Abstract
Key Message
Pinus radiata D. Don growing in the central North Island of New Zealand did not show full winter cambial dormancy. While there was a brief cessation of cambial cell division during June (winter), cell wall thickening, secondary wall deposition, and lignification of tracheids continued throughout the year.
Context
The xylogenesis of Pinus radiata showing only partial winter dormancy has not previously been reported.
Aims
To verify the absence of winter dormancy of P. radiata growing in the mild cool climate of the central North Island. To characterise the intra-annual dynamics of xylem cell formation (cell division and enlargement as well as cell wall thickening and lignification) and growth rates and identify the main drivers of growth.
Methods
Xylogenesis was monitored by microcore sampling while radial growth was monitored by dendrometers, which was then related to rainfall and temperature.
Results
Xylem cell formation started by mid-July and continued until late May of the following year and peaked in spring and early autumn with minimum activity in Southern Hemisphere winter solstice (June 21, DOY 172). A higher correlation was found between the radial stem growth and temperature than with rainfall.
Conclusion
There was a winter period of about 30 days where there was no cambial cell division and the differentiation of latewood cells was stalled. This slow-down was supported by dendrometer measurements. This is likely due to the relatively mild winter temperatures and absence of drought conditions that are characteristic of the central North Island climate.
Similar content being viewed by others
1 Introduction
Pinus radiata D. Don is the dominant commercial tree species in New Zealand: 90% of New Zealand’s 1.7 million ha (6.4% of land area) commercial forestry estate is comprised of P. radiata (FOA 2016). Forestry plays an important role for the country’s economy with it being the third largest export earner from the primary sector (MPI 2019). However, comparatively little is known about the details of the xylogenesis process of this species and how the process is influenced by the environment. Xylem ring width and structure depends on xylem cell differentiation dynamics, particularly on the duration and rate of cambial cell production, which in turn are driven by environmental factors (Larson 1969; Savidge 2000). To achieve the New Zealand forestry sector’s vision of doubling export earnings from P. radiata forests by 2022 (GCFF 2014), the productivity of existing forests needs to increase. A more detailed knowledge of the xylogenesis process of New Zealand grown P. radiata will help the forestry industry to develop key management inteventions that will not only increase productivity, but will mitigate impact on desirable wood properties.
Cambial activity ensures the perennial life of trees through regular renewal of functional xylem and phloem (Larson 1994). The annual course of cambial activity is generally related to alternating contrasting seasons (cold and warm or dry and wet) (Denne and Dodd 1981). In temperate regions, the cambium is typically dormant during winter and active during spring and summer. In tropical regions, it may be dormant during the dry season and be active during the wet season (Fromm 2013). In temperate and cold environments, temperature is the main climatic factor affecting xylogenesis, mainly in spring and autumn (Vaganov et al. 2005; Rossi et al. 2007; Deslauriers et al. 2008; Rossi et al. 2008; Seo et al. 2008; Begum et al., 2013).
The largest plantation forest (2900 km2) in New Zealand is located in the central North Island, which has a warm temperate climate. The cambial cells of P. radiata growing in this region do not show the ultrastructural characteristics (Barnett 1971; Barnett 1973) usually used to define dormancy (Prislan et al., 2013). Dendrometer measurements of P. radiata growing in the central North Island support these observations since they showed a slow but continued increase in stem diameter during winter (Tennent 1986). Jackson studied growth of 5-year-old P. radiata in Kaingaroa Forest, and showed maximum rates of stem increment in January, February and March, following the October to December peak in height growth (Jackson et al. 1976). The year-round growth based on height and diameter measurements of P. radiata growing in Victoria, Australia (Skene 1969) and continuous height growth in Hawaii (Lanner 1966) has also been reported. This year-round growth is closely related to the polycyclic activity of the apical bud of P. radiata (Bollmann and Sweet 1976).
These observations are in contrast to what occurs in other Pinus species growing in temperate climates, which showed a clear winter-dormancy (Murmanis 1971; Samuels et al., 2006). There were clear differences between the winter and summer cambial cells, at the ultrastructural level. Cambial cells of dormant trees contained numerous small vacuoles, whereas the cambial cells of active trees only had one large central vacuole (Murmanis 1971; Prislan et al. 2013). At a cellular level, a dormant cambium can be defined as radially narrow and compactly arranged cells, located between the secondary phloem cells and the thick-walled secondary xylem cells (Begum et al. 2013). An increasing number of cambial cells and the appearance of first enlarging cells immediately adjacent to cambium are signs of cambium reactivation (Gričar et al. 2006; Rathgeber et al., 2011)
A method of microcore embedding and confocal image processing was developed to study the wood formation process of fast-growing P. radiata with wider xylem differentiation zones (Dickson et al., 2017). This study applies the method over a full year to develop an understanding of xylogenesis of New Zealand grown P. radiata at two locations. The first location is the same as the 2017 study while the second location is a 13-year old commercial forestry stand. We tested the hypotheses that (1) under mild climatic conditions, cambial cell division and xylem differentiation can continue all year round and (2) the rate of cambial cell division and xylem differentiation are similar despite differences in age, climate, stand structure, and management.
2 Material and methods
2.1 Study sites
The first site was at the Scion Nursery, Rotorua (latitude 38.156406° S and longitude 176.269614° E, 300 m a.s.l.), in the central North Island of New Zealand. Trees were randomly selected from a group of 6-year-old trees planted at a spacing of approximately 2.5 × 2.5 m (Table 1) at an approximate tree density of 1,600 stems per hectare (SPH). Point dendrometers (BestTech Australia Pty. Ltd) were used to monitor the stem radial growth. Xylogenesis was monitored (microcore sampling) from April 2014 to May 2015 in the same trees monitored by dendrometers. The stem diameters at breast height (DBH, 1.4 m) and heights are given in Table 1.
The second site, Kaingaroa, was approximately 53 km south-east of the Rotorua site. Kaingaroa Forest (latitude 38.59075° S, longitude 176.494533° E, 514 m a.s.l.), is also in the central North Island. The stand was 13-year old and were planted in 2003 at 747 SPH and thinned to 374 SPH in 2015. At 374 SPH, spacing between the trees was approximately 5 × 5 m. Xylogenesis was monitored (microcore sampling) from July 2016 to June 2017 for two randomly selected trees from each of the four 20 m × 20 m square plots (total of eight trees) (Table 1).
2.2 Climate
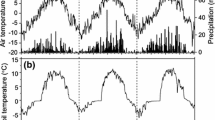
New Zealand’s central North Island region has a warm, temperate climate. The warmest months are January, February and March, where the daily mean maximum temperatures can reach 27 °C, while the coolest months are June to August, with the mean daily minimum temperature averaging 5 °C. The central North Island has an average annual rainfall of 1650 mm with a range of 1300 to 2200 mm. Rainfall is spread throughout the year, with the winter months receiving slightly more (Chappell 2013).
The climatic data (daily averages of temperature, rainfall, humidity, solar radiation and wind speed) at the two sites (Table 2) were obtained from nearby meteorological stations (500 m from Rotorua and 5 km from Kaingaroa). Daily average temperature and the rainfall trends were similar in both sites (Fig. 1) and showed typical patterns for the central North Island (Chappell 2013).
2.3 Radial growth data using point dendrometers
Point dendrometers were installed at breast height (1.4 m) on all of trees in Rotorua, 2 weeks before the experiment to allow for a period of adjustment. Dendrometers were mounted using 4-mm stainless steel threaded rods inserted into the wood. To ensure a smooth contact between the sensor rod and trunk, the outer bark was lightly brushed off. Each dendrometer was individually calibrated and a 1.25-mm change in radius corresponded to a sensor output of approximately 1 V. Raw data were recorded every 15 min using a data logger (Model C8850, Campbell Scientific Inc., USA).
We followed the daily maximum approach to remove daily fluctuations caused by stem shrinkage and swelling (Bouriaud et al., 2005). The daily growth rate (μm/day) was considered to be the maximum from 24-h radial measurements obtained from 00:00 to 24:00 h on a daily basis.
2.4 Collection of microcores
Microcores (2 mm in diameter and 15 mm long) were collected approximately every 2 weeks. They were collected at breast height using a Trephor tool (Vitzani, Peraroli di Cadore (BL), Italy) as described in (Rossi et al. 2006a), following an ascending spiral pattern and spacing each sampling about 2 cm apart to avoid wound reaction (Makinen et al. 2008). The microcores were stored in FAA (Formaldehyde:Glacial acetic acid:Ethanol, 5:5:90) at 5 °C. They were transferred to 96% ethanol prior to embedding in LR white resin (London Resin Company, Reading, England), as described in (Dickson et al. 2017)
2.5 Determining xylem formation zones from microcore images
Microcores were prepared for confocal microscopy, and the images of transverse surface of microcores were captured directly from the block face as described in Dickson et al. (2017). Four to five microcores were set in each of the resin blocks. The entire resin block was fixed to a glass slide with double-sided tape; a drop of 80% glycerol was placed on the exposed surface to be imaged and mounted with a #1.5 cover glass. Imaging was performed using an upright Leica TCS SP5 confocal laser scanning microscope. Excitation was at 355 and 488 nm, and emission bands were 380–480 nm (blue), 493–538 nm (green) and 625–800 nm (red). A number of overlapping images were gathered for each microcore so the full region of xylem formation zones was covered ranging from the phloem to mature wood. Images were stitched together to form a montage image (Image Composite Editor, Microsoft Corporation). Mean cell dimensions (radial width, cell wall thickness) and lignin autofluorescence were recorded at 30-μm intervals along the length of the image, which varied between 2500 and 4000 μm. Image processing was performed using ImageJ (1.51p) (Abramoff 2004).
Our approach combines the cambium (cell division zone) with the cell enlargement zone. We refer to this as the CE zone (Fig. 2). Changes in radial cell width and wall thickness were used for locating the cambium and cell enlarging zones on the image (Fig. 2) (Dickson et al., 2017). The cambium was defined by cells with narrow radial widths and thin walls and enlarging zone with cells radial width at least twice that of cambial cells (Rossi et al. 2009). The beginning of the CE zone was defined by the minimum radial width that extended through the enlarging zone to the point where cell-wall thickening begins to increase from the minimum value. Cells in the phase of wall thickening and lignification were identified as LT zone. The LT zone starts at the point where lignin auto-fluorescence increases from the minimum value and ends when the signal plateaus (Fig. 2). The end of the LT zone is also verified by the absence of cellular content (Dickson et al., 2017). The width of the zone of mature tracheids was not measured. This is because the growth rings of radiata pine are wide and in very fast-growing trees are often wider than the 15-mm length of the Trephor tool used for sampling.
Active cambial zone from a microcore sampled in 01.10.2014 showing the cambium and cell enlargement zone (CE) and the wall thickening and lignification zone (LT). The line labelled ‘lignin’ represents the normalized relative lignin autofluorescence. The yellow line labelled ‘width’, the normalized relative cell radial width; and the white line labelled ‘thickness’, the normalized relative wall thickness
2.6 Statistical analysis
To determine the correlation between the daily maximum radial growth (dendrometers) and temperature, rainfall and solar radiation, a correlation technique known as repeated measures correlation technique (rmcorr) was used (Bakdash and Marusich 2017). Repeated measures correlations (rrm) were performed using the rmcorr R package (Anonymous 2017) as described in (Bakdash and Marusich 2017). Widely used correlation techniques (e.g. Pearson) to determine the correlation between individual variables, assume independence of error between observations. However, this assumption is violated when each individual provides more than one canonical data point. Rmcorr accounts for non-independence using analysis of covariance (ANCOVA) to statistically adjust for inter-individual variability.
Our microcore data measure the width of a zone at a given date and do not record the onset of the various phases. Therefore, comparison with the dendrometer data is done on a growth rate basis rather than accumulated growth. To investigate if there was a relationship between dendrometer data and cambial zone widths, the daily maximum radial growth was averaged over time periods consisting of the number of previous days (previous 2 days, 3 days, etc….90 days). The resultant mean daily maximum growth rates (μm/day) were correlated with the CE zone width (figure not shown) and LT zone width (Fig 5a), to find the time period with the highest r-squared with zone width.
3 Results
3.1 Seasonal cambial dynamics
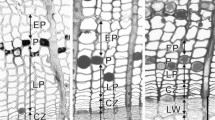
The wood formation was monitored from mid winter, and at both sites, the cambium was active for approximately 335 out of 365 days. The cambium had the minimum width and number of cells (12–14) during the whole of June (Fig. 3a), showing minimal activity. Adjacent to the cambial and enlarging cells were incompletely lignified latewood tracheids (from the previous year), with cell contents still present suggesting xylogenesis continued throughout the winter (Fig. 3a, b). By late August, lignification of previous years latewood tracheids had reached completion, with the loss of cell contents (Fig 3d).
(a–f). Seasonal changes in cambial zones. The dotted white line represents the approximate position of the growth ring boundary (previous season’s latewood) in the images it overlaps. “C” refers to the cambium, “E” refers to cell expansion, “T” refers to cell wall thickening, “LT” refers to cell wall thickening and lignification, “M” refers to mature xylem. These refer specifically to the higher-magnification insets. The scale bars in the insets are 100 μm
When sampling began in early-July (DOY 186), 2 weeks after the winter solstice (DOY 172), new-season xylogenesis (earlywood formation) had begun and 2–3 enlarging cells were present per radial file (Fig. 3b). Two weeks after the first enlarging cells were observed, wall thickening and lignification of the newly formed cells began (Fig 3c). By late September, fully lignified earlywood tracheids had been formed (Fig. 3e). From initial cell division and enlargement, it took about 2 months for the tracheids to reach full maturity. Xylem production continued until the end of the following May, with its activity peaking at the spring equinox and again at early autumn.
3.2 Site comparisons—zone widths and radial growth
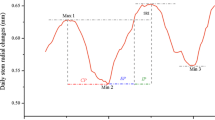
Both sites showed similar seasonal cambium dynamics (Fig. 4a, c), even though sampling periods at each site occurred in different years. For both sites, the CE and LT zones were at a minimum near the winter solstice. There was also a corresponding minimum for the dendrometer data for Rotorua (Fig 4b). The average LT zone widths in June were approximately 520 ± 188 and 400 ± 107 μm for Rotorua and Kaingaroa, respectively. LT zone width showed two periods of increase (Fig 4a, c). The first, prior to the spring equinox, is in line with increasing temperature and solar irradiance after the winter solstice. The second began approximately mid-way between the spring equinox and summer solstice and peaked in early autumn. These two periods were also shown by dendrometer data from Rotorua, averaged over the previous 2 weeks (the approximate length of time between cell enlargement and cell wall thickening, see above).
Cambial activity. a CE and LT zone widths from April 2014 to May 2015 for Rotorua. b Associated dendrometer data (2-week moving average—only 1 point shown per week for clarity). c CE and LT zone widths from July 2016 to June 2017 for Kaingaroa site. Solid arrows indicate apparent phases of increased growth. Error bars represent 95% confidence intervals. WS winter solstice, SE summer equinox, SS summer solstice, AE autumnal equinox and WS winter solstice
The CE zone widths also peaked around the time of the spring equinox (Fig. 4a, c). At an equivalent date, the widths of the LT zones of the Rotorua trees were greater than those of the Kaingaroa trees. At the spring peak (September 24, DOY 267), the width of the LT zone for Rotorua was 1865 μm, while for the trees growing in Kaingaroa, it was 1077 μm. At the early autumn peak, the LT zone widths were 1986 and 1867 for Rotorua and Kaingaroa, respectively.
3.3 Stem radius variations (Dendrometer data)
Figure 4b shows weekly averages of daily maximum radial growth recorded at Rotorua from April 2014 to April 2015. Radial growth showed a clear increase after the winter solstice (DOY 172) with a pronounced first period of maximal increment in spring around mid-September, followed by a second, less-marked increment maximum in summer. As seen from the climate data (Fig 1), there were no clear drought periods nor rainfall extremes during the study likely to influence the dendrometer data. Instead, the pattern observed in radial increment was closely related with the LT zone width data for Rotorua, with the exception of the peak at DOY 349.
3.4 Relating stem radial growth rate to LT zone width
There was a good correlation between the rate of xylem cell formation (LT zone width) and the radial stem growth rate (μm/day) when averaged over the previous 57 days (R2 = 0.66) (Fig. 5a). Figure 5b shows that the correlation between the LT width and the radial growth rate initially increases as the number previous days (over which it is averaged) increases. Averaging over greater than the previous 57 days resulted in a decrease in the correlation (Fig. 5b). The width of the CE zone was poorly correlated (R2 = 0.18) with the radial growth rate. The peak correlation was at 20 previous days (Fig. 5b).
3.5 Radial growth and climate relationships
The daily maximum radial growth increments extracted from the radial stem growth (dendrometer data, April 2014 to April 2015) were correlated to daily temperature and rainfall. When all trees were considered, radial growth was better correlated to temperature than with rainfall or solar radiation; however, all correlations were significant at p = 0.05 (Table 3; Fig. 6a, b, c, d). The rainfall data was highly positively skewed due to the number of days with no rainfall, which may have impacted the correlation with radial growth (Fig. 6c).
Individual observations of daily maximum radial growth and climate data (different colour dots for individual trees data points) and repeated measures intra-class correlations for individual tress (coloured lines for individual trees) between dendrometer daily radial maximum and air temperature (a), probe temperature (b), rainfall (c) and solar radiation (d)
4 Discussion
4.1 Seasonal cambial dynamics
We hypothesised that P. radiata cambial cell division and xylem differentiation would continue all year round with the mild climatic conditions of New Zealand. However, results showed that the cambium was only active for 335 days of the year, with a brief cessation of cambial cell division from late May (autumn) until the end of June (winter). Cambial dormancy is usually identified by ultrastructure changes in winter and summer cambial cells (Barnett 1973). However, this study showed P. radiata cambium inactivity was characterised by a minimum number of cambial cells or a minimum CE zone width and by the absence of enlarging cells next to cambium (Fig 3(a)). Barnett (1971) has reported 16-20 cells in the cambium of 5-year old P. radiata sampled in June 24th (winter solstice), which is comparable with this study’s observation of 14–16 minimum number of cambial cells in a radial file during the May to June relative inactivity. A previous study in Australia also found P. radiata cambial activity for at least 335 days of the year, with minimum activity during the month of June (Skene 1969). The study was located at Mount Macedon in the State of Victoria, which has a similar mild climate to the central North Island (Cfb according to the Köppen-Geiger climate classification). Skene’s study found cell division and radial growth had virtually ceased by late May, although development of the secondary wall of the tracheid continued until mid-July, and cell division appeared to restart by about late July. Thus, there was only a very short period where no tracheid development occurred.
The study found that the previous year’s tracheids continue to undergo xylem differentiation (Fig 3(a–c)), adjacent to the cambium zone during the months of June–August. This indicated that xylem differentiation (cell wall thickening, secondary wall deposition and lignification) possibly continues throughout the winter, albeit, at a greatly reduced rate. These results are consistent with Barnett’s (1971) results, “the most surprising feature of the 24 June sample is the absence of the characteristic secondary thickening from those cells (formed in the previous year) that were expected by this time to be fully differentiated latewood tracheids.” Thus, xylem differentiation appeared to continue during the winter period, which was in agreement with dendrometer radial growth. We hypothesize that was likely due to the relatively mild winter minimum daily average temperatures (~ 5 °C) at both sites and the absence of significant drought periods throughout the year. The mild winter temperatures of this study are similar to a reported threshold temperature of 6 to 8 °C for the xylogenesis of conifers (Rossi et al., 2007). Tracheid differentiation continued through the winter in Aleppo pines growing in the Mediterranean region when the average winter temperatures were around 10 °C (De Luis et al., 2007). Year-round growth and activity has been found in P. radiata growing in Australia, in terms of general physiological activity, including bud elongation and internode extension (Cremer 1973). It is unclear if winter xylem differentiation is common for New Zealand grown P. radiata throughout the country, or if it is specific to the mild climatic conditions of the central North Island. To further investigate this phenomenon, more research would be required over a range of climates. Our results on the presence of previous-season latewood tracheids with cell contents, which show incomplete cell lignification during quiescence, have also been previously reported. The last-formed tracheids had not completed development of their secondary wall by winter and did not lay down the S3 layer until after the resurgence of cambial activity in spring (Skene 1969; Donaldson 1991; Donaldson 1992).
In temperate and cold-winter environments, there is evidence that temperature is a key factor for the onset of cambium activity, mainly in spring in field experiments (Vaganov et al. 2005; Begum et al. 2008; Deslauriers et al. 2008; Rossi et al. 2008), or with temperature manipulation experiments (Begum et al. 2018). In contrast, the results of this study appear to indicate that P. radiata cambial reactivation was not triggered by the temperature alone. The average daily temperature at both sites remained at a yearly minimum 5 °C in June through to mid-July, while new season xylogenesis had begun by early July. Previous studies on height and diameter increment (Jackson et al. 1976; Tennent 1986) have also observed an acceleration from minimum winter rates before there was a general rise in mean temperatures. This suggests factors other than temperature can also trigger the break of winter dormancy in trees such as photoperiod (Jenkins et al. 1977; Rossi et al. 2006a, b; Lupi et al., 2012), and endogenous factors such as hormonal signals (Larson 1962; Jenkins and Shepherd 1975). More detailed research is required to identify the triggers of P. radiata cambial reactivation.
Xylem cell formation peaked in spring equinox and early autumn as shown by the maximum LT zone widths. The first peak of xylem cell formation, which occurred at the spring equinox, was most likely governed by the increasing temperatures and solar irradiance after the winter solstice. Pinus pinaster growing in a Mediterranean climate showed a bimodal pattern of stem radial increment, in spring and in autumn; however, the second peak was due to the stem hydration after the summer drought (Vieira et al. 2014). In our case, the second peak in xylem activity was not related to stem hydration, as there were no severe droughts during the study period. The second peak of xylem differentiation around autumn is an important period, where the large number of thick-walled cells is formed. After the summer solstice when stem extension stops and buds are set, photosynthates are directed to developing tracheids (Jenkins 1976). Cell walls tend to be thickest in autumn, as a greater proportion of available photosynthate is translocated to the cambial region. A causal link between formation of earlywood/latewood and sugar availability in the xylem has been postulated by Carteni et al., 2018. During this period, exogenous factors such as drought or fertilisation would be expected to have a marked influence on the productivity of the cambium and the thickening of tracheids. If the cambium can be manipulated to increase the proportion of thick-walled latewood in the ring, this will lead to overall ring-density gains (Locosselli 2018).
4.2 Zone width and radial growth
New Zealand-grown P. radiata cambial activity cannot be represented as a cumulative number of cells over the entire growth ring as the annual growth rings wider than can be sampled by microcoring. Consequently, we used an alternative method that uses the widths of xylem formation zones (CE and LT), as described in Dickson et al. (2017).
Trees at the Rotorua site were 7 years younger than the Kaingaroa site, and had a greater stem increment growth rate (as measured at DBH). The Kaingaroa site is at a higher elevation and has a known soil nitrogen deficiency. These and other site factors likely impacted the productivity of the Kaingaroa site when compared to the Rotorua site. Further, age may be a factor as younger trees have demonstrated to have higher rates of cell production than older trees (Vieira et al., 2018).
The differences we have shown in CE and LT zone widths, at a given date, were linked to differences in diameter growth of trees growing in two study sites (Fig. 4). At equivalent sampling dates, the widths of the LT zone for Rotorua trees were greater than the widths of the LT zone for Kaingaroa trees. Increased diameter growth and ring widths have been linked to an increased annual cell production (Vaganov et al., 2005; Rathgeber et al., 2011). Wide tree rings can be a result of a longer duration of cell production (early onset/late termination) (Lupi et al., 2010) or a higher rate of cell production by cambial division (Vieira et al., 2014). In both sites, the onset and termination of cambial activity occurred at around the same time; thus, we hypothesise the elevated diameter growth rate in Rotorua is largely a result of higher rates of cell production. Despite the hypothesized site differences, as well as age and tree density, the timing of intra-annual dynamics of xylem cell formation was similar. This suggests this is strongly genetically controlled; therefore, the timing of P. radiata cambial dynamics is likely similar at different ages and potentially different climates. If this is the case, management interventions for improving wood quality could be applied at the same time across different aged stands and different forests.
Due to the limitation of the length of the trephor tool used, it was unable to include P. radiata mature tracheids in the xylem differentiation zone. However, there was a strong correlation (R2 = 0.66) between the LT zone widths and radial growth rates averaged over different time periods (Fig. 5a). The correlation between CE width and growth rate was weak. This may, in part, be due to the more delicate and easily deformed nature of this tissue (Dickson et al., 2017); meaning, it was more difficult to gain an accurate measure of the width of the CE zone. The highest correlation between LT width and radial growth rate averaged over 57 days. This suggests that the width of the LT zone relates to growth rates occurring over the previous 1 to 2 months. A previous study reported that woody biomass production lags behind stem girth increase by over 1 month in conifers growing in the Northern Hemisphere (Cuny et al., 2015).
4.3 Implications of this study
The results of this study have provided knowledge on distinctive seasonal patterns of cambial activity of New Zealand-grown P. radiata grown under mild climatic conditions. This knowledge will be valuable for commercial forestry operations to optimise the timing (e.g. spring vs autumn) of management interventions (e.g. fertilizer, growth hormones) to increase latewood growth. Thus, the forest managers will be able to either not degrade or even improve desirable wood properties with accelerated growth. This will be essential for the New Zealand forest industry to meet their goals of increasing productivity and profitability of existing plantations.
The study’s novel results on xylem cell formation contribute insights on fine-scale variability in growth and wood properties of P. radiata. Seasonal xylogenesis data will be useful in developing mechanistic wood formation models such as eCambium (Drew and Downes 2015), in parameter estimation, and beyond this, predicting tree- and forest-level wood quality across many scenarios, which will be ultimately used by researchers, policy makers and forest industry owners.
5 Conclusions
Pinus radiata growing in the central North Island of New Zealand did not show full winter dormancy. An increase in cambium cell division/enlargement and diameter growth was observed not long after the winter solstice. Xylem cell formation peaked in spring and early autumn, and the minimum activity was in June coinciding with the winter solstice. Despite a 30-day period of no cambial cell division during the month of June (winter), lignification and cell wall thickening appears to continue throughout the year.
P. radiata radial stem growth was more highly correlated with temperature than with rainfall. Xylem differentiation appears to continue during the winter period in agreement with dendrometer radial growth, likely due to the relatively mild winter temperatures (~ 5–10 °C) and absence of drought conditions which are characteristic of the mild climate of the central North Island.
The cambium dynamics of P. radiata growing in central North Island differs from the classical cambium dormancy shown in temperate species, makes it an interesting model system for gaining better insights to the process of xylogenesis and early wood/late wood formation.
Availability of dataset
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abramoff MD (2004) Image processing with Image J. Biophoton Int 11:36–42
Anonymous (2017) R Core Team, rmcorr R package http://cran.r-project.org/web/packages/rmcorr. Accessed 04/04/2018
Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:1–13
Barnett JR (1971) Winter activity in the cambium of Pinus radiata. NZ J Forestry Sci 1:208–222
Barnett JR (1973) Seasonal variation in the ultrastructure of the cambium in New Zealand grown Pinus radiata D. Don. Ann Bot 37:1005–1011
Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R (2008) Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii x P. grandidentata) under natural conditions. Tree Physiol 28:1813–1819
Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R (2013) Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol Plant 147:46–54. https://doi.org/10.1111/j.1399-3054.2012.01663.x
Begum S, Kudo K, Rahman MH, Nkaba S, Yamagishi Y, Nebeshima E, Nugroho WD, Oribe Y, Kitin P, IJin H-O, Funada R (2018) Climate change and the regulation of wood formation in trees by temperature. Trees 32:3–5
Bollmann MP, Sweet GW (1976) Bud morphogenesis of Pinus radiata in New Zealand. 1. The initiation and extension of leading shoot of one clone in two sites. NZ J Forestry Sci 6:379–392
Bouriaud O, Leban J-M, Bert D, Deleuze C (2005) Intra-annual variations of climate influence growth and wood density of Norway spruce. Tree Physiol 25:651–660
Carteni F, Deslauriers A, Sergio R, Morin H, De Micco V, Mazzoleni S, Ginnino F (2018) The physiological mechanism behind earlywood-latewood transition: a process based modelling approach. Front Plant Sci 9:1053. https://doi.org/10.3389/fpls.2018.01053
Chappell PR (2013) The climate and weather of Bay of plenty, New Zealand. NIWA Science and Technology Series No 62, Bay of Plenty, pp. 1-40
Cremer KW (1973) Seasonal variation of height development in Pinus radiata near Canberra. Aust Forest Res 6:31–52
Cuny HE, Rathgeber CBK, Frank D, Fonti P, Makinen H, Prislan P, Rossi S, Del Castillo EM, Campelo F, Vavrci H, Camarero JJ, Bryukhanova MV, Jyske T, Gricar J, Gryc V, De Luis M, Vieira J, Cufar K, Kirdyanov AV, Oberhuber W, Treml V, Huang JG, Li X, Swidrak I, Deslauriers A, Liang E, Nojd P, Gruber A, Nabais C, Morin H, Krause C, King G, Fournier M (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat Plants 1:1–6. https://doi.org/10.1038/nplants.2015.160
De Luis M, Gricar J, Katarina C, Jose R (2007) Seasonal dynamics of wood formation in Pinus halepensis from dry and semi-arid ecosystems in spain. IAWA J 28:389–404
Denne M, Dodd R (1981) Environmental control of xylem differentiation, xylem cell development. In: Barnett JR (ed) Xylem cell development. Castle House, Kent, pp 236–255
Deslauriers A, Rossi S, Anfodillo T, Saracino A (2008) Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol 28:863–871
Dickson AR, Nanayakkara B, Sellier D, Meason D, Donaldson L, Brownlie R (2017) Fluorescence imaging of cambial zones to study wood formation in Pinus radiata D. Don. Trees 31:479–490. https://doi.org/10.1007/s00468-016-1469-3
Donaldson LA (1991) Seasonal changes in lignin distribution during tracheid development in Pinus radiata Wood Sci Technol 25:15–24
Donaldson L (1992) Lignin distribution during latewood formation in Pinus radiata D. Don. IAWA Bull 13:381–387
Drew DM, Downes G (2015) A model of stem growth and wood formation in Pinus radiata. Trees 29:1395–1413
FOA (2016) https://www.nzfoa.org.nz/resources/publications/facts-and-figures. Forest Owners Association. Accessed 24 Apr 2019
Fromm J (2013) Xylem development in trees: from cambial divisions to mature wood cells In: Fromm J (ed) Cellular Aspects of Wood Formation. Springer, Heidelberg, pp 3–40
GCFF (2014) Growing confidence in forestry’s future, https://gcff.nz/. New Zealand. Accessed 10 Oct 2018
Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P (2006) Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway Spruce (Picea abies). Ann Bot 97:943–951. https://doi.org/10.1093/aob/mcl050
Jackson DS, Gifford HH, Chittenden J (1976) Environmental variables influencing the increment of Pinus radiata: (2) effects of seasonal drought on height and diameter increment. NZ J Forestry Sci 5:265–286
Jenkins PA (1976) Seasonal trends in translocation of 14C photosynthate and their association with wood formation in radiata pine seedlings. NZ J Forestry Sci 5:62–73
Jenkins PA, Shepherd PR (1975) Seasonal changes in levels of indole-acetic acid and abscisic acid in stem tissues of Pinus radiata. NZ J Forestry Sci 4:511–519
Jenkins PA, Hellmers H, Edge EA, Rook DA, Burdon RD (1977) Influence of photoperiod of growth and wood formation of Pinus radiata. NZ J Forestry Sci 7:172–191
Lanner RM (1966) The phenology and growth habits of pines in Hawaii. US For Serv Res Paper PSW-29
Larson PR (1962) Auxin gradients and the regulation of cambial acvitity. In: Kozlowski TT (ed) Tree Growth. The Ronald Press, New York, pp 97–117
Larson PR (1969) Wood formation and the concept of wood quality. Yale University School of For Bull 74:1–53
Larson PR (1994) The vascular cambium development and structure. Springer-Verlag, Berlin
Locosselli GM (2018) The cambium activity in a changing world. Trees 32(1):1–2. https://doi.org/10.1007/s00468-017-1616-5
Lupi C, Morin H, Deslauriers A, Rossi S (2010) Xylem phenology and wood production: resolving the chicken-or-egg dilemma. Plant Cell Environ 33:1721–1730. https://doi.org/10.1111/j.1365-3040.2010.02176.x
Lupi C, Morin H, Deslauriers A, Rossi S (2012) Xylogenesis in black spruce: does soil temperature matter? Tree Physiol 32:74–82
Makinen H, Seo JW, Nojd P, Schmitt U, Jalkanen R (2008) Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. Eur J For Res 127:235–245. https://doi.org/10.1007/s10342-007-0199-x
MPI (2019) https://www.mpi.govt.nz/news-and-resources/open-data-and-forecasting/situation-and-outlook-for-primary-industries-data/. Ministry of Primary Industries. Accessed 24 Apr 2019
Murmanis L (1971) Structural changes in the vascular cambium of Pinus strobus L. during an annual cycle. Ann Bot 35:133–142
Prislan P, Cufar K, Koch G, Schmitt U, Gricar J (2013) Review of cellular and subcellular changes in the cambium. IAWA J 34:391–407
Rathgeber CBK, Rossi S, Bontemps JD (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438. https://doi.org/10.1093/aob/mcr168
Rossi S, Anfodillo T, Menardi R (2006a) Trephor: A new tool for sampling microcores from tree stems. IAWA J 27:89–97
Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M (2006b) Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol 170:301–310. https://doi.org/10.1111/j.1469-8137.2006.01660.x
Rossi S, Deslauriers A, Anfodillo T, Carraro V (2007) Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152:1–12. https://doi.org/10.1007/s00442-006-0625-7
Rossi S, Deslauriers A, Gricar J, Seo JW, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707. https://doi.org/10.1111/j.1466-8238.2008.00417.x
Rossi S, Simard S, Rathgeber CBK, Deslauriers A, De Zan C (2009) Effects of a 20-day-long dry period on cambial and apical meristem growth in Abies balsamea seedlings. Trees-Struct Funct 23:85–93. https://doi.org/10.1007/s00468-008-0257-0
Samuels AL, Kaneda M, Rensing KH (2006) The cell biology of wood formation: from cambial divisions to mature secondary xylem. Can J Bot 84:631–639. https://doi.org/10.1139/b06-065
Savidge RA (2000) Intrinsic regulation of cambial growth. J Plant Growth Regul 20:52–77
Seo J-W, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U (2008) Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiol 28:105–112
Skene DS (1969) The period of time taken by cambial derivatives to grow and differentiate into trachieds in Pinus radiata. Ann Bot 33:253–262
Tennent RB (1986) Intra-annual growth of young Pinus radiata in New Zealand. NZ J Forestry Sci 16:166–175
Vaganov EA, Huges MK, Shashkin AV (2005) Growth dynamics of conifer tree rings. Springer, Heidelburg
Vieira J, Rossi S, Campelo F, Freitas H, Nabais C (2014) Xylogenesis of Pinus pinaster under a Mediterranean climate. Ann For Sci 71:71–80. https://doi.org/10.1007/s13595-013-0341-5
Vieira J, Carvalho A, Campelo F (2018) Xylogenesis in the early life stages of maritime pine. For Ecol Manag 424:71–77. https://doi.org/10.1016/j.foreco.2018.04.037
Acknowledgements
Authors acknowledge Alex Manig’s help in microcore sampling, Rod Brownlie for his expertise in dendrometers and Damien Sellier for helping in tree measurements and dendrometer data collation. Rowland Burdon, Lloyd Donaldson for critical review of manuscript and Rowland Burdon for useful suggestions. Michelle Harnett for editorial review.
Funding
This research was supported by the ‘Growing Confidence in Forestry’s Future’ research programme (C04X1306) which is jointly funded by the New Zealand Ministry of Business, Innovation and Employment and the New Zealand Forest Growers Levy Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Patrick Fonti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is part of the topical collection on Wood formation and tree adaptation to climate
Contribution of the co-authors
BN: Experimental design, sample selection and collection, sample processing, microscopy, image analysis, data analysis, analysis of results, and writing the manuscript. AD: Experimental design, sample processing, microscopy, image analysis, data analysis, analysis of results, and writing the manuscript. DM: Experimental design and editing the manuscript.
Rights and permissions
About this article
Cite this article
Nanayakkara, B., Dickson, A.R. & Meason, D.F. Xylogenesis of Pinus radiata D. Don growing in New Zealand. Annals of Forest Science 76, 74 (2019). https://doi.org/10.1007/s13595-019-0859-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-019-0859-2