Na]+; HRESIMS m/z 259.1307 [M + Na]+ (calcd for C14H20NaO3, 259.1310).
3.4.2 (2R,4S,5R,7R)-2-Hydroxyeremophila-9, 11-dien-8-one (2)
White solid; [α] 23D − 79 (c 0.12, MeOH); UV (MeOH) λmax (logε) 240 (3.08) nm; ECD (c 0.018, MeOH): ∆ε239 nm − 6.83, ∆ε201 nm + 7.36; 1H and 13C NMR data, see Table 1; ESIMS m/z 257 [M + Na]+; HRESIMS m/z 257.1512 [M + Na]+ (calcd for C15H22NaO2, 257.1518).
3.4.3 (1R,4S,5S,7R,11R)-13-Hydroxydaphnauran-9-en-8-one (3)
White solid; [α] 26D − 37 (c 0.07, MeOH); UV(MeOH) λmax (logε) 390 (1.85), 246 (3.97), 201 (3.70) nm; ECD (c 0.018, MeOH): ∆ε322 nm − 3.78, ∆ε249 nm + 12.15; 1H and 13C NMR data, see Table 2; ESIMS m/z 257 [M + Na]+; HRESIMS m/z 257.1516 [M + Na]+ (calcd for C15H22NaO2, 257.1518).
3.4.4 (4R,5S,7R,8S,10S,13R)-8,13-Dihydroxyrotunda-1,11-dien-3-one (4)
Yellow oil; [α] 27D − 78 (c 0.09, MeOH); UV (MeOH) λmax (logε) 237 (3.87), 196 (3.73) nm; ECD (c 0.009, MeOH): ∆ε226 nm − 6.46, ∆ε197 nm + 12.42; IR νmax (KBr) 3424, 3071, 2970, 2936, 2877, 1687, 1597, 1457, 1187, 1043, 1027, 975 cm−1; 1H and 13C NMR data, see Table 2; ESIMS m/z 271 [M + Na]+; HRESIMS m/z 271.1314 [M + Na]+ (calcd for C15H20NaO3, 271.1310).
3.4.5 (5S,6S,7S,8R)-8-Chloro-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydrochromone (15)
Yellow solid; [α] 26D + 12 (c 0.12, MeOH); ECD (c 0.009, MeOH): ∆ε305 nm + 0.32, ∆ε232 nm + 1.33; ESIMS m/z 359 [M + Na]+, 695 [2 M + Na]+.
3.5 Computational Methods
All DFT and TD-DFT calculations were carried out at 298 K in the gas phase with Gaussian 09 [21]. Conformational searches were carried out at the molecular mechanics level of theory employing MMFF force fields. The conformers with relative energy within 10 kcal/mol of the lowest-energy conformer were selected and further geometry optimized at the B3LYP/6–311 + + G (2d, p) level. All the lowest-energy conformers, which correspond to 99% of the total Boltzmann distribution, were selected for ECD spectra calculation. The Boltzmann factor for each conformer was calculated based on Gibbs free energy. Vibrational analysis at the B3LYP/6–311 + + G (2d, p) level of theory resulted in no imaginary frequencies, confirming the considered conformers as real minima. TDDFT was employed to calculate excitation energy (in nm) and rotatory strength R in dipole velocity form, at the B3LYP/6–311 + + G (2d, p) level [22].
3.6 Biological Assays
3.6.1 Corticosterone-Induced Damage in PC12 Cellular Assay
Poorly differentiated PC12 cells (Cell Bank of Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) and incubated with 5% CO2 at 37 ºC. The poorly differentiated PC12 cells were divided into the following groups: blank (untreated), negative control (150 μM CORT), positive control (150 μM CORT plus 10 μM desipramine), and compounds (20 μM of each tested compound plus 150 μM CORT). Briefly, the poorly differentiated PC12 cells were seeded into 96-well culture plates at a density of 1 × 104 cells/well. After culturing for 24 h, compounds were added to the wells. After 48 h, MTS solution was added to each well. The absorbance was measured at 492 nm using a Thermo Multiskan FC [15].
3.6.2 MPP+-Induced Damage in PC12 Cellular Assay
Poorly differentiated PC12 cells were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and incubated at 5% CO2 and 37 ºC. The cells were divided into the following groups: blank (untreated), negative control (750 µM MPP+), positive control (0.2 µM vitamin E plus 750 μM MPP+), and compounds (20 μM of each tested compound plus 750 μM MPP+). Briefly, poorly differentiated PC12 cells were seeded into 96-well culture plates at a density of 1 × 104 cells/well. After 23 h of culture, each compound or vitamin E was added to the wells. One hour later, MPP+ was added. At 24 h after MPP+ exposure, MTS was added; and two hours later, absorbance at 492 nm was read using a Thermo Multiskan FC.
3.6.3 BACE1-Inhibitory Activity Assay
The BACE-inhibitory assay was carried out according to the manufacturer described protocol available from Sigma-Aldrich [20]. Detection was based on fluorescence resonance energy transfer (FRET) technology, by which the enhanced fluorescence signal can be observed after the substrate is cleaved by BACE1. In the positive control group, 76 μL of buffer, 20 μL of 50 μM BACE1 substrate solution, 2 μL of 0.3 units/μL BACE1 enzyme solution, and 2 μL of LY2886721 were added. The final concentration of LY2886721 was 0.2 μM. In the compound groups, 76 μL of buffer, 20 μL of 50 μM BACE1 substrate solution, 2 μL of 0.3 units/μL BACE1 enzyme solution, and 2 μL of tested sample solution were added. The final concentration of compounds was 20 μM. After adding the enzyme, the zero point of the fluorescence value (excitation 320 nm, absorption 405 nm) was measured immediately with FlexStation 3. Then, the plate was incubated for 2 h at 37 ºC, and the fluorescence value was measured again by FlexStation 3.
4 Conclusion
Four new and 13 known compounds were isolated from the resinous heartwood of A. sinensis. Six compounds showed significant protective effects on CORT-induced injury in PC12 cells, while one compound exhibited a significant protective effect on MPP+-induced injury in PC12 cells. These active compounds are worth further evaluation for neuroprotective activities.
References
Editorial Board of Chinese Pharmacopoeia, Chinese Pharmacopoeia (China Medical Science Press, Beijing, 2020), Vol. 1, pp. 192–193
M. Gao, X. Han, Y. Sun, H. Chen, Y. Yang, Y. Liu, H. Meng, Z. Gao, Y. Xu, Z. Zhang, J. Han, RSC Adv. 9, 4113–4130 (2019)
A.N. Kristanti, M. Tanjung, N.S. Aminah, Mini-Rev. Org. Chem. 15, 36–55 (2018)
S. Wang, Z. Yu, C. Wang, C. Wu, P. Guo, J. Wei, Molecules 23, 342 (2018)
Q. He, D.-B. Hu, L. Zhang, M.-Y. Xia, H. Yan, X.-N. Li, J.-F. Luo, Y.-S. Wang, J.-H. Yang, Y.-H. Wang, Phytochemistry 181, 112554 (2021)
F.R. Chang, S.T. Huang, C.C. Liaw, M.H. Yen, T.L. Hwang, C.Y. Chen, M.F. Hou, S.S. Yuan, Y.B. Cheng, Y.C. Wu, Phytochemistry 131, 124–129 (2016)
S.Z. Huang, X.N. Li, Q.Y. Ma, H.F. Dai, L.C. Li, X.H. Cai, Y.Q. Liu, J. Zhou, Y.X. Zhao, Tetrahedron Lett. 55, 3693–3696 (2014)
S. Ahn, M. Chi Thanh, J.M. Choi, S. An, M. Lee, L. Van Thi Hong, J.J. Pyo, J. Lee, M.S. Choi, S.W. Kwon, J.H. Park, M. Noh, J. Nat. Prod. 82, 259–264 (2019)
T. Yagura, M. Ito, F. Kiuchi, G. Honda, Y. Shimada, Chem. Pharm. Bull. 51, 560–564 (2003)
K. Hashimoto, S. Nakahara, T. Inoue, Y. Sumida, M. Takahashi, Y. Masada, Chem. Pharm. Bull. 33, 5088–5091 (1985)
Y. Shimada, T. Tominaga, T. Konishi, S. Kiyosawa, Chem. Pharm. Bull. 30, 3791–3795 (1982)
K. Iwagoe, T. Konishi, S. Kiyosawa, Y. Shimada, K. Miyahara, T. Kawasaki, Chem. Pharm. Bull. 36, 2417–2422 (1988)
Y. Shimada, T. Konishi, S. Kiyosawa, M. Nishi, K. Miyahara, T. Kawasaki, Chem. Pharm. Bull. 34, 2766–2773 (1986)
T. Mukaiyama, T. Takuwa, K. Yamane, S. Imachi, Bull. Chem. Soc. Jpn. 76, 813–823 (2003)
B.P. Jiang, Y.M. Liu, L. Le, Z.Y. Li, J.Y. Si, X.M. Liu, Q. Chang, R.L. Pan, Cell. Physiol. Biochem. 34, 1015–1026 (2014)
L.L. Ji, X. Wang, J.J. Li, X.J. Zhong, B. Zhang, J. Juan, X.Y. Shang, Molecules 24, 625 (2019)
W. Liu, S. Kong, Q. Xie, J. Su, W. Li, H. Guo, S. Li, X. Feng, Z. Su, Y. Xu, X. Lai, Int. J. Mol. Med. 35, 739–746 (2015)
K.H. Lin, C.Y. Li, Y.M. Hsu, C.H. Tsai, F.J. Tsai, C.H. Tang, J.S. Yang, Z.H. Wang, M.C. Yin, Food Chem. Toxicol. 133, 110765 (2019)
H. Hampel, R. Vassar, B. De Strooper, J. Hardy, M. Willem, N. Singh, J. Zhou, R. Yan, E. Vanmechelen, A. De Vos, Biol. Psychiatry 89, 745–756 (2021)
Sigma-Aldrich, https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/cs0010bul.pdf. Accessed 8 April 2021
M.J. Frisch, Gaussian 09. Rev.Cl (M.J. Frisch, et al. Gaussian, Inc., Pittsburgh PA, 2009)
N.L. Tun, D.-B. Hu, M.-Y. Xia, D.-D. Zhang, J. Yang, T.N. Oo, Y.-H. Wang, X.-F. Yang, Nat. Prod. Bioprospect. 9, 243–249 (2019)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (nos. 31960480, 81960629, 21662040, and 21462048) and the Joint Special Project of Local Undergraduate Universities in Yunnan Province, China (no. 2018FH001-024).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, SY., Hu, DB., Xia, MY. et al. Sesquiterpenoids and 2-(2-Phenylethyl)chromone Derivatives from the Resinous Heartwood of Aquilaria sinensis. Nat. Prod. Bioprospect. 11, 545–555 (2021). https://doi.org/10.1007/s13659-021-00313-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-021-00313-0