Abstract
In India, Adenanthera pavonina is traditionally used in the treatment of diabetes mellitus and lipid disorders. In the present study, the antihyperglycaemic and lipid lowering effect of Adenanthera pavonina seed aqueous extract (APSAE) was evaluated using streptozotocin induced diabetes in rats. Streptozotocin was given at the dose of 55 mg/kg, i.p. After induction of diabetes, APSAE was administered for 30 days p. o. and simultaneously different biochemical parameters like plasma glucose, HbA1c, serum triglyceride, cholesterol, LDL-cholesterol and HDL-cholesterol were estimated. Diabetic control showed significant increase (P < 0.01) in plasma glucose, serum triglyceride, cholesterol, LDL-cholesterol and significant decrease (P < 0.01) in serum HDL-cholesterol and HbA1c. Treatment with APSAE showed significant reduction (P < 0.01) in plasma glucose when compared with diabetic control. The elevated levels of serum triglyceride and cholesterol levels were significantly reduced (P < 0.01) by APSAE. APSAE treatment for 30 days showed significant decrease in serum LDL-cholesterol (P < 0.01) and significant increase in serum HDL cholesterol level (P < 0.01). Moreover, diabetic control there was significant decrease in HbA1c which was significantly increased (P < 0.05) by treatment with APSAE. Hence, from the result obtained in the present study it can be confirmed that Adenanthera pavonina has the potential to treat diabetes condition and associated lipid disorders.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic disease caused by inherited or acquired deficiency in insulin secretion and by decreased responsiveness of the organs to secreted insulin (Matsui et al. 2007). DM is currently one of the most costly and burdensome chronic diseases and is a condition that is increasing in epidemic proportions throughout the world (King et al. 1998). Diabetes affects about 5 % of the global population (WHO traditional medicine strategy 2005) and the management of diabetes without any side effects is still a challenge to the medical system (Chakraborty and Rajagopalan 2002; Kameswararao et al. 2003). Renewed attention in recent decades to alternative medicines and natural therapies has stimulated a new wave of research interest in traditional practices. The plant kingdom has become a target for the search for new drugs and biologically active “lead” compounds (Evans 1996). Ethno botanical information indicates that more than 800 plants are used as traditional remedies for the treatment of diabetes (Pushparaj et al. 2000; Alarcon-Aguilara et al. 1998), but only a few have received scientific scrutiny.
Adenanthera pavonina Linn. (Family: Leguminosae-Mimosaceae), is a deciduous tree, 18–24 m tall, bole erect and 60 cm in diameter (Bouquet and Debray 1974). Many species of adenanthera, including Adenanthera pavonina, have been used as traditional herbal medicine against a variety of diseases including diabetes, lipid disorders, diarrhoea, haemorrhage from the stomach, haematuria and as anti-inflammatory agent in gout. The seed contains an anti-inflammatory active principle, O-acetylethanolamine. The leaves contain octacosanol, dulcitol, glucosides of betasitosterol and stigmasterol. The bark contains sitgmasterol glucoside (Khare 2007). Traditionally, the ground seed is widely used for the treatment of various human ailments such as treatment of boils, inflammation, blood disorders, arthritis, rheumatism, cholera, paralysis, epilepsy, convulsion, spasm and indigestion (Burkill 1966; Balogun and Fetuga 2004). Phytochemically, the seed and pod contain glycosides, saponins and steroids (Howes 1974; Yadav et al. 1976). A new five-membered lactone ring compound, pavonin was isolated from the methanol soluble part of A. pavonina (S.A.Muhammad et al. 2005), oil extracted from the seed has been reported to have membrane-stabilizing activity by reducing lytic effect on erythrocytes, exhibited by many intravenous drugs (Anna et al. 2006). The methanol seed extract has also been reported to demonstrate anti-inflammatory and analgesic activities (Olajide et al. 2004).
On the basis of reported activities and chemical constituents the aqueous extract of seed was chosen. Therefore, taking into consideration the reported pharmacological activities of Adenanthera pavonina Linn. the present study is planned to investigate antihyperglycaemic and lipid lowering potential in Stz- induced diabetic rats.
Materials and methods
Plant material
Seeds of Adenanthera pavonina were collected during March 2009 from the Mahatma Phule Krishi Vidyapeeth, Rahuri, Maharashtra, India. The plant was authenticated by Dr. P.G. Diwakar, Joint Director, Botanical Survey of India, Pune as Adenanthera pavonina Linn. (Mimosaceae) with a voucher specimen (BSI/WRC/Tech/2010/463) kept in herbarium, BSI, Pune.
Preparation of extract
The seeds were washed with distilled water, shed dried and latter powdered. This powder was then defatted with petroleum ether which was further macerated with distilled water for 72 h with occasional shaking. It was then filtered and evaporated. The yield of APSAE was 2.5 % w/w.
Preliminary phytochemical screening
The preliminary phytochemical screening of APSAE was carried out for qualitative identification of type of phytoconstituents present (Latha and Pari 2004; Kokate 1994).
Animals
Healthy adult male wistar rats weighing 150-200 g and Swiss albino mice weighing 25–30 g were obtained from in house breed at the animal house of M.E.S. College of Pharmacy, Sonai and were housed in polypropylene cages lined with husk in standard environmental conditions (Temperature 25 ± 2°C; relative humidity 55 ± 10 %; and 12:12 light: dark cycle,). The animals were fed on a standard pellet diet (Amrut rat and mice feed, Sangli, India) and water ad libitum.
Animals were acclimatized to the laboratory condition for at least 8 days prior to the experiment and were maintained in a well ventilated animal house. The experimental protocol was approved by the Institutional animal Ethical Committee (MESCOP/IAEC/07/2010) and the care of the laboratory animals was taken as per the current CPCSEA regulation.
Experimental design
Acute toxicity study (OECD 425, 2001)
Acute toxicity of APSAE was done using Swiss albino mice (25–30 g) according to the procedure of Organization for Economic Co-operation and Development (OECD) guideline no. 425 (OECD, 2001). The animals were fasted overnight prior to the experiment and maintained under standard conditions. Animals were observed for general behavioral change and mortality for a period of 14 days post treatment.
Effect of APSAE on normoglycaemic rats
The rats were divided into four groups of 6 animals (n = 6) each. Group I served as control and received distilled water. Group II, III and IV received APSAE orally at doses 50,100 and 200 mg/kg/day b.wt. Blood glucose levels were determined at 0,1,2,3 and 4 h following treatment by retro-orbital plexus of the eye under mild ether anesthesia.
Oral glucose tolerance test in normal rats (OGTT)
The rats were divided into four groups of 6 animals (n = 6) each. Group I served as control and received distilled water. Group II, III and IV received APSAE orally at doses 50,100 and 200 mg/kg/day b.wt. All the animals were given glucose (2 g/kg) 30 min after dosing. Blood samples were collected from the retro-orbital plexus of the eye just prior (0 h) and 1, 2, 3 and 4 h. after the glucose loading and blood glucose levels were estimated.
Induction of diabetes
Diabetes was induced in rats by single intraperitoneal injection of STZ (55 mg/kg b.wt.) dissolved in freshly prepared 0.01 M citrate buffer, pH 4.5. (Gupta et al. 2004) after 72 h rats with marked hyperglycemia (fasting blood glucose ≥250 mg/dl) were selected and used for the study.
Treatment schedule
Total of 36 Wistar rats were used (30 Diabetic surviving and 06 nondiabetics). The rats were divided into six groups of 6 animals (n = 6) each as follows- The solution of APSAE was prepared with 1 % gum acacia, an emulsifying agent. Glibenclamide was served as a reference standard. Group-I (Nondiabetic Control) animals were received only 1 % gum acacia (1 ml/kg/day, p.o.). Group-II (Diabetic Control) animals were diabetic and received 1 % gum acacia (1 ml/kg/day, p.o.). Group-III (Diabetic + Glibenclamide) animals were diabetic and received glibenclamide (0.25 mg/kg/day, p.o.) (Sun Pharmaceuticals Ltd, India). Groups IV, V, VI animals were diabetic and received three different doses of APSAE 50, 100 and 200 mg/kg, p.o. respectively. All the animals received above treatment for 30 days.
Evaluation of antihyperglycaemic activity
Antihyperglycaemic activity of APSAE was evaluated by estimation of blood glucose levels and body weight measurement on 1st, 10th, 20th and 30th day of the study by the glucose oxidase/peroxidase (GOD/POD) (Trinder 1969) method using a standard kit obtained from Span Diagnostics, India.
Evaluation of antihyperlipidaemic activity
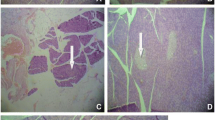
At the end of the experiment, the animals from each group were sacrificed by cervical dislocation and blood and organs were collected to estimate various biochemical and histological studies (Chakrabarti et al. 2005). Blood was collected from the heart and allowed to clot and the serum was separated by centrifuged at 3,500 rpm for 10 min. Serum was assayed either immediately or stored at -20°C. The tissue like pancreas was collected and used for histological studies. Serum samples were analyzed spectrophotometrically for triglycerides, total cholesterol, high density lipoprotein (HDL-C), using their respective kits UV- visible spectrophotometer (Jasco V-630, Japan), VLDL-C and LDL-C were calculated as per Friedwald’s equation (Richterich and Colmbo 1981).\( \matrix{ {{\text{VLDL}} = {\text{TG}}/{5}}; \\ {{\text{LDL}} = {\text{TC}} - \left( {{\text{HDL}} + {\text{VLDL}}} \right)} \\ }<!end array> \).
(Alayash et al. 1988; Burstein et al. 1970; Friedwald et al. 1972).
Estimation of glycated hemoglobin
After 30 days of treatment, the 12 h fasted rats were sacrificed by cervical decapitation, blood was withdrawn by retro orbital puncture under light ether anesthesia and the glycated hemoglobin was estimated (Sadasivam and Manickam 1996).
Statistical analysis
The results were expressed as mean ± S.E.M., statistical difference was doone by using one-way analysis of varience (ANOVA) followed by Dunnette’s multiple comparison test. A difference in the mean P value <0.05 was considered as statistically significant.
Results
Preliminary phytochemical Screening
The study showed the presence of steroids, terpenoids, alkaloids, tannins, phenolic compounds, flavonoids, Sugars and amino acids.
Acute toxicity study
Oral administration of APSAE was found safe up to dose of 2,000 mg/kg, p.o. produced no signs of toxicity. However, from 5 g/kg APSAE caused slow movement of animal, decreased aggressiveness, altered touch and pain sensibility but did not cause any negative behavioral changes such as excitement, respiratory distress, convulsions or coma. No mortality was observed up to 14 days. Hence, the median lethal dose (LD50) of the APSAE was then greater than 2,000 mg/kg body weight. Therefore doses 50,100 and 200 mg/kg b.wt. were selected for all in vivo experiments.
Effect of APSAE in normoglycaemic rats
The results from the study exhibited that there was no significant effect observed on normoglycaemic rats when treated with the single dose of APSAE at 50,100 and 200 mg/kg b.wt. (Table 1).
Oral glucose tolerance test in normal rats (OGTT)
The results from the study indicated that the APSAE at 50,100 and 200 mg/kg and glibenclamide (0.25 mg/kg) reduced the blood glucose level (hyperglycemia due to glucose load 2 g/kg p.o.) significantly after 3 h of oral administration, when compared to diabetic control group (Table 2).
Antihyperglycaemic activity
The results from the repeated administration of APSAE daily up to 30 days exhibited significant antihyperglycaemic activity in stz-induced diabetic rats, whilst there was no significant effect observed on normoglycaemic rats. However, at the end of 30 days of treatment, there was a 74.27 %, 69.55 %, 71.45 % and 72.66 % (p < 0.01) decrease of serum glucose levels with the glibenclamide and APSAE (50,100 and 200 mg/kg) respectively when compared with diabetic control group (Table 3).
Antihyperlipidaemic activity
The results from the repeated administration of APSAE daily up to 30 days exhibited significant reduction in lipid profile in stz-induced diabetic rats. Lipid profile of animals showed significant reductions (p < 0.01) of 13.82 %, 18.08 % and 22.34 % CHL (cholesterol), 44.21 %, 51.57 % and 60.00 % LDL, 11.60 %, 18.13 % and 18.86 % VLDL (Very Low density lipoproteins) and 27.43 %, 30.08 % and 31.85 % TG after treatment with APSAE 50,100 and 200 mg/kg respectively when compared with diabetic control rats. There was also a significant (p < 0.01) increase of 54.12 %, 66.62 % and 70.75 % HDL in the APSAE treated diabetic rats in comparison of diabetic control rats, where a fall in HDL level (Table 4).
Changes in body weight
At the end of 30 days treatment, the body weight of normal rats, APSAE and standard drug treated group, increased significantly, whereas body weight of diabetic control group decreased (Table 5).
Changes of serum glycosylated hemoglobin
After 30 days of treatment with APSAE, it was observed that animals treated with APSAE showed a significant decrease in glycosylated hemoglobin levels when compared to diabetic control groups (Table 6).
Discussion
The use of traditional medicine and medicinal plants in most developing countries, as a normative basis for the maintenance of good health has been widely observed (Tiwari and Madhusudanarao 2002). Diabetes mellitus is probably the fastest growing metabolic disease in the world and as knowledge of the heterogeneous nature of the disease increases so does the need for more challenging and appropriate therapies. Traditional plant remedies have been used for centuries in the treatment of diabetes (Akhtar and Ali 1984; Kesari et al. 2005; 2007; Rai et al. 2007), but only a few have been scientifically evaluated. Therefore, we have investigated the effect of Adenanthera pavonina seed aqueous extract on glycemic control and serum lipid profile in STZ-induced diabetic rats. APSAE showed a dose dependent effect on fasting blood glucose at 50,100 and 200 mg/kg b.wt. in diabetic rats. So, detailed studies were carried out with the dose of 50,100 and 200 mg APSAE mg/kg b.wt. The capacity of APSAE to decrease the elevated blood glucose to normal level is an essential trigger for the liver to revert to its normal homeostasis during experimental diabetes. Lower levels of total hemoglobin observed in diabetic rats might be due to the increased formation of HbA1c. In uncontrolled or poorly controlled diabetes, there is an increased glycosylation of a number of proteins including hemoglobin and crystalline of lens (Alberti and Keen 1982). HbA1c was found to increase in patients with diabetes mellitus and the amount of increase was directly proportional to the fasting blood glucose levels (Pari and Saravanan 2002) therefore, measurement of HbA1c is supposed to be very sensitive index for glycemic control. Treatment with APSAE showed a significant decrease in the glycated hemoglobin levels, which could be due to an improvement in insulin secretion. Induction of diabetes with STZ is associated with the characteristic loss of body weight, which is due to increased muscle wasting (Swanston-Flatt et al. 1990), and due to loss of tissue proteins (Chatterjea and Shinde 1976). Diabetic rats treated with the APSAE showed an increase in body weight when compared to the untreated diabetic rats which may be due to its protective effect in controlling muscle wasting i.e. reversal of gluconeogenesis and may also be due to the improvement in glycemic control. Increased levels of triglycerides and cholesterol during diabetes lead to cardiovascular complications. In this study, STZ-induced diabetic mellitus characterized by hyperglycemia caused a significant rise in serum lipids. These findings indicate that diabetes mellitus is accompanied by increased risk of atherosclerosis and coronary artery diseases. Lowering of serum lipid levels through dietary or drug therapy seems to be associated with a decrease in the risk of vascular disease (Rhoads et al. 1976). In the present study, the APSAE significantly reduced the triglyceride, total cholesterol, LDL and VLDL cholesterol levels with an increase of HDL cholesterol in treated diabetic rats as compared to untreated diabetic rats so, these changes are could be beneficial in preventing diabetic complications as well as in improving lipid metabolism in diabetics (Gupta et al. 2005). The significant control of the levels of serum lipids in the APSAE treated diabetic rats could be directly attributed to improvement in glycemic control upon APSAE therapy. Hence, these findings demonstrate that Adenanthera pavonina could be beneficial to treat diabetes mellitus and complications owing to its antihyperglycaemic and lipid lowering effect. Further studies are necessary to substantiate above claim and to work out exact mechanism of action involved in antihyperglycaemic and antihyperlipidaemic activity of this plant.
References
Akhtar MS, Ali MR (1984) Study of antidiabetic effect of a compound medicinal plant prescription in normal and diabetic rabbits. J Pak Med Assoc 34:239–244
Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL (1998) Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethnopharmacol 61:101–10
Alayash AI, el-Hassan AM, Omer R, Bonaventura J (1988) Glycosylated hemoglobin: an indicator of long term blood glucose in domestic sheep and goats. Comp Biochem Physiol A 90:229–231
Alberti KGMM, Keen H (1982) The biochemistry and the complications of diabetes. In: Jarrett J (ed) complications of diabetes, vol 43. Edward Arnold Ltd, London, pp 231–270
Anna J, Robert Z, Arkadiusz K (2006) Emulsions of oil from Adenanthera pavonina L. seeds and their protective effect. J Cell Mol Biol 3:1425
Balogun AM, Fetuga BM (2004) Fatty acid composition of seed oils of some members of the leguminosae Family. Food Chem 17:175–82
Bouquet A, Debray M (1974) Medicinal plants in Ivory Coast. Document Orstom France 32:1–4
Burkill IH (1966) A dictionary of the economic products of the Malay Peninsula Edited by: Ministry of Agriculture (Malaysia). Crown Agents for the colonies London, 839
Burstein M, Scholnichk HR, Morfin R (1970) Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res 11:583–595
Chakrabarti S, Biswas TK, Seal T, Rokeya B, Ali L, Azad Khan AK (2005) Antidiabetic activity of Caesalpinia bonducella F. in chronic type 2 diabetic model in Long-Evans rats and evaluation of insulin secretagogue property of its fractions on isolated islets. J Ethnopharmacol 97(1):117–122
Chakraborty R, Rajagopalan R (2002) Diabetes and insulin resistance associated disorders: disease and therapy. Curr Sci 83:1533–8
Chatterjea MN, Shinde R (1976) Diabetes mellitus, textbooks of medical biochemistry, 5th edn. Jaypee Brothers Medical Publishers Ltd., New Delhi
Evans WC (1996) Trease and Evans’s Pharmacognosy, 14th edn. London, Saunders, pp 124–43
Friedwald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low density lipoproteincholesterol in plasma without the use of the preparative ultracentrifuge. Clin Chem 18:499–502
Gupta S, Kataria M, Gupta PK, Murganandan S, Yashroy RC (2004) Protective role of extracts of neem seeds in diabetes caused by Streptozotocin in rats. J Ethnopharmacol 90:185–189
Gupta RK, Kesari AN, Watal G, Murthy PS, Chandra R, Maithal K, Tandon V (2005) Hypoglycemic and antidiabetic effect of aqueous extract of leaves of Annona squamosa. Curr Sci 88:1244–1254
Howes FN (1974) A dictionary of useful everyday plants and their common names. Cambridge University Press, 15
Kameswararao B, Kesavulu MM, Apparao C (2003) Evaluation of antidiabetic effect of Momordica cymbalaria fruit in alloxan-diabetic rats. Fitoterapia 74:7–13
Kesari AN, Gupta RK, Watal G (2005) Hypoglycemic effects of Murraya koenigii on normal and alloxan diabetic rabbits. J Ethnopharmacol 97:247–251
Kesari AN, Kesari S, Singh SK, Gupta RK, Watal G (2007) Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J Ethnopharmacol 112:305–311
Khare CP (2007) Indian medicinal plants—an illustrated dictionary. Springer-Verlag; Berlin; 601
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025; prevalence, numerical estimates, and projections. Diab Care 21:1414–31
Kokate CK (1994) Practical pharmacognosy. 4th ed. Vallabh Prakashan New Delhi
Latha M, Pari L (2004) Effect of an aqueous extract of Scoparia dulcis on blood glucose, plasma insulin and some polyol pathway enzymes in experimental rat diabetes. Braz J Med Biol Res 37(4):577–586
Matsui T, Tanaka T, Tamura S, Toshima A, Miyata Y, Tanaka K (2007) Alphaglucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem 55:99–105
Muhammad SA, Farman A, Iqbal A, Muhammad KP (2005) Pavonin: a new five membered lactone from Adenanthera pavonina Linn. (Mimiaceae). Nat Prod Res 9:37–40
Olajide AO, Echianu CA, Adedapo AD, Makinde JM (2004) Anti-inflammatory studies on Adenanthera pavonina seed extract. Inflammopharmacology 3(12):196–202
Pari L, Saravanan G (2002) Antidiabetic effect of Cogent db, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 131:19–25
Pushparaj P, Tan CH, Tan BKH (2000) Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol 72:69–76
Rai PK, Rai NK, Rai AK, Watal G (2007) Role of LIBS in elemental analysis of Psidium guajava responsible for glycemic potential. Instrum Sci Technol 35:507–522
Rhoads GG, Gulbrandse CL, Kagan A (1976) Serum lipoproteins and coronary artery disease in a population study of Hawaiian Japanese men. New Engl J Med 294:293–298
Richterich N, Colmbo LP (1981) Clin chemistry. Wiley, Toronto, pp 432–7
Sadasivam S, Manickam A (1996) Methods in Biochemistry, 2nd edn. New Age International Pvt. Ltd., New Delhi
Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR (1990) Traditional plant treatment for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia 33:462–464
Tiwari AK, Madhusudanarao J (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 83:30–38
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
WHO (2002–2005) Traditional medicine strategy. WHO Publications; 1–6
Yadav N, Misra G, Nigram SK (1976) Triterpenoids from Adenanthera pavonina bark. Plant Med 29:176–178
Acknowledgement
The first author is sincerely thank to Principal, M.E.S. College of Pharmacy, Prashant Patil Gadakh Secretary, Mula Education Society, Sonai and Department of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan, India for encouragement and availing of the laboratory facilities during the course of investigation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pandhare, R.B., Sangameswaran, B., Mohite, P.B. et al. Anti-hyperglycaemic and lipid lowering potential of Adenanthera pavonina Linn. in streptozotocin induced diabetic rats. Orient Pharm Exp Med 12, 197–203 (2012). https://doi.org/10.1007/s13596-012-0074-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-012-0074-2