Abstract
Key message
Radial growth of silver and red maples was investigated across three forests in northwest Ohio following the outbreak of the invasive emerald ash borer. The growth response of maples was driven by an advancement in canopy class and disturbance severity.
Context
Forest disturbances resulting in species-specific diffuse mortality cause shifts in aboveground and belowground competition. This competition may differentially affect non-impacted trees, depending on crown class, disturbance severity, and species-specific responses.
Aims
The purpose of this study is to elucidate the primary drivers of silver and red maple (Acer saccharinum and A. rubrum) growth following emerald ash borer (EAB, Agrilus planipennis)-induced ash tree (Fraxinus spp.) mortality in riparian forests of northwest Ohio.
Methods
Using dendroecological approaches, we analyzed the pattern of radial growth in red and silver maples in conjunction with the EAB outbreak.
Results
This study revealed growth rates of maples increased 72% following EAB arrival and trees advancing in crown class grew 41% faster than those not advancing. The growth response varied by initial crown class, with trees in the intermediate class responding most dramatically. Furthermore, the positive correlation between relative basal area of ash and the radial growth response of maples indicates the important role of disturbance severity in post-disturbance dynamics.
Conclusion
These findings suggest that, although advancement in crown class may allow predictions of “winners” in forest succession post-disturbance, even trees not changing crown class benefit from decreased competition. Results from this study provide a detailed account of radial growth responses in maples following EAB-induced ash mortality and lend insight into the future canopy composition of ash-dominated riparian forests.
Similar content being viewed by others
1 Introduction
Disturbance agents alter forest successional dynamics, forcing remaining species to fill the niche(s) created after disturbance. Winners and losers are governed in part by species interactions, environmental conditions, differential species performance, and lottery competition (Gleason 1917; Waring and Running 2010; Flower and Gonzalez-Meler 2015; Lienard et al. 2015). Forest pest disturbances that target specific genera differ from abiotic disturbances (e.g., wind throw, flooding, or fire), which can occur rapidly and randomly affect trees of any genus. Such differences between biotic and abiotic disturbances may result in new and unique successional trajectories (Flower and Gonzalez-Meler 2015). Forest responses to disturbances such as the emerald ash borer (EAB, Agrilus planipennis Fairmaire) and other diffuse mortality agents vary with the magnitude, severity, and type of disturbance. These disturbances alter the successional trajectory and productivity of the affected forests, with considerable ramifications for their future diversity and structure (Denslow 1980; Forrester et al. 2003; Kurz et al. 2008; Schäfer et al. 2010; Flower et al. 2013a; Flower and Gonzalez-Meler 2015). In part because of reduced belowground competition for nutrients and reduced aboveground competition for light (Foster et al. 2012), non-host residual tree species will benefit from such disturbances. Previous research has found that in forests of the western USA, eruptive bark beetle outbreaks that impact conifers have led to the rapid release of co-occurring aspen (Populus tremuloides Michx.) (Bretfeld et al. 2015); yet, the impacts of natural disturbance severity on such releases remain largely unknown particularly in deciduous mixed-species temperate forests. A study by Zhang et al. (1999) in mixed Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), subalpine fir (Abies lasiocarpa (Hook.) Nutt.), and interior spruce (Picea spp.) stands of British Columbia, Canada, highlights a pattern of above-average growth in Douglas-fir that is attributable to severe outbreaks of spruce beetle (Dendroctonus rufipennis (Kirby)). In mountain pine beetle (MPB, Dendroctonus ponderosae Hopkins)-impacted regions of central British Columbia, the radial growth of residual tree species has shown positive responses to the MPB-induced mortality (Hawkins et al. 2013). However, the enhanced growth in these MPB-impacted systems has been dominated by understory relative to overstory trees and young stands (<80 years old) relative to older stands (>80 years old) (Hawkins et al. 2013). With an estimated 7.5 billion ash trees in the continental USA (Flower et al. 2013a), many of which occur in the ecologically sensitive riparian areas, EAB could drastically alter the structure and composition of temperate forests of the eastern USA which are witnessing increasing maple (Acer spp.) dominance (Abrams 2005; Hart et al. 2012).
Emerald ash borer is a wood-boring insect from eastern Asia that selectively targets trees in the genus Fraxinus and likely other species in the family Oleaceae (Cappaert et al. 2005; Cipollini 2015). EAB was accidently introduced to the Detroit, Michigan, USA, region in the 1990s and was subsequently discovered in 2002 (Haack et al. 2002; Siegert et al. 2014). EAB has rapidly spread from its epicenter to 30 eastern US states and two southeastern Canadian provinces (USDA-APHIS 2016). Despite foliar feeding by adults, the most devastating life stage of the insect is its larval form (Anulewicz et al. 2008; Flower et al. 2013b). Larvae emerge from eggs deposited onto stems and branches and bore through the tree’s bark, feeding on the tree’s phloem (Lyons et al. 2005). At high-enough densities, the serpentine galleries created by larvae disrupt water and nutrient flow and eventually girdle the tree, resulting in the tree’s mortality (Flower et al. 2011, 2013b).
Forests near the epicenter have exhibited significant loss of canopy ash, with cases reporting over 95 to 99% ash mortality (Kashian and Witter 2011; Knight et al. 2012; Flower et al. 2013a). In conjunction with this EAB-induced ash mortality, maple and elm (Ulmus spp.) have exhibited the highest relative growth rates, indicating potential growth responses in post-EAB forests (Flower et al. 2013a). Yet, these high growth rates may be more indicative of species-specific characteristics than post-disturbance responses, suggesting the need for dendroecological approaches to untangle shifts in growth dynamics.
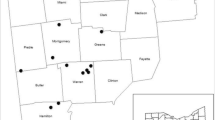
The objectives of this study were twofold. First, we were interested in quantifying the radial growth response of silver and red maple (Acer saccharinum L. and A. rubrum L.) to the EAB-induced ash decline in northwestern Ohio (Fig. 1). Maples are not only of high ecological importance in riparian forests of Ohio but also the most prevalent genus after Fraxinus spp. within the study sites. Second, we sought to determine which specific characteristics (i.e., forest, initial crown class, changes in crown class, or disturbance severity) explained red and silver maple growth release during the EAB disturbance.
Map of the study site locations (main panel) and Ohio (inset) denoting the physiographic regions (from Brockman, 1998)
2 Material and methods
2.1 Study site and experimental design
This research was conducted in 13 permanent research plots established in 2005 across three green ash (Fraxinus pennsylvanica Marsh.)-dominated forests in northwestern Ohio: Oak Openings Metropark (n = 3), Fallen Timbers Metropark (n = 4), and Maumee Bay State Park (n = 6) (Fig. 1; also see Flower et al. 2013a for a more detailed site description). These forests lie in the western region of Ohio’s Huron-Erie Lake Plain physiographic region near the historic Great Black Swamp (Brockman 1998) and exhibit glacial lake sediments and similar hydrological conditions; see Table 1 for additional forest stand characteristics. The permanent forest plots (11.28 m radius) are located ~100 km from the epicenter of the EAB infestation and aside from the presence of ash in the canopy were randomly placed >50 m apart within the same forest stand. Plots were annually monitored for tree growth (all trees >10 cm diameter at breast height; DBH 1.37 m) and crown class (dominant, codominant, intermediate, and suppressed; as per Oliver and Larson (1996)). From DBH, we computed the relative basal area of ash, which we use synonymously as the proportion of canopy disturbed through EAB-induced ash dieback (i.e., disturbance severity).
In order to assess the progression of EAB and the health of ash trees, we annually assessed ash trees (n = 526) for their ash canopy condition (AC; a visual canopy health classification tightly linked to EAB larval gallery cover). Ash canopy condition (or canopy health) is assigned to one of five categories, where a 1 is healthy and a 5 is dead, described in more detail in Flower et al. (2013b) and Smith (2006). Additional EAB symptoms such as D-shaped galleries, epicormic sprouting, and woodpecker damage were also counted annually (for more information on these monitoring approaches, see Knight et al. (2014)). Exit holes from all ash trees >10 cm DBH were counted annually in a window on each stem between 1.25 and 1.75 m in height.
In the spring of 2011, we identified two release groups from the crown class data. Maples that showed crown class advancement between 2005 and 2010 were assigned to the “crown advancement” (CA) group. Those that did not advance in crown class were assigned to the “non-crown advancement” (NCA) group. Trees in the CA group (n = 36) had changed crown class in one of six ways: from suppressed to either intermediate, codominant, or dominant; from intermediate to either codominant or dominant; or from codominant to dominant. An advancement in crown class indicates that the canopy of the CA trees received greater sun exposure due to the death of surrounding trees (see Fig. 2). An increase of two levels (e.g., from suppressed to codominant) was rare within the sample population, occurring in three of the 36 CA trees. Maples in the NCA group largely remained in the same crown class (n = 32) or declined in crown class (n = 4) within the 5-year observation period. Additionally, we excluded trees recorded as dominant in 2005 because we would expect no significant change in the allocation of light resources for these individuals as ash mortality transpired. Individual silver (n = 34) and red (n = 38) maples were initially paired in a random fashion such that CA and NCA pairs were selected from within each forest and to correspond with a tree of the same 10-cm DBH class. As such, there was no size difference between trees in the CA and NCA groups.
Idealized forest profiles depicting a pre-disturbance community (A) and a post-disturbance community (B). In the pre-disturbance community, note that three sub-canopy maple trees (shaded grey) are suppressed (S) by adjacent live trees. In the post-disturbance community, the tree on the left remains suppressed (or does not exhibit crown class advancement, NCA, because its neighbors remain alive), while the previously suppressed tree in the middle moves from suppressed to intermediate and the previously suppressed tree on the right had its neighbors die and subsequently it is no longer suppressed, but codominant (C, and thus these two trees exhibit crown class advancement or CA)
To assess changes in the pattern of red and silver maple radial growth in conjunction with the EAB outbreak, 5-mm increment cores (extracted with a 50-cm increment borer, Haglöf Sweden, Långsele, Sweden) were collected in August of 2011 (at breast height, 1.37 m) from either the east or west side of 72 maples. All sampled trees were free from signs of physical injury, symptoms of disease, and insect damage. Each core was air-dried in paper straws, mounted, and progressively sanded from 200- to 500-grit sandpaper (Stokes and Smiley 1968). Annual radial increment growth from 1982 to 2010 was measured using WinDENDRO™ (v.2002a Regent Instruments Inc., Quebec City, Canada, 2003), with a measurement precision of 0.01 mm. For brevity, only data from 1990 to 2010 is presented herein. Statistical crossdating approaches have typically been deployed in scenarios where trees are dead and the year of the last growth ring is unknown, or when trying to recreate historical disturbance events or the influences of abiotic factors which result in small anomalies in growth. Formal crossdating analysis was not necessary in our study as the trees were all alive when they were cored, so the final year of growth was known. The measured ring-width series were plotted with skeleton plots, and all ring width series were visually cross-dated (Fritts 1976; Jones et al. 2009). The presence of a drought in 1988 (Palmer drought severity index of −4.06) was used as a pointer year, and radial growth in 83% of the study trees were impacted by this drought (mean annual growth increment was reduced by 29%). Measurements of several cores with poor or indistinguishable growth rings were enhanced through dying with phloroglucinol, which stains lignin red while leaving the cellulose unstained (Stokes and Smiley 1968). Furthermore, as the focal area across northwest Ohio was relatively small and topographically uniform, we assumed that any climatic influence was similar for all stands and samples. As such, we assume that variability in tree growth was not a climate change impact but instead associated with the impact of EAB-induced ash mortality.
Because of the distance between sites and the nature of EAB dispersal, not all sites became infested with EAB in the same year. Therefore, “pre-EAB” and “post-EAB” periods were determined at the plot level from the presence of EAB exit holes and other EAB symptoms (i.e., woodpecker damage and plot-level canopy thinning greater than AC condition 2). Pre-EAB growth was calculated as the average annual growth increment during the 5-year period prior to EAB arrival. The post-EAB period was the number of years following EAB arrival, and post-EAB growth was the average annual growth increment across this period. As EAB-infested trees have been linked to ash canopy decline (Flower et al. 2013b) and we predict the residual trees will respond to this canopy decline, we included the year in which EAB arrived in the post-EAB period. For example, if an EAB exit hole was discovered in 2007, we considered EAB to have arrived in 2006; thus, the pre-EAB period would be from 2001 to 2005 and the post-EAB period from 2006 to 2010.
2.2 Data analysis
First, we conducted a repeated measure analysis of variance (RMANOVA; α = 0.05) to test for a differential growth response between red and silver maples over time following the EAB-induced mortality. No significant difference between species was detected (P = 0.320); as such, red and silver maples were pooled for future analyses. Next, we conducted two separate but complimentary analyses to investigate silver maple growth during the EAB outbreak. For the first analysis, we used an analysis of variance with repeated measures (RMANOVA, α = 0.05) to investigate growth differences between the release groups, the pre-EAB and post-EAB periods (i.e., time), and the time × release group interaction. Plot was not significant and was excluded from the model. Following the RMANOVA, we conducted a univariate F-test to identify any significant differences between the release groups (CA versus NCA) during the pre- and post-EAB periods.
To further untangle factors that affected maple growth in response to ash decline, we created a single dependent variable, hereafter called growth response, by taking the post-EAB growth rate and subtracting the pre-EAB growth rate. Such an approach gives us the actual growth difference between pre- and post-invasion periods, making it easy to compare the growth response of trees. Although most of the individual trees increased average yearly growth post-EAB and thus have a positive growth response, some trees experienced a marginal decline in growth and thus have a negative growth response. Using the growth response as the dependent variable, a hierarchical mixed-effect model was constructed with forest (random factor), plot nested in forest (random factor), release group (CA and NCA), and initial crown class (i.e., from 2005, pre-EAB-induced mortality) as fixed factors and relative basal area of ash (i.e., disturbance severity) as a covariate. The estimation method used for the model was restricted maximum likelihood with the Kenward-Rogers denominator degrees of freedom adjustment. The covariance parameters associated with the random effects were variance components with a compound symmetry structuring for the error term.
To assess EAB-induced potential differences between release groups in the annual growth increment over time, we utilized a RMANOVA. Release group was treated as a main effect and the annual growth increment from 2005 to 2010 was the repeated measure and the time × release group interaction was used to assess differential responses between groups over time. A significant time × release group interaction suggests the presence of differential growth responses between groups (with the CA exhibiting more rapid growth relative to the NCA group in multiple years). In order to assess the presence of a lag in growth in the NCA group relative to the CA group, we lagged the annual growth increment in the NCA group by −1 and −2 years and reanalyzed the data set using the same model. We considered the lag to have been resolved when the time × release group interaction was no longer significant. All statistics were analyzed using SYSTAT (2007) statistical software (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Pre-disturbance versus post-disturbance growth patterns
Before the outbreak of EAB in 2005, maple growth was similar between release groups. Both groups exhibited stable growth patterns, from 1990 to 2000, and a slowing growth rate from 2000 to 2005, indicative of canopy closure (Fig. 3). Following the EAB outbreak, trees in both the CA and NCA groups responded rapidly, exhibiting increased growth rates (Fig. 3). Statistical analysis indicates that prior to EAB-induced decline, maples from both the CA and NCA groups had similar growth rates (0.127 ± 0.016 and 0.131 ± 0.014 cm year−1, mean ± SE, respectively) (Fig. 4; RMANOVA F 1,70 = 0.05, P = 0.824). The progression of growth over time from the “pre-EAB” to “post-EAB” period was also significantly different from growth rates during the pre-EAB period, with growth rates increasing over 72% (Fig. 4; RMANOVA F 1,70 = 50.369, P < 0.001). Maples from both the CA and NCA groups exhibited enhanced growth rates following the EAB outbreak (0.185 ± 0.022 and 0.262 ± 0.030 cm year−1, respectively). A significant time × release group interaction was observed (RMANOVA F 1,70 = 7.482, P = 0.008) in which trees in the CA group responded more positively (99% larger growth increment post-EAB) to the EAB-induced ash mortality relative to those in the NCA group (41%). As a result, post-EAB growth between release groups differed significantly (RMANOVA F 1,70 = 4.124, P = 0.046).
Mean annual radial increment growth of maples during the “pre-EAB” (5 years before observance) and “post-EAB” for both the canopy advancing group (CA, black bars) and the non-canopy advancement group (NCA, grey bars). The double asterisk indicates significant differences between the “pre-EAB” and “post-EAB” periods (P < 0.001), while the single asterisk denotes the significant difference between the CA and NCA groups in the “post-EAB” period (P = 0.046). Error bar denotes standard error
3.2 Factors affecting maple growth response
Further analysis revealed that basal area of ash (disturbance severity), crown class, and other factors affected the maple growth response following EAB-induced mortality. Consistent with the RMANOVA results, release group remained a significant factor, with trees in the CA group exhibiting a larger growth response than those in the NCA group (F 1,56 = 10.938, P = 0.002, overall model adj. R 2 = 0.582). Relative basal area of ash covaried with the maple growth response, with focal trees in less severely disturbed areas showing lower growth responses than trees in areas of high disturbance severity (Fig. 5; F 1,56 = 5.456, P = 0.023). Analysis of the initial crown class data indicates that trees in the intermediate canopy positions exhibited a larger canopy release than did those in suppressed and codominant positions, suggesting that trees in different crown classes respond differently to disturbance (Fig. 6; F 2,56 = 3.239, P = 0.047).
The relationship between the relative basal area of ash (%) and the differential growth response (“post-EAB” growth average − “pre-EAB” growth average) of maple following ash decline (R 2 = 0.196; P = 0.023). The dashed line denotes the linear relationship, and the solid line differentiates between individuals that did (+) and did not (−) exhibit a growth release. Grey symbols indicate trees in the non-canopy advancing (NCA) group while black symbols denote individuals in the canopy advancing (CA) group
3.3 Ash canopy decline and maple growth
Annual monitoring of the visual ash canopy condition of 526 ash trees within the study plots indicated relatively healthy ash canopies in 2005 and 2006, then a gradual decline in canopy health, culminating in widespread mortality by 2010 (Fig. 7). Significant temporal variability in the annual growth increment of maples occurred following the arrival of EAB and the subsequent EAB-induced ash decline (2005–2010; F = 20.836, P < 0.001). Furthermore, a significant difference in the temporal response was observed between release groups, with maples in the CA group exhibiting higher annual growth increments relative to those in the NCA group (Fig. 7; F = 2.935, P = 0.013). The significant time × release group interaction indicates a potential lag in the growth response of the NCA group. Shifting annual growth increment response in the NCA group by 1 year in the NCA group did not resolve the significance of the time × release group interaction (F = 2.55, P = 0.039), but shifting it by 2 years did (F = 0.357, P = 0.784). These results indicate that the release of maples in the CA group occurred concurrently with the decline in ash and that these trees were positioned to capitalize on the reduced competition for light (and likely other) resources with ash and able to advance in canopy position as ash declined. In contrast, the release of maples in the NCA group lagged the CA group by 2 years indicating that this group required higher levels of ash canopy decline and thus light to stimulate growth.
4 Discussion
The purpose of our study was to evaluate a potential growth release of residual red and silver maple trees (the second most abundant tree species after ash) in response to the EAB outbreak in northwestern Ohio at the turn of the twenty-first century. As expected, our findings show a significant increase in the radial annual growth increment of maples in both crown-advancing and non-crown-advancing release groups between the pre- and post-EAB periods coinciding with the EAB-induced ash tree mortality. Our results suggest that the annual growth increment in both the CA and NCA groups increased progressively during the 5-year period, which coincides with EAB arrival and EAB-induced mortality. This enhanced growth is consistent with a release from competitive suppression associated with competition for light, nutrients, and water. Similar results have been observed in response to pest outbreaks (Hadley and Veblen 1993; Zhang et al. 1999; Bretfeld et al. 2015; Sangüesa-Barreda et al. 2015), natural disturbances (Canham 1988; Merrens and Peart 1992), and manipulative experiments, such as those following prescribed burning and mechanical removal (North et al. 1996; Plauborg 2004; Thorpe et al. 2007; Anning and McCarthy 2013). Furthermore, the increasing annual growth increment after EAB arrival may highlight the gradual redistribution of light and nutrients to residual trees from ash, peaking in 2010 when mean ash canopy condition is nearly dead. Similarly, a study by Devine and Harrington (2013) focused on Oregon white oak (Quercus garryana Douglas ex Hook) growth responses at different time periods (1–3, 4–5, 6–10 years) following three silvicultural treatments (thinning, individual tree release or half release, and stand release or full release). They found significant differences in oak growth responses between treatments and across time, but temporal differences were dominated by increasing growth rates in the full release or most severe disturbance treatment. Our study is unique relative to the aforementioned body of research in part because we paired high-resolution growth data from maples with annual measures of EAB-induced ash tree mortality in a natural setting.
The EAB-induced growth responses include an immediate response observed in the CA group and a 2-year lagged response observed in the NCA group. The rapid response post-disturbance and the growth enhancement associated with disturbance severity while not surprising are underrepresented in the literature as they relate to contemporary natural disturbance events in temperate deciduous systems. Plauborg (2004) highlighted differential radial growth responses to thinning severity, focal species, and site quality. Plauborg (2004) reveals a tight species-specific disturbance severity effect driving increased radial growth, yet the immediacy of this response was not a focus. Thorpe et al. (2007) report a short (2-year) lag time of no radial growth response in black spruce (Picea mariana Mill.) after harvest, followed by a period of radial growth between 3 and 9 years. The different temporal patterns of the growth response may be due in part to the shade-tolerance levels of the two focal species. Black spruce is shade tolerant, and red and silver maples have intermediate shade tolerance, which permits the maples to respond more quickly (Burns and Honkala 1990). Thorpe et al. (2007) showed that the magnitude of the growth response depended on tree age (higher for younger relative to older trees). Our findings are somewhat consistent with their results in that the maples in intermediate canopy positions exhibited higher growth responses relative to those in the suppressed and codominant positions. Jones et al. (2009) witnessed similar growth responses of shade-tolerant and shade-intolerant species 3–15 years post-harvest. Our results depicting an immediate growth response of red and silver maples in the CA group to the EAB disturbance reveal how resilience in the productivity of forests impacted by pests and pathogens may be mediated by the plasticity in the response of non-impacted species to the increased abundance of light, nutrients, and water (Flower and Gonzalez-Meler 2015). The immediate growth response of the CA group in this natural system may have occurred in part because these sites did not have the disturbances associated with harvesting (e.g., soil compaction and nitrogen leaching). Furthermore, the EAB disturbance is a progressive mortality event leading to tree-level mortality in ~2 years and stand-level ash mortality in ~5 years unlike largely instantaneous silvicultural disturbances. As ash trees are attacked by EAB, the canopy slowly thins and water and nutrient uptake is impaired over a few years prior to the mortality of the ash tree (Flower et al. 2011; Flower et al. 2013b). The gradual increase in aboveground and belowground resources may allow residual trees to make physiological adjustments prior to the mortality of the ash trees that would facilitate a rapid growth response when ash mortality occurs. For example, a gradual increase in water availability could prevent new water stress associated with light saturation. This “priming” effect caused by gradual decline and mortality of ash trees may lead to very different forest responses than would sudden disturbances. Thus, our study suggests that not only is the spatial pattern of disturbance (i.e., diffuse mortality) important, the temporal pattern of disturbance (i.e., gradual) may also be a critical influence on forest successional responses.
Our finding that disturbance severity is positively related to maple growth supports the findings of Flower et al. (2013a) in which relative growth rates of all residual tree species following EAB disturbance in Toledo, in northwestern Ohio, were positively related to disturbance severity. Furthermore, it supports the post-disturbance data highlighting growth release of residual trees. Further research on the extent of this pattern across species is needed to better understand the extent to which EAB may alter forest successional patterns (e.g., how the response varies across a gradient from shade tolerance to shade intolerance). Additionally, future research should investigate the longevity of the growth response, particularly as it relates to disturbance severity.
5 Conclusion
Host-specific invasive pests create patterns of diffuse mortality in mixed-species forest stands. Our study of the radial growth response of red and silver maple trees to ash mortality brought on by the invasive emerald ash borer yields insights into understanding how trees respond to diffuse mortality. Understanding residual tree growth responses to disturbances that result in diffuse canopy mortality can facilitate better models of post-disturbance successional dynamics and yield better predictions that can shape informed management. Such knowledge is particularly relevant in light of the increased prevalence of pest and pathogen outbreaks that result in species-specific mortality events (Aukema et al. 2010).
Red and silver maple trees that experienced increased exposure to sunlight (measured by advancement in crown class) had the greatest increases in growth. Thus, by predicting the potential for crown class advancement after disturbance, it is possible for managers to predict the biggest “winners” in forest succession following diffuse mortality. Furthermore, trees that did not advance in crown class also experienced some increase in growth following ash mortality, suggesting that the overall stand may benefit from decreases in aboveground and belowground competition following diffuse mortality of ash. Stands with greater ash density had the greatest increases in growth after the onset of ash mortality. The radial growth response of maples occurred in tandem with the thinning of ash canopies caused by EAB infestation.
The rapid growth response of certain tree species in EAB-affected stands may promote structural complexity and maintain closed-canopy conditions that resist the proliferation of invasive plant species and sustain ecosystem services. Pre-disturbance vegetation surveys and the management of ash stands may help to ensure an adequate abundance of trees in different successional cohorts which will respond positively to diffuse disturbances to enhance the future health of these stands and increase their resilience (Mitchell et al. 2016).
References
Abrams MD (2005) Prescribing fire in eastern oak forests: is time running out? North J Appl For 22:190–196
Anning AK, McCarthy BC (2013) Long-term effects of prescribed fire and thinning on residual tree growth in mixed-oak forests of southern Ohio. Ecosystems 16:1473–1486. doi:10.1007/s10021-013-9696-6
Anulewicz AC, McCullough DG, Cappaert DL, Poland TM (2008) Host range of the emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) in North America: results of multiple-choice field experiments. Environ Entomol 37:230–241. doi:10.1603/0046-225X(2008)37
Aukema JE, McCullough DG, Von Holle B et al (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60:886–897. doi:10.1525/bio.2010.60.11.5
Bretfeld M, Doerner JP, Franklin SB (2015) Radial growth response and vegetative sprouting of aspen following release from competition due to insect-induced conifer mortality. For Ecol Manag 347:96–106. doi:10.1016/j.foreco.2015.03.006
Brockman S (1998) Physiographic regions of Ohio. Division of Geological Survey, Columbus
Burns R, Honkala B (1990) Silvics of North America, vol. 2: hardwoods. Agriculture handbook 654. United States Department of Agriculture Forest Service, Washington, DC
Canham CD (1988) Growth and canopy architecture of shade-tolerant trees: response to canopy gaps. Ecology 69:786–795
Cappaert D, Mccullough DG, Poland TM, Siegert NW (2005) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51:152–165
Cipollini D (2015) White fringetree as a novel larval host for emerald ash borer. J Econ Entomol 108:370–375. doi:10.1093/jee/tou026
Denslow J (1980) Patterns of plant species diversity during succession under different disturbance regimes. Oecologia 46:18–21
Devine WD, Harrington CA (2013) Restoration release of overtopped Oregon white oak increases 10-year growth and acorn production. For Ecol Manag 291:87–95. doi:10.1016/j.foreco.2012.10.053
Flower CE, Gonzalez-Meler MA (2015) Responses of temperate forest productivity to insect and pathogen disturbances. Annu Rev Plant Biol 66:547–569. doi:10.1146/annurev-arplant-043014-115540
Flower CE, Knight KS, Gonzalez-Meler MA (2013a) Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol Invasions 15:931–944. doi:10.1007/s10530-012-0341-7
Flower CE, Knight KS, Rebbeck J, Gonzalez-Meler MA (2013b) The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For Ecol Manag 303:143–147. doi:10.1016/j.foreco.2013.04.017
Flower CE, Lynch DJ, Knight KS, González-Meler MA (2011) EAB induced tree mortality impacts tree water use and ecosystem respiration in an experimental forest. In: Proceedings of the emerald ash borer research and technology development meeting. Port Huron MI, 12–13 Oct 2011, FHTET-2011–06, United States Department of Agriculture Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virgina. pp 115–116
Forrester JA, McGee GG, Mitchell MJ (2003) Effects of beech bark disease on aboveground biomass and species composition in a mature northern hardwood forest, 1985 to 2000. J Torrey Bot Soc 130:70–78. doi:10.2307/3557531
Foster DR, Aber JD, Melillo JM et al (2012) Forest response to disturbance and anthropogenic stress. Bioscience 47:437–445
Fritts HC (1976) Tree rings and climate. Academic Press, London, U.K.
Gleason HA (1917) The structure and development of the plant association. Bull Torrey Bot Club:463–481
Haack RA, Jendek E, Liu H et al (2002) The emerald ash borer: a new exotic pest in North America. Newsl Michigan Entomol Soc 47:1–5
Hadley KS, Veblen TT (1993) Stand response to western spruce budworm and Douglas-fir bark beetle outbreaks, Colorado Front Range. Can J For Res 23:479–491. doi:10.1139/x93-066
Hart JL, Buchanan ML, Clark SL, Torreano SJ (2012) Canopy accession strategies and climate-growth relationships in Acer rubrum. For Ecol Manag 282:124–132. doi:10.1016/j.foreco.2012.06.033
Hawkins CDB, Dhar A, Balliet NA (2013) Radial growth of residual overstory trees and understory saplings after mountain pine beetle attack in central British Columbia. For Ecol Manag 310:348–356. doi:10.1016/j.foreco.2013.08.035
Jones T, Domke G, Thomas S (2009) Canopy tree growth responses following selection harvest in seven species varying in shade tolerance. Can J For Res 39:430–440. doi:10.1139/X08-186
Kashian DM, Witter JA (2011) Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For Ecol Manag 261:480–488. doi:10.1016/j.foreco.2010.10.033
Knight KS, Flash BP, Kappler RH, et al. (2014) Monitoring ash (Fraxinus spp.) decline and emerald ash borer (Agrilus planipennis) symptoms in infested areas. United States Department of Agriculture Forest Service, Gen. Tech. Rep. NRS-139
Knight KS, Herms D, Plumb R, et al. (2012) Dynamics of surviving ash (Fraxinus spp.) populations in areas long infested by emerald ash borer (Agrilus planipennis). In: Sniezko R, Yanchuk A, Kliejunas J, et al. (eds) Proceedings of the 4th international workshop on genetics of host-parasite interactions in forestry: disease and insect resistance in forest trees, Eugene, Ore., 31 July–5 August 2011. United States Department of Agriculture Forest Service. Gen. Tech. Rep. PSW-GTR-240, pp 143–52
Kurz WA, Dymond CC, Stinson G et al (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990. doi:10.1038/nature06777
Lienard J, Florescu I, Strigul N (2015) An appraisal of the classic forest succession paradigm with the shade tolerance index. PLoS One 10:e0117138. doi:10.1371/journal.pone.0117138
Lyons DB, Jones G., Wainio-Keizer K (2005) The biology and phenology of the emerald ash borer, Agilus planipennis. In: Gottschalk KW (ed) XV United States Department of Agriculture Interagency Research Forum on gypsy moth and other invasive species, Annapolis, Maryland, 13–16 January 2004. United States Department of Agriculture Forest Service, Gen. Tech. Rep. NE-332, p 54
Merrens EJ, Peart DR (1992) Effects of hurricane damage on individual growth and stand structure in a hardwood forest in New Hampshire, USA. J Ecol 80:787–795
Mitchell RJ, Pakeman RJ, Broome A et al (2016) How to replicate the functions and biodiversity of a threatened tree species? The Case of Fraxinus excelsior in Britain Ecosystems doi. doi:10.1007/s10021-015-9953-y
North M, Chen J, Smith G et al (1996) Initial response of understory plant diversity and overstory tree diameter growth. Northwest Sci 70:24–35
Oliver CD, Larson BC (1996) Forest stand dynamics, update edi. Wiley, New York
Plauborg KU (2004) Analysis of radial growth responses to changes in stand density for four tree species. For Ecol Manag 188:65–75. doi:10.1016/j.foreco.2003.07.022
Sangüesa-Barreda G, Linares JC, Camarero JJ (2015) Reduced growth sensitivity to climate in bark-beetle infested Aleppo pines: connecting climatic and biotic drivers of forest dieback. For Ecol Manag 357:126–137. doi:10.1016/j.foreco.2015.08.017
Schäfer KVR, Clark KL, Skowronski N, Hamerlynck EP (2010) Impact of insect defoliation on forest carbon balance as assessed with a canopy assimilation model. Glob Chang Biol 16:546–560. doi:10.1111/j.1365-2486.2009.02037.x
Siegert NW, McCullough DG, Liebhold AM, Telewski FW (2014) Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers Distrib 20:847–858. doi:10.1111/ddi.12212
Smith A (2006) Effects of community structure on forest susceptibility and response to the emerald ash borer invasion of the Huron River watershed in southeast Michigan. The Ohio State University
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. Arizona Press, Tuscon
SYSTAT (2007) SYSTAT 12.0 for Windows
Thorpe HC, Thomas SC, Caspersen JP (2007) Residual-tree growth responses to partial stand harvest in the black spruce (Picea mariana) boreal forest. Can J For Res 37:1563–1571. doi:10.1139/X07-148
USDA-APHIS (2016) Emerald ash borer quarantine map. http://www.emeraldashborer.info/documents/MultiState_EABpos.pdf. Accessed 8 November 2016
Waring RH, Running SW (2010) Forest ecosystems: analysis at multiple scales, 3rd edn. Elsevier, San Diego, USA
Zhang Q, Alfaro RI, Hebda RJ (1999) Dendroecological studies of tree growth, climate and spruce beetle outbreaks in central British Columbia, Canada. For Ecol Manag 121:215–225. doi:10.1016/S0378-1127(98)00552-0
Acknowledgements
We thank the Toledo Metroparks and the Maumee Bay State Park for their assistance and cooperation. In addition, a special thanks to Tim Fox for his assistance both in the field and in the lab. Thank you to D.A. Herms and R. Williams for their input on the project as well as friendly reviews from the lab of P.S. Curtis. Finally, we would like to thank the reviewers for their thoughtful comments. Funding was provided by The Ohio State University, School of Environment and Natural Resources MENR Project Minigrant, USDA Forest Service Northern Research Station, USDA Animal and Plant Health Inspection Service, and the USDA NRI Competitive Grants, Weedy and Invasive Species Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Aurelien SALLE
Contribution of the co-authors
Kyle Costilow and Dr. Charles Flower conducted the field work; Dr. Kathleen Knight supervised the MS thesis. All co-authors shared equally in the data analysis and preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Costilow, K., Knight, K. & Flower, C. Disturbance severity and canopy position control the radial growth response of maple trees (Acer spp.) in forests of northwest Ohio impacted by emerald ash borer (Agrilus planipennis). Annals of Forest Science 74, 10 (2017). https://doi.org/10.1007/s13595-016-0602-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-016-0602-1