Abstract
A review is presented of the steels used to produce perhaps the two most famous swords in history, the Japanese sword and the Muslim crucible Damascus sword. The review is based largely on the authors experience in producing blades of these materials, bloomery steel blades by T.Z., and crucible Damascus blades by J.V. and the late bladesmith Alfred Pendray. Brief summaries are presented of the history of when and how the swords were made, our modern understanding of their metallurgical microstructures, their chemical composition, relative strength, resistance to breakage (toughness) and ability to hold an edge. Both swords were superior to the more ancient swords they competed with. All were made from bloomery iron. A smelted bloom contains large amounts of slag inclusions and varying amounts of carbon. The slag inclusions reduced the toughness of the ancient steel and the non-uniform carbon composition of the bloom produced wide variations in strength. It is explained how these deficiencies were overcome in the production of both swords. Comparing the properties of the sword microstructures after processing them to shape, it is concluded that the Japanese sword was superior.
Similar content being viewed by others
Introduction
Throughout human history the sword has been one of the principal weapons of waring armies starting long ago with bronze swords and extending into the iron age which began around 1000 BC. They continued in that role until finally being replaced by guns a few centuries ago. Even today military dress uniforms in European and Asian countries generally include displays of swords. This is less common in the Americas. However, in the US Navy swords are describable for all commissioned officers on active duty but are not required for officers below the level of Lieutenant.
There is a huge literature on the many different sword types developed in human history. See, for example, Sword on the Wikipedia web site. Deciding which swords were the best is very difficult as there are so many variables. For example, which is best for thrusting versus slashing? Which style, regarding cross-sectional shape, length, curvature was best? Is cutting ability more important than toughness? In this paper we limit our evaluation to the microstructures of the blades, and the corresponding physical properties of strength and toughness (resistance to fracture) of the materials.
There are two particular types of swords that have obtained the reputation of being the best, the Japanese sword and the crucible Damascus sword of the Muslim world. Both swords were said to be extremely sharp, have good toughness, and display attractive surface patterns produced by unique internal microstructures. Both were made from a type of iron called bloomery iron [1, 2], first produced by ancient smiths around 1000 BC.
In this paper we review the techniques by which the two swords were made, their resulting physical properties, metallurgical microstructures, and as just mentioned the resulting physical properties of these microstructures: strength and toughness. The two authors have firsthand experience in producing modern reproductions of short blades of the Samurai type, T.Z., and crucible Damascus blades, J.V. (with the aid of the late bladesmith Al Pendray). The microstructure of a short blade is virtually identical to that of a full-length sword blade.
Bloomery Iron
Iron was first made by our ancestors starting around 1000 BC. This was an amazing development considering it was made from rocks or sand, and iron melts at an extremely high temperature, 1538 °C (2800 °F). The simple bloomery furnaces used to make this iron (now called bloomery iron) often produced nearly pure iron. Iron swords were inferior to bronze swords because an iron sword is not as strong (or hard) as a cold-worked bronze sword. However recent work [3] indicates that the first blacksmiths learned how to convert the bloomery iron to steel by carburization in charcoal furnaces, probably within just a few decades, and bronze swords were replaced by steel swords shortly after 1000 BC.
Smelting of iron ore was first developed utilizing bowl and shaft furnaces. Figure 1 shows a schematic of a generalized shaft furnace taken from Rostoker [1]. The ore could be obtained from rocks or sand, rich in iron oxides. The rocks were broken into small pieces and the furnace was first filled with crushed charcoal. Air was blown through a slanted tube at the bottom called a tuyere. After lighting the charcoal and heating the furnace a bit, alternating layers of charcoal and ore were added at the top. A cavity forms at the mouth of the tuyere, and as Fig. 1 shows a mixture of solid iron particles and liquid slag drops fall through the cavity and collect on the bottom of the furnace. The ores also contain silica and other non-iron oxides. At temperatures attained in the furnace, these along with the iron oxide of the ore interact and form a liquid called a slag. The slag pool on the bloom keeps the iron particles from oxidizing. At the high temperature of the furnace bottom they quickly weld together to form an iron-rich bloom containing pores infiltrated with slag. The primary oxides in bloomery slags are FeO, Fe2O3 and oxides of Si, Ca, Mn, Al and Mg [2-Table 9.1a]. A mixture of CO/CO2 gas forms when charcoal burns in air. Carbon monoxide, CO, will reduce iron oxides to iron, but carbon dioxide, CO2, will not. By increasing the air flow, the heat generated increases and raises the furnace temperature. But, at higher blowing rates the amount of CO2 is increased lowering the amount of CO in the CO/CO2 mixture. At a temperature of around 1300 °C, well below the 1538 °C melting temperature of iron, the low CO level cannot reduce iron oxide. Consequently, the iron produced in a bloomery furnace is formed as small solid particles. Rostoker [1, p. 97] has presented data shown in Table 1 taken from the experimental studies of Tylecote [2] showing the effect of the major processing variable, the fuel/ore ratio, on the %C in the bloom metal. Baraclough [4] reviews old literature showing that in addition to high fuel/ore ratios, increased %C in the blooms is produced with higher blowing rate, lower Si and P and higher Mn in the ore. Author T.Z.’s experiments producing bloomery iron have shown that there are additional variables affecting the % C level in the blooms: variation in tuyere angle, charcoal quality, furnace dimensions and ore quality. Additional experiments will be done before work is submitted for publication.
A generalized shaft furnace [1]
In most early bloomery furnaces, both the fuel/ore ratio and the air flow rate were low and early blooms usually had carbon composition levels under around 0.1%. As seen in Table 1, at higher fuel to ore ratios the %C in the iron blooms increases significantly. In the western world in the time period between 1400 and 1600 AD, furnaces like the Stücköften furnace used both high fuel/ore ratios and air flow rates and produced blooms with increased carbon levels [1]. The %C in them was non-uniform varying from low values to a bit above 2.1%, producing regions of low- to high-carbon steel and cast iron. By the early second millennia AD the Japanese had developed the tartara furnace to the point where it produced tonnage levels of bloomery iron with similar large variations in carbon compositions [5]. They called the product tamahagane, precious steel. It is still made in Japan, and videos illustrating the method are available on the web: e.g., https://japaninsider.com/how-japanese-knives-are-made-with-japans-rarest-steel/
The blooms of bloomery furnaces look like an irregular shaped sponge. They consist of the iron plus a significant amount of entrained slag as well as void cavities. Consequently, there was a problem in forming high-quality blades from the forged blooms as the slag content needed to be reduced and the %C homogenized. To produce high-quality blades the final carbon content had to be homogenized to around 0.6 to 0.8% in the portions of the blade near the cutting edge.
Early Western European swords were made from low-carbon bloomery iron by first forging thin strips and then carburizing them in charcoal furnaces. Several strips were piled on top of each other and then forge welded together in a process often called piling. Because the swords were made from bloomery iron, which contains significant levels of slag inclusions, the finished blades contained high levels of slag stringers produced upon forging by elongation of the remnant slag in the iron. Consequently, the swords suffered from low toughness. The Japanese swords and the Muslim crucible Damascus swords, both made from bloomery iron, overcame these problems in two very different ways.
The Crucible Damascus Blade
First, it is important to realize that currently there are two different types of blades that are called Damascus blades. One type is made from pieces of two different types of steel forged welded together forming alternate layers of the two steels. The patterns on these blades result from the fact that because of their chemical composition difference, one steel will etch significantly lighter than the other. These blades are currently being made by hundreds of bladesmiths worldwide from many kinds of steel. These blades will be called pattern-welded blades, as the surface patterns result from the number and size of the welded layers and how the blade surface is deformed, as well as how the blade blanks may have been twisted prior to forging. They date from the early part of the first millennium to the present and will not be discussed further here.
The method of making crucible Damascus blades which display the characteristic surface patterns found on museum blades was only demonstrated by recent research [6,7,8]. In contrast, the method of making Japanese swords with their characteristic surface patterns has been available in the Japanese literature since the early first millennium [5].
The crucible Damascus blades were forged from a single ingot of steel which had been produced by melting in a crucible. Hence, they are called crucible Damascus steel here. (They have several other names in the literature: wootz, oriental, genuine Damascus steel and in Russia: poulad and bulat steel.)
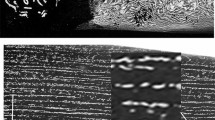
Dr Leo Figiel had a hobby of collecting crucible Damascus steel swords during his travels in India. He had perhaps one of the best collections of these swords in the world. His book [9] presents beautiful photographs of most of his blades. Figure 2 shows a photograph donated by Dr Leo Figiel to J.V. and bladesmith Al Pendray of the sword labeled ps4. Figure 3 shows the surface of another Figiel blade, labeled ps 18 [9]. (The designation ps 4 and 18 appear in reference [9].) Both blades date to the 1690s. Some of the higher-quality blades found in museums have the interesting surface pattern called either the Mohammad ladder pattern or the Kirk Nardaran pattern shown in Fig. 3. It is made by a skilled bladesmith producing undulations in the surface of the blade during the forging process. See reference [6] for a discussion of two techniques for producing the ladder pattern in a crucible Damascus blade. The wavy pattern can be made on flat surfaces by using peening forging dies, which are dies having arrays of shot peens on their surface. See reference [8] for a modern reproduction by Finnish bladesmith Niko Hynninen using peening dies.
Blade ps 4 from [9]. Reprinted with permission from Mowbray Publishing
Surface of blade ps 18 [9] having a Mohammad ladder pattern. Reprinted with permission from Mowbray Publishing
In the early 1990s Leo Figiel gave Al Pendray a short blade. It was roughly 16 cm (6.4 inches) long and was highly corroded everywhere on both sides. It identified the author with a gold inlay on one side. After Al polished and etched the surface, up to the gold inlay on this side and fully on the reverse side, a strong pattern appeared on the blade as shown in Fig. 4. The top shows the side of the blade with the inlay and the bottom the opposite side. The blade has an excellent aligned array of internal bands of pearlite/cementite cluster sheets on cross-sectioned micrographs as is shown in Fig. 5. The left image of Fig. 5 illustrates the excellent alignment of the bands on a transverse section. (The band alignment is often not as good as on a longitudinal section, but it is in this blade.) The right image, at a higher magnification, illustrates both the typical size and shape of the cementite particles in the cluster sheets. It shows the intersection of these cementite sheets with the blade surface and illustrates why the band spacing is larger on the surface than on a perpendicular section of the blade.
Transverse section of Figiel blade (Fig. 4). Left: low magnification Right: high mag of region box
Even though British scientists witnessed how Indian bladesmiths made the small steel ingots (which the British called wootz ingots) from which the blades were forged, western smiths were not able to reproduce them. A problem with understanding how the crucible Damascus blades were made is that there was very little literature on their production. There are 4 reports dating from 1807 to 1839 [10,11,12,13] by the English scientists who observed production of the so-called wootz ingots. Yet, it was not until recent times that blades, matching both the surface patterns and the well-aligned banded internal microstructure, have been successfully produced by bladesmiths [6,7,8]. The early wootz ingots were produced by placing a mixture of bloomery iron, wood chips and green leaves in small, sealed crucibles and heating them above the melting temperature of the mixture for short times and cooling back to room temperature. The crucible Damascus swords and blades were made from these small ingots by directly forging them from the ingots. The surface patterns revealed by polishing and etching resulted from the aligned banded internal microstructure somehow generated during the forging process. Western smiths and scientists had not been able to produce blades that matched the surface patterns and internal microstructure.
In the mid-80s J.V. began a study of crucible Damascus steels and the mystery of how they were made after reading a report by C.S. Smith [14]. He began a collaborative effort with bladesmith Al Pendray. After a 7-year effort Pendray was able to consistently make blades matching the surface patterns and internal microstructure of the ancient blades [15]. Figure 6 presents one of the first blades made by Pendray showing a good match to the patterns of ancient blades as well as a weak Mohammed ladder pattern. Figure 7 presents a micrograph of a longitudinal section cut from the forged bar just beyond its end. It demonstrates a fairly good match to the internal microstructure of the museum quality crucible Damascus blade shown in Fig. 5.
Internal microstructure of blade of Fig. 6
Pendray’s technique was similar to the Indian technique described above except that whereas their slag was produced from the bloomery iron and wood, he employed broken glass to produce the slag. In addition, the percent carbon was adjusted by adding small pieces of charcoal instead of wood. The chemical composition of high-quality crucible Damascus blades had been determined in a 1924 study by French scientist, B. Zschokke [16]. He showed them to be remarkably clean hypereutectoid steels with an average composition for his 3 best blades of 1.65 C, 0.066 Si, 0.017 Mn, 0.14 P and 0.014 S, where the numbers are in units of weight percent. The composition of Pendray’s ingots was controlled in early experiments by adding a combination of high-purity iron, low-carbon steels and charcoal. Chemical analysis of the ingots gave results close to that of Zschokke’s report. However, blades forged from the ingots only occasionally produced the characteristic surface patterns, at around a 5–10% frequency, because the cementite particles were randomly oriented. They were not aligned into the banded sheet structures that produce the surface patterns. Later a lucky break occurred when Pendray began using a quite pure high-carbon cast iron called Sorel iron that is used in ductile cast iron foundries. Suddenly, the cementite particles became banded after forging, and the success rate went to near 100%. Emission spectrograph and mass spectrograph analysis showed the blades contained 3 carbide-forming elements, V, Cr and Ti. Subsequent studies [6] adding controlled amounts of V provided strong evidence that band formation required the presence of quite small levels of a carbide-forming element to be present in the steel.
A study [17] was done to determine the level of various different carbide-forming element additions to pure iron needed to produce banding. The carbide-forming elements were added to a mixture of electrolytic iron and graphite, and blades forged from the resulting small ingots were evaluated. The minimum composition of an element added to the ingots producing strong or weak banding is listed in Table 2.
Shortly after these results were discovered, J.V. proposed a mechanism for how the carbide formers produced the aligned bands in the forging process. Wrought hypoeutectic steels, %C less than 0.7%, have been well studied since around 1900. Wrought steels contain aligned alternation bands of pearlite and ferrite and are commonly called ferrite/pearlite-banded steels. The aligned bands form on a single air cool from above the A3 temperature where the microstructure is all austenite. The mechanism for formation of this aligned banded structure is well established, and it is related to the dendritic mode of solidification of steel, plus the presence of low levels of elements like Mn and P in commercial steels. J.V.’s proposed mechanism argues that the pearlite/cementite bands of the hypereutectoid Damascus steels also result indirectly from the dendritic solidification mode. It proposes a different cause for the selective formation of the cementite cluster sheet bands than the established mechanism for the formation of the ferrite bands of hypoeutectic steels. It is related to the fact that the carbide-forming elements reduce the solutioning rate of the cementite carbides during the heat-up portion of the forging cycles. During the forging process the carbides dissolve on heat-up and grow on cool-down. At the maximum forging temperature, the smaller carbides will disappear and not reform on cooling. The vast majority of the carbides formed in the ingot lie in between the sheets of iron dendrites in any given grain of iron. Forging causes these sheets to align in the forging plane. The carbide-forming elements reduce the rate of dissolution of carbides on each heat-up of the forging cycles. Because there are more carbides between the dendrite sheets, they are able to survive the maximum cycle temperature and end up concentrated between aligned dendrite sheets after forging, thereby forming the aligned cementite cluster sheets that produce the surface patterns. The mechanism has been discussed more fully in [18, 19]. The most recent paper [19] presents new data on the role of ingot cooling rate in controlling band spacing in the blades. It also presents data from literature studies on the role of carbide-forming elements in controlling maximum hardness in tempered steels by reducing coarsening of the carbides during tempering. The latter data also show V and Mo to be the best carbide-forming element additions at reducing the coarsening rate of the carbides.
The Japanese Blade
Unlike the crucible Damascus blade there is a large literature on how the Japanese blades were made dating back to the early first millennium, see reference [5] for an excellent review. Figure 8 presents a picture of a typical Japanese blade displaying a wavy surface pattern located above the cutting edge which the Japanese call the hamon. The blades are made from the bloomery iron of the tartara furnace discussed above which the Japanese call tamahagane steel. The smelted bloom has carbon compositions varying from low to cast iron levels in a non-uniform distribution and significant amounts of slag inclusions. A skilled bladesmiths is able to remove most all of the slag and homogenize and control the carbon level using a complicated forging procedure to produce the swords. The swords are composite structures consisting of 2 or sometimes 3 different strips of steel forge welded together. Author, T.Z. has developed theses skills and made over a hundred blades displaying the hamon pattern. Figure 9 presents an example in the form of a razor blade.
The Japanese swords have a composite structure consisting of an outer region called the kawagane and an inner region called the shingane as shown in the sketch of a Kobuse style blade in Fig. 10. The carbon content in the kawagane region is around 0.7 wt.% and 0.5% in the shingane region. Some Japanese blades consist of a composite of three layers with the third layer called the hagani. It extends from the end of the shingone region to the tip edge of the blade. The outer shape is similar to the kebuse style of Fig. 10 [5].
To produce the steel used in the layers the smith uses a complex process of making it from the tamahagane steel of the tartara furnace. Pieces are forged, quenched and fractured into small segments of around a centimeter or two on a side. By examining the fracture surfaces of these pieces, they are sorted into low-carbon and high-carbon piles. The % carbon in them is sometimes adjusted by reheating in a forge in a process called oroshigane.
Author T.Z. has developed the skill to make bloomery iron from the black sand of Lake Superior which is similar in structure to the Japanese tamahagane bloomery iron. Figure 11 shows a sketch of the furnace he developed to utilize the oroshigane process. After placing the pieces of high-carbon iron in the lower part of the furnace, the high level of CO2 there removes carbon from the iron. Alternately, placing the pieces of low carbon in the top of the top of the furnace where the level of CO is high carburizes the iron.
The next steps in making a blade involve placing piles of either the low- or high-carbon pieces of oroshigane on a piece of sheet steel with a long handle and forge welding together. The original block is forged to twice its original length, notched, folded back on itself and forge welded again. This process is repeated many times. The number of layers increases exponentially with the number of folds and after 10 folds reaches 1024 layers. In this process the carbon distribution is homogenized, and the slag content reduced. The slag is viscous at the forging temperature, and the compressive stresses of forging are able to eject most of it from the steel. The slag is not completely removed but as shown below, it is present as small, separated particles having sizes on the order of 10 to 50 micron diameter.
In the heat-treated Kobuse blade construction, shown in Fig. 10, the outer region below the hamon will be martensite and the outer region above the hamon as well as the inner region of the shingane will be fine pearlite. Consequently, the visible hamon on the surface locates the martensite/pearlite transition on the blade surface. To control the shape of the hamon, prior to heat treatment, the blade is coated with a clay mixture from top to bottom of the blade with the coating thicker at the top. Figure 12 presents an example of the appearance of one of T.Z’s razors after the coating has dried. The edge of the blade is then heated to a temperature slightly above the Ac3 temperature of the steel and quenched in a long trough of water. (The Ac3 temperature is the temperature above which all the carbon has dissolved into the face-centered cubic iron.) The coating causes the top part of the blade to cool more slowly than the bottom and thereby generates pearlite in the top region and martensite in the bottom region. This results because of the combined effects of the thickness variation of the blade from spine to edge and the variation of the hardenability of the steel from both cooling rate and %C level. After removal of the remnant clay coating, polishing and etching the blade piece of Fig. 12 would end up with a hamon pattern similar to that shown in Fig. 9.
In some cases a skilled bladesmith is able to control the temperature distribution in the blade prior to quenching adequately to produce a light-colored pattern above the hamon called a utsuri pattern. Figure 9 shows such a pattern as the light vertical streaks. Figure 9 of reference [5] presents an example on a Japanese blade where the utsuri pattern appears as an undulating band below the spine. The metallurgical cause of this pattern formation has not been determined [5]. A fascinating feature of the Japanese blade is how its curvature is automatically produced during the quenching process. There are videos on the web; [21] is one example, showing the shape change of the blade viewed through a transparent-sided quench trough when the blade is immersed in water. Upon immersion a straight blade first curves to a small convex shape along the edge due to the increased contraction on cooling along the edge. The contraction is followed by a reversal of curvature to the final larger convex shape along the edge due to the increased specific volume of martensite forming along the edge.
Examination of One Japanese Sword Blade
Kevin Cashen loaned the authors a short section of a Koto style Japanese blade (time period prior to 1596 AD) that had a transverse section mounted for metallographic study. After polishing to a mirror finish a light scratch was made longitudinally centered on the cutting edge with 6 more transverse scratches spaced out going toward the spine. Figure 13 presents a micrograph of the as-polished surface at one of the transverse scratches. The residual slag inclusions in the blade show up as the dark regions on the as-polished surface. On none of the surface area of this blade were any slag inclusions observed in the form of stringers, all were found to be small isolated spherical particles. The average area fraction of slag inclusions at the scratch intersection locations was measured to be 1.1%. The results show that the slag inclusions in this sword would not have produced a significant reduction in its toughness.
Figure 14 presents a micrograph of the transverse section of the sword after a nital etch. Metallographic examination showed the microstructure of the sword was martensite near the dark etching tip and pearlite over the major portion of the remainder going toward the spine.
Microhardness tests were made at the 6 transverse scratch intersections using a 2-kg load on a machine calibrated with a 2-kg load test block. Figure 15 presents the results. The hardness profile is what one expects with HV values running from around 600 (Rc = 55) near the cutting edge of the blade, typical of a quenched and tempered 0.7%C steel to values typical of pearlite hardness near the spine of the blade. The results of Figs. 12, 13 and 14 show that this Japanese blade would have had excellent wear resistance and if properly sharpened excellent cutting ability. Both are significantly superior to a crucible Damascus sword and no loss of toughness due to slag inclusions.
Hardness vs from cutting edge of Fig. 14 blade
The Japanese Sword Versus the Crucible Damascus Sword
Based on the microstructures of the two blades, one can determine their hardness and wear resistance at the cutting edge, but their relative toughness is difficult to evaluate. Because the crucible Damascus blades have no slag inclusions and the Japanese blades have very little slag remaining the toughness of both blades should not be reduced by included slag. Also, both blades should not suffer from a reduction of toughness or loss of edge hardness due to non-uniform carbon composition along the blade. Regarding relative toughness one must compare the toughness of a eutectoid pearlitic carbon steel containing parallel sheets of fine cementite particles in the crucible Damascus steel, to a tempered martensite/pearlite composite steel blade of the Japanese blade. In addition, the Japanese blades often contained a very tough low-carbon core.
The superior sword would be the one having both:
-
(1)
Superior ability to retain edge sharpness and cutting ability in battle
-
(2)
Adequate toughness to avoid breakage in battle.
The author, J.V., has a collection of short lengths of four 17-18th century crucible Damascus sword pieces which display excellent surface patterns and well-aligned bands of pearlite/cementite cluster sheets having %C ranging from 1.0 to 1.79%. HV measurements were taken on the 4 blades as described above. The average values of the hardness on the 4 blades ranged from 275 to 330 HV. These hardness values are well below the level of even a severally tempered Japanese blade. In view of these data there can be little question that the Japanese blade with its tempered martensite microstructure would be the superior sword in category (1).
Zschokke [16] did bending tests comparing well-banded crucible Damascus blades and modern steel blades made in Solingen Germany. The Damascus blades showed inferior bending deflection despite having hardness lower than the Solingen blades, 183 − 248 HV vs. 347−463 HV. These data show that the crucible Damascus blade rates are low in category (2).
In consulting work of author J.V. for the Marshalltown Trowel company, data were obtained that are relevant to category (2). The trowel blade requires high hardness to reduce wear in forming concrete, and good toughness to reduce breakage when masons use their trowels to strike bricks, breaking them to a desired length. The trowel blades are made from fine grained steels of compositions around 0.8 %C. Towels made by quenching to martensite and tempering to hardness of 52 Rockwell C (544 Vickers) show an optimum combination of wear resistance and toughness. These data support the conclusion that if the Japanese blades were adequately tempered, they would rate high in category (2).
Yaso et. al. [22] have published a relevant study in which they made measurements of two Japanese swords employing bending tests. Borth swords were of the kobuse shape shown in Fig. 10, one made 600 years ago in the Muromachi era and the other in 1945. Both displayed a hamon and micrographs showed a transition to martensite at the hamon. Electron probe microanalysis gave carbon compositions of 0.67 and 0.71 % at the sharp edge, 0.48 and 0.69% on the side planes and 0.02 and 0.01% in the core areas of the Old sword and Modern sword, respectively. The conclusions from the bending tests were that the toughness’s of the swords were in the range of high-performance tool steels.
The above data on the Kyoto blade show that the Japanese swords were tempered significantly to improve toughness. This result offers support to the conclusion that the Japanese blade would have been tempered adequately to avoid breakage during the blows experienced in battle. This result, coupled with the conclusions of [22], show that the Japanese sword should be rated the superior sword in category (2) as well as in category (1).
Hence, it is our conclusion that the Japanese sword would have been the superior sword in battle.
References
W. Rostoker, B. Bronson, Pre-Industrial Iron. Its Technology and Ethnology, Archeomaterials Monograph No 1: Philadelphia, PA (1990)
R.F. Tylecote, J.N. Austin, A.E. Wrath, Mechanism of the bloomery process in shaft furnaces. JISI. 209(5), 342–363 (1971)
J.D. Verhoeven, A.H. Pendray, W.E. Dauksch, Did the first iron blacksmiths learn to carburize iron? Part II, Experiments show that it is likely they did. J. Metals. 68, 2256–2260 (2016)
K.C. Barraclough, Steelmaking Before Bessemer. Crucible Steel, the Growth of Technology, vol 2 (The Metals Society, London, 1984)
LH. Kapp, Y. Yoshishara, The Craft of the Japanese Sword, Kodansha, New York (1987)
J.D. Verhoeven, A.H. Pendray, W.E. Dauksch, The key role of impurities in ancient Damascus swords. J. Metals. 50(9), 58–64 (1998)
J. Perttula, Wootz Damascus steel of ancient orient. Scand. J. Met. 33, 92–97 (2004)
N. Hynninen, J.D. Verhoeven, W.E. Dauksch, A new method for making crucible Damascus steel Blades. J. Metals. 74, 2484–2491 (2022)
L.S. Figiel, On Damascus Steel (Atlanta Arts Press, Atlantis, 1991)
F. Buchanan, A Journey from Madras through the Countries o f Mysore Canara and Malabar, vol II (Publisher, London, 1807)
B. Heyne, Tracts: Historical and Statistical on India (1814).
D. Voysey, Description of the native manufacture of steel in Southern India. Trans. R. Asiatic Soc., pp. 245–247 (1832)
J.S. Heath, On Indian Iron and steel. J. R. Asiatic Soc. 5, 390–397 (1839)
C.S. Smith, Four Outstanding Researches in Metallurgical History (ASTM, Philadelphia, 1963), pp.17–23
Verhoeven J.D. https://www.mse.iastate.edu/news/john-verhoeven/
B. Zschokke, Du Damasse et lamas de Damas. Rev. Met. 21, 635–669 (1924)
J.D. Verhoeven, F. Laabs, A.H. Pendray, W.E. Dauksch, Microsegregation and banding in hypereutectoid steel: damascus steel. ISS Trans. Iron Steelmak. 25(11), 65–74 (1998)
J.D. Verhoeven, Genuine Damascus Steel: A type of banded microstructure in hypereutectoid steels. Steels Res. 73, 347–355 (2002)
J.D. Verhoeven, A.H. Pendray, W.E. Dauksch, S.R. Wagstaff, Damascus steel revisited. JOM. 70(7), 1331–1336 (2018)
Blade for a Sword (Katana), The Metropolitan Museum of Art, Gift of Etsuko O. Morris and John H. Morris Jr., in memory of Dr. Frederick M. Pedersen, (2007)
M. Yaso, Y. Minagi, T. Takawa, K. Kubota, T. Kanaizumi, T. Ohba, S. Morito, T. Hyaashi, Study of Metallurgy and Mechanical Properties of Japanese Swords: Materials Science Forum (vols. 738-739, 222-227, 2013). ISSN: 1662-9732.
Acknowledgements
Kevin Casen supplied the authors with the Kyoto style blade piece that was examined in the study. The important reference [22] was pointed out to the authors by Marc Cibella, Production Coordinator of this journal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verhoeven, J.D., Zowada, T. Comparison of Two Swords of Antiquity: The Japanese Sword and the Muslim Crucible Damascus Sword. Metallogr. Microstruct. Anal. 12, 934–943 (2023). https://doi.org/10.1007/s13632-023-01018-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-023-01018-1