Abstract
Phenological development is critical for crop adaptation. Phenology models are typically driven by temperature and photoperiod, but chickpea phenology is also modulated by soil water, which is not captured in these models. This study is aimed at evaluating the hypotheses that accounting for soil water improves (i) the prediction of flowering, pod-set, and flowering-to-pod-set interval in chickpea and (ii) the computation of yield-reducing frost and heat events after flowering. To test these hypotheses, we compared three variants of the Agricultural Production System Simulator (APSIM): (i) APSIMc, which models development with no temperature threshold for pod-set; (ii) APSIMx, which sets a threshold of 15 °C for pod-set; and (iii) APSIMw, derived from APSIMc with an algorithm to moderate the developmental rate as a function of soil water, in addition to temperature and photoperiod common to all three models. Comparison of modelled and actual flowering and pod-set of a common cheque cultivar PBA BoundaryA in 54 diverse environments showed that accuracy and precision were superior for APSIMw. Because of improved prediction of flowering and pod-set timing, APSIMw improved the computation of the frequency of post-flowering frosts compared to APSIMc and APSIMx. The number of heat events was similar for all three models. We conclude that accounting for water effects on plant development can allow better matching between phenology and environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chickpea is the world’s second most important food legume (FAOSTAT 2021) and shares nutritional and agronomic benefits with other legumes, including protein-rich seed, biological nitrogen fixation, and rotational advantages in cereal-pulse systems contributing to sustainability (Pyett et al. 2019; Palmero et al. 2022; Cutforth et al. 2009; Naderi et al. 2021; Rani and Krishna 2016; Gan et al. 2011; Saget et al. 2020). However, chickpea yield is low, hovering around 1 t/ha globally (FAOSTAT 2021), and the rate of increase in chickpea yield has been slower than for other winter crops (Joshi and Rao 2017).
The critical period for the yield of chickpea spans ~ 800 °Cd, centred at 100 °Cd after flowering (Lake and Sadras 2014). Abiotic stresses coinciding with this period constrain chickpea yield (Anwar et al. 2021; Richards et al. 2019; Peake et al. 2021; Lake and Sadras 2017, 2016). As a result, avoiding exposure to these stresses is a top priority for agronomists and breeders. Therefore, matching chickpea’s timing of the critical period to the environment is central to managing the trade-offs between low temperature for fast-developing phenotypes and high temperature and drought for their slower-developing counterparts (Lake et al. 2021; Anwar et al. 2021; Berger et al. 2004, 2003). Experiments combining varieties and sowing dates, complemented with modelling, are commonly used to investigate the relationship between timing of phenology and stress (Anwar et al. 2021; Richards et al. 2020, 2022; Peake et al. 2021; Jenkins and Brill 2012).
Experimental and modelling evidence support a substantial effect of water supply on the reproductive development of chickpea (Krishnamurthy et al. 2011; Johansen et al. 1994; Ramamoorthy et al. 2016; Singh 1991; Chauhan et al. 2019). Recent research revealed temperature-dependent and temperature-independent effects of plant water status on the reproductive development of chickpea (Li et al. 2022). Crops develop faster in drier soils relative to wetter soils, and this effect is genotype-dependent (Li et al. 2022).
Chickpea models in DSSAT (Decision Support System for Agrotechnology Transfer) and APSIM (Agricultural Production Systems sIMulator) do not account for the effects of soil water on phenology (Singh and Virmani 1996; Holzworth et al. 2014; Robertson et al. 2002; Boote et al. 2018). Chauhan et al. (2019) have advanced a model that captures the dynamic effect of soil water on flowering by moderating the thermal time experienced by the crop. This model needed broader testing, primarily at higher latitudes where delayed flowering was more commonly reported (Kumar and Abbo 2001; Berger et al. 2012). In addition, we needed to know if this model can also help explain the failure of chickpeas to set pods in some environments (Berger et al. 2012; Rani et al. 2020). Pod-set seems to fail below a daily mean temperature threshold of 15 or 21 °C (Berger et al. 2012; Croser et al. 2003) and frost compounds this issue (Chauhan et al. 2022).
This study is aimed at evaluating the hypotheses that accounting for soil water improves (i) the prediction of flowering, pod-set, and flowering-to-pod-set interval in chickpea and (ii) the computation of yield-reducing frost and heat events after flowering.
2 Methods
2.1 Field experiments
We grew the commercial variety PBA BoundaryA in experiments that combined sowing dates ranging from 28 June 2013 to 1 July 2020, and 10 locations spread between 26.6 and 34.6 °S and 138.7 and 151.8° W in south-eastern Australia (Table 1 and Fig. 1). Daily weather data from the nearest Bureau of Meteorology weather stations were sourced from the SILO website (https://longpaddock.qld.gov.au/silo/point-data/). Some experiments were irrigated before sowing or during the growing period to improve crop growth in hot and/or dry seasons. We checked the crops at least twice a week to 50% flowering when 50% and when half of the plants in a plot had at least one open flower (referred to as ‘flowering’ hereafter) and 50% pod-set when half of the plants in the plot had at least one visible pod and were expressed as days after sowing (DAS) (“pod-set” hereafter).
2.2 Model comparison
We modelled chickpea flowering and pod-set using three variants of the APSIM model (https://www.apsim.info/) (Holzworth et al. 2014, 2018). We used (i) APSIMc version 7.10, which models crop development with no temperature threshold for the pod-set; (ii) APSIMx, which sets a threshold of 15 °C for the pod-set based on the experimental observations (Clarke and Siddique 2004); and (iii) APSIMw, which incorporates an algorithm into the APSIMc model, to moderate the crop development rate by a function of soil water, in addition to temperature and photoperiod common to all three models. APSIMw does not use threshold temperature for pod-set. We included APSIMc and more recent APSIMx because these are currently the benchmark models available for chickpea. The phenology model in the APSIMc was described by Robertson et al. (2002). The APSIMx, described by Holzworth et al. (2022), uses different temperature and photoperiod parameters and thermal time requirements than APSIMc (Supplementary Tables 1 and 2). In all three models, thermal time was used to drive phenological development, calculated using a standard set of three cardinal temperatures: base = 0 °C, optimum = 30 °C, and maximum = 40 °C. The daily thermal time is accumulated into a thermal time sum, and reaching a particular target determines the phase’s duration. In APSIMw, thermal time accumulation is moderated as function of soil water. The inputs for all three models include crop management (sowing date, irrigation, and variety), daily weather data (minimum and maximum temperature, global solar radiation, and rainfall), and cultivar parameters. Cultivar parameters for PBA Boundary were obtained in experiments conducted from 2013 to 2017 (Chauhan et al. 2019).
Chauhan et al. (2019) fully described the rationale and algorithms to account for soil water effect on flowering in APSIMw, which assumes that the cultivar PBA BoundaryA (i) has a unique thermal time requirement to commence flowering and pod-set, (ii) has no temperature threshold for pod-set, and (iii) soil water moderates the thermal time accumulation to flowering and pod-set. To incorporate the effect of soil water on flowering and pod-set, we used the following two equations in the manager module of APSIMc:
TT (°Cd) is the daily thermal time, and TTm (°Cd) is the thermal time scaled by fractional available soil water (FASW) in the surface 60 cm layer from the emergence stage, which is called stage 3 in APSIMc. TTm equals TT when FASW ≤ 0.65, leading to faster thermal time accumulation when FASW ≤ 0.65. In all three models, the daily mean ambient temperature up to 30 °C adds TT of 30 °Cd and declined proportionally to become 0 °Cd when it reached the ceiling temperature of 40 °C (Robertson et al. 2002). The parameter ‘a’ in Equation 1 is a constant set at 1.65 through manual optimisation, and FASW ≥ 0.65 represents readily available water.
FASW in Equation 1 was computed in the manager module of APSIMw as the ratio of the available soil water, which is the difference between actual soil water content and the lower limit of soil water content, to the potentially available soil water, which is the difference between the upper and lower limits of soil water content, in the top 60 cm layer, as given in Equation 2.
where sw_dep(i) is the soil water content (mm) present in the soil at the time of measurement, ll15_dep(i) is the soil water (mm) content corresponding to a soil water potential of 1.5 MPa, and dul_dep(i) is the soil water content (mm) at 0.033 MPa in each layer (i) in the top 60 cm soil surface layers. The parameters for this equation were obtained from the soil cascading water balance model, capturing soil water infiltration, movement, evaporation, runoff, drainage, extractable soil water, and the total available water. Soil-specific parameters used to calculate the water budget were obtained through systematic soil sampling and characterisation in the APSoil database (Dalgliesh et al. 2012).
We calibrated a base thermal time requirement of 200 °Cd for simulating the pod-set. APSIMc does not simulate pod-set, but as the transition to this stage was driven by only temperature (Robertson et al. 2002), we, therefore, considered that the model will simulate pod-set when the thermal time target of 200 °C over the thermal target set for flowering was achieved. This target for pod-set in APSIMc was not modified by soil water and photoperiod or temperature threshold of 15 °C. This phase was assumed to be unresponsive to photoperiod in APSIMw as well. The time (days) taken to reach the thermal time target was increased depending upon the scaling of daily thermal time in Equation 1.
Flowering and pod-set were also predicted using the default parameters in APSIMx. The model assumes thermal time targets for flowering and a calendar day requirement for pod-set (Supplementary Table 3). The pod-set in the model was triggered when the crop experienced five days of temp above 16 °C or ten consecutive days at 15 °C after flowering.
For simulations, the initial soil water was set to 60% on the day of sowing except for those locations where pre-sowing irrigation was applied (Table 1). At these locations, soil water was initialised at 20% plant available water a day before the pre-sowing irrigation.
2.3 Frequencies of post-flowering frost and heat stress
Frost and heat frequencies were observed and calculated from weather data with actual and modelled flowering times using APSIMc, APSIMx, and APSIMw. Frost frequency was computed as the number of days with minimum temperature <=0 °C at 1.2 m height (Chauhan et al. 2022) and heat stress frequency as the number of days with maximum temperature >=32 °C (Devasirvatham et al. 2012).
2.4 Model performance evaluation
We compared actual and modelled flowering and pod-set with least square linear regressions and a series of parameters, including coefficient of determination (R2), the normalised root means square error (NRMSE) as precision parameters, and Lin’ concordance correlation (LinCCC) and Willmott index as model performance (accuracy) parameters. The relationship between observed (x variables)/simulated flowering and pod-set (as y variables) was quantified using a linear regression with the R programme (Team 2021). The normalised root mean square error (NRMSE) was computed using the following equations in the same programme.
where \({S}_{i}\) and \({O}_{i}\) are the simulated and the observed value, respectively; \(\overline{O }\) is the mean of the observed values; n is the number of observed values. NRMSE is expressed in % when multiplied by 100. A lower value of NRMSE indicates better precision. The Willmott index agreement proposed by Willmott et al. (2012) was computed using the following equation.
The resulting value of 1 indicated a perfect match, and 0 indicated no agreement. LinCCC (Lin 1989), another index of agreement, was computed in Excel using the following equation:
where \({\mu }_{x}\) and \({\mu }_{y}\) are the means of two variables (simulated and observed, respectively), \({\upsigma }_{x}^{2}\) and \({\upsigma }_{y}^{2}\) are the corresponding variances (simulated and observed, respectively), and ρ is the correlation coefficient between the two variables.
McBride (2005) suggested the following guidelines to infer a model’s predictive performance:
-
ρc < 0.90: poor
-
ρc > 0.90 to 0.95: moderate
-
ρc > 0.95 to 0.99: substantial
-
ρc > 0.99 is almost perfect.
3 Results
3.1 Weather
Across ten locations, the ambient mean maximum temperature ranged between 15.8 and 22.7 °C, and the minimum ambient temperature was 1.3 and 9.4 °C (Supplementary Table 3). Narrabri was the warmest location, whilst Wagga Wagga was the coolest. Breeza was the driest location, with only 71 mm in-season rainfall, and Wagga Wagga was the wettest, with up to 300 mm in-season rainfall.
3.2 Observed timing of flowering and pod-set in fifty-four site-sowing date-year combinations
Time to 50% flowering ranged between 61 and 154 DAS (Fig. 2a) and pod-set between 70 and 169 DAS (Fig. 2b). Time between pod-set and flowering varied from 5 to 55 days (Fig. 2c). The longer time from flowering to pod-set occurred in earlier sowings.
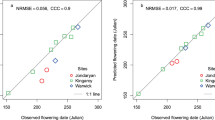
3.3 Prediction of flowering time
The predicted vs observed regression line was closer to the 1:1 line (ideal fit) for APSIMw than for APSIMc and APSIMx (Fig. 3a–c). Model precision quantified with R2 and the measurement error ranked APSIMw > APSIMx > APSIMc (Fig. 3a–c). The LinCCC and Wilmott indexes showed that there was a poorer agreement between the observed and simulated flowering times for APSIMc and APSIMx (Fig. 3a and b) than for APSIMw (Fig. 3c). The prediction of flowering times by APSIMw was generally better than APSIMc and APSIMx for early, mid, and late sowing times with predicted mean and standard deviations more closely reflecting the observed values (Supplementary Table 4).
Comparison of observed flowering (a, b, and c) and pod-set (d, e, and f) and predictions with APSIMc (a and d), APSIMx (b and e), and APSIMw (c and f) for cultivar PBA BoundaryA across ten locations (n = 54). The default model in APSIMc and APSIMx has different photoperiod and temperature parameters. The insets are the coefficient of determination of the linear relationship; normalised root means square error (NRMSE), Lin’s concordance correlation coefficient (LinCCC), and Willmott’s index (WI).
3.4 Prediction of pod-set
The regression line related to the predicted and observed time of the pod-set was closer to the 1:1 line for APSIMx and APSIMw than for APSIMc (Fig. 3d–f). The precision (R2, NRMSE) ranked APSIMw > APSIMx > APSIMc. The LinCCC and Wilmott indexes showed there was a poorer agreement between the observed and simulated pod-set for APSIMc and APSIMx (Fig. 3d and e) than APSIMw (Fig. 3f). Prediction of pod-set by APSIMw was better than APSIMc and APSIMx for all three ranges of sowings (Supplementary Table 4). The standard deviation across different sowing groups was similar between the observed and modelled values for APSIMw but different for APSIMc and APSIMx.
3.5 Flowering to pod-set interval
APSIMw reported the highest model performance for flowering to pod-set interval simulation (Fig. 4) though not as accurate as for flowering and pod-set. The values of precision parameters, including R2 (0.62) and NRMSE (0.38), were reasonable. The flowering and pod-set interval in APSIMc and APSIMx ranged from negative to positive values indicating both models, in a few cases, predicted pod-set to occur earlier than actual flowering, highlighting their limitations.
Comparison of observed flowering-to-pod-set interval and predictions with APSIMc (a), APSIMx (b), and APSIMw (c) for cultivar PBA BoundaryA (n = 54). The insets are the coefficient of determination of the linear relationship (R2), normalised root means square error (NRMSE), Lin’s concordance correlation coefficient (LinCCC), and Willmott’s index (WI).
3.6 Frequencies of post-flowering frosts and heat events
The APSIMw predicted post-flowering frosts and heat events with reasonable accuracy, especially for locations with higher frequencies of events (Fig. 5). The R2 for the prediction of frost was highest and significant only with APSIMw (0.88). The R2 of prediction for heat stress was significant only with APSIMw (0.99) and APSIMc (0.93) and not with APSIMx. Frost events after flowering calculated using default phenology models within APSIMc and APSIMx were overestimated, but heat stress events were comparable to APSIMw. Heat events after flowering were similar with APSIMw and APSIMc and slightly over or under-predicted by APSIMx. Within early, mid, and late sowing windows, frost and heat stress frequencies were identical with a similar range of variation (Supplementary Table 4). Heat stress frequencies of APSIMc were also identical to APSIMw.
4 Discussion
Photoperiod and temperature are the primary drivers of chickpea phenology (Roberts et al. 1985; Soltani et al. 2006). More recently, evidence has emerged on the role of soil water content on chickpea development. Trials in India and Australia show a positive relationship between water stress and time to flowering (Li et al. 2022; Sihag et al. 2019). Vadez et al. (2013) found no association between chickpea time to flowering (biological days) and latitude as expected from variation in temperature and photoperiod but showed that flowering was positively related to the amount of rainfall. Other studies showed that soil water, which varies with rainfall, evaporative demand, and soil characteristics, influences the phenological development of chickpea (Ramamoorthy et al. 2016; Chauhan et al. 2019; Richards et al. 2020).
In our study, the inclusion of soil water improved the prediction of flowering and pod-set over predictions based on ambient temperature and photoperiod alone (Fig. 3). The prediction of the timing of flowering could be further improved with site-specific weather and soil attributes and genotype-specific parameters (Chauhan et al. 2019). Additionally, there could be an error in visually recording flowering time due to some subjectivity of individuals recording these events (Maphosa et al. 2020). A standard protocol was applied to reduce bias in recording phenology.
In this study, we extended the effect of soil water influencing pod-set timing. Currently, pod-set in the chickpea crop is mainly limited by the mean ambient temperature below 15 °C when soil moisture is adequate (Croser et al. 2003). The later work from Western Australia indicated this threshold to be 21 °C (Berger et al. 2012). In Kingaroy, pod-set occurred even when the mean ambient temperature was <15 °C (Yash Chauhan, unpublished results). This observation suggests that caution is needed in using temperature thresholds, mainly when temperature interacts with soil water and day length. APSIMx predicted the time to pod-set better than APSIMc by delaying pod-set until the average temperature of 10 consecutive days exceeded 15 °C (i.e., 150 °Cd for the first pod initiation). In our study, we set a minimum thermal time target of 200 °Cd for pod-set for PBA BoundaryA, and thermal time was scaled by soil water to account for the delay. This thermal time target of 200 °Cd could be a varietal trait, but its scaling by soil water could also vary amongst varieties which remain to be investigated. APSIMw’s ability to predict flowering and pod-set has been verified with three other chickpea varieties, including PBA HattrickA, Amethyst, and Tyson (Yashvir Chauhan, DAF, unpublished, 2023).
Improved phenological prediction by accounting for soil moisture is consistent with empirical observations (see Introduction). Therefore, water availability has a dual role: in growth (Anwar et al. 2003) and development (Chauhan et al. 2019). Wet soil delays flowering and increases the number of pod-bearing nodes and hence grain number later in the season (Lake and Sadras 2016). Alternatively, earlier flowering in dry soil will reduce the risk of drought and heat during the critical period, at the expense of increased risk of lower temperature compromising the yield potential (Li et al. 2022). Soil water promotes the production of infertile pseudo flowers (Smithson et al. 1985). The production of these infertile flowers could lead to a delayed pod-set (Roberts et al. 1985).
For this reason, farmers in India do not irrigate chickpea when it is close to flowering (Khanna-Chopra and Sinha 1987). These hard-to-notice pseudo-flowers can also result in misinterpretation of flowering time (Roberts et al. 1985). Given that short-photoperiod (<12 h) and low night temperature also promote the development of pseudo-flowers (Roberts et al. 1980; Or et al. 1999), we speculate that the effect of water availability might be partially associated with genes modulating the formation of fertile flowers in response to photoperiod and low temperature. These pseudo-flowers can also be produced under very high temperatures (Jumrani and Bhatia 2014). Saini et al. (2022) reported that chickpea plants primed with drought at the vegetative stage had better reproductive functioning. The connection between pseudo-flower production in wetter soils and low to elevated temperatures and short photoperiods should be investigated as it could provide a better understanding of the ecological basis of adaptation of chickpea.
The gap between flowering and pod-set varies both within and across locations and with sowing time (Fig. 2). When this gap increases, the growing duration also increases, which results in the exposure of the crop to a greater degree of drought and heat stress (Maphosa et al. 2020; Graham et al. 2022). Reducing chickpea sensitivity to low temperatures has been the focus of research efforts in Australia (Croser et al. 2003; Berger et al. 2005). Our study provides indirect evidence that soil water influences this gap. The prediction of the time between flowering and pod-set was more accurate with APSIMw (Fig. 4) than the other two models, including APSIMx, that incorporate a temperature threshold. In the context of the failed attempts to improve pod-set under low temperatures, focusing on podding in response to soil water could be more fruitful, as significant intraspecific variation has been reported (Li et al. 2022).
The implication of the accuracy and precision in predicting phenology became more apparent when we computed frequencies of post-flowering frost and heat events with and without soil water input. The number of post-flowering frosts computed was threefold more with APSIMc than their actual occurrences, as this model predicted flowering much earlier than the actual observed date. Flowering and pod-set prediction without considering soil water effects would lead to recommending delayed sowing to reduce the frequency of yield-reducing frosts. The significant delays in sowing could lead to a lower yield than what could be obtained when frequencies of frosts are predicted more accurately.
Research on how soil water affects chickpea phenology has been reported sporadically. This study highlights the need for more systematic research to understand its impact on chickpea’s adaptation to climate, including its interactions with other drivers of phenology like photoperiod and temperature. By interpreting the observed responses of a single cultivar in a range of environments using a modelling approach, this study provides a compelling case for investigating various chickpea cultivars to determine if phenological responses differ in response to soil water and whether this information can be used to improve cultivar performance and agronomy.
5 Conclusions
The study highlights the importance of accounting for soil water in predicting the timing of flowering and pod-set in chickpea. A better prediction of the critical period would improve the pairing of sowing date and cultivar to manage the trade-offs between frost, drought, and heat stress. Priorities for trait-based crop improvement would benefit from considering the effect of soil water modulating chickpea development. An additional focus on the effects of soil water on chickpea phenology (Saini et al. 2022) can contribute to reducing the flowering-to-pod gap that is critical for yield, in addition to ongoing research focus on increasing chilling tolerance of cultivated chickpea (Mir et al. 2019). Our results suggest that accounting for the effect of soil water on phenological development will improve not only modelling but also allow for better management of the risk of frost and heat stress management, agronomy and possibly breeding.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Code availability
Not applicable.
References
Anwar MR, McKenzie B, Hill G (2003) Water-use efficiency and the effect of water deficits on crop growth and yield of Kabuli chickpea (Cicer arietinum L.) in a cool-temperate subhumid climate. J Agric Sci 141(3–4):285–301
Anwar MR, Luckett DJ, Chauhan YS, Ip RHL, Maphosa L, Simpson M, Warren A, Raman R, Richards MF, Pengilley G (2021) Modelling the effects of cold temperature during the reproductive stage on the yield of chickpea (Cicer arietinum L.). Int J Biometeorol 66:115–125
Berger J, Turner N, Siddique K, Knights E, Brinsmead R, Mock I, Edmondson C, Khan T (2004) Genotype by environment studies across Australia reveal the importance of phenology for chickpea (Cicer arietinum L.) improvement. Aust J Agric Res 55(10):1071–1084
Berger JD, Buck R, Henzell JM, Turner NC (2005) Evolution in the genus Cicer—vernalisation response and low temperature pod set in chickpea (C. arietinum L.) and its annual wild relatives. Aust J Agric Res 56(11):1191–1200
Berger J, Kumar S, Nayyar H, Street K, Sandhu JS, Henzell J, Kaur J, Clarke H (2012) Temperature-stratified screening of chickpea (Cicer arietinum L.) genetic resource collections reveals very limited reproductive chilling tolerance compared to its annual wild relatives. Field Crop Res 126:119–129
Berger J, Turner NC, French R (2003) The role of phenology in adaptation of chickpea to drought. In: Australian Agronomy Conference, Geelong, Victoria, Australia, 2-6 February 2003. Australian Agronomy Society, p 4
Boote K, Prasad V, Allen L Jr, Singh P, Jones J (2018) Modeling sensitivity of grain yield to elevated temperature in the DSSAT crop models for peanut, soybean, dry bean, chickpea, sorghum, and millet. Eur J Agron 100:99–109
Chauhan YS, Ryan M, Chandra S, Sadras VO (2019) Accounting for soil moisture improves prediction of flowering time in chickpea and wheat. Sci Rep 9(1):7510
Chauhan YS, Allard S, Krosch S, Ryan M, Rachaputi R (2022) Relationships of frequencies of extreme low temperatures with grain yield of some Australian commercial chickpea cultivars. Int J Biometeorol 66(10):2105–2115
Clarke H, Siddique K (2004) Response of chickpea genotypes to low temperature stress during reproductive development. Field Crop Res 90(2–3):323–334
Croser J, Clarke H, Siddique K, Khan T (2003) Low-temperature stress: implications for chickpea (Cicer arietinum L.) improvement. Crit Rev Plant Sci 22(2):185–219
Cutforth H, Angadi S, McConkey B, Entz M, Ulrich D, Volkmar K, Miller P, Brandt S (2009) Comparing plant water relations for wheat with alternative pulse and oilseed crops grown in the semiarid Canadian prairie. Can J Plant Sci 89(5):823–835
Dalgliesh N, Cocks B, Horan H APSoil-providing soils information to consultants, farmers and researchers. In: 16th Australian Agronomy Conference, Armidale, NSW, 2012.
Devasirvatham V, Tan D, Gaur P, Raju T, Trethowan R (2012) High temperature tolerance in chickpea and its implications for plant improvement. Crop Pasture Sci 63(5):419–428
FAOSTAT (2021) FAO. Italy, Rome
Gan Y, Liang C, Hamel C, Cutforth H, Wang H (2011) Strategies for reducing the carbon footprint of field crops for semiarid areas. A Review. Agron Sustain Dev 31(4):643–656
Graham N, Raman R, Warren1 A, Anwar M (2022) Timing of flowering and pod initiation influences yield potential in chickpeas. GRDC Grains Research Online Update paper, 25 February 2022. Available at https://www.icanrural.com.au/documents/Northern%20GRDC%20Grains%20Research%20Updates%20online%202022%20week%202.pdf#page=109
Holzworth DP, Huth NI, deVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C (2014) APSIM–evolution towards a new generation of agricultural systems simulation. Environ Model Softw 62:327–350
Holzworth D, Huth NI, Fainges J, Brown H, Zurcher E, Cichota R, Verrall S, Herrmann NI, Zheng B, Snow V (2018) APSIM next generation: overcoming challenges in modernising a farming systems model. Environ Model Softw 103:43–51
Holzworth D, Huth N, Holzworth D (2022) A new generation of APSIM. Paper presented at the Proceedings of the 20th Australian Agronomy Conference, Toowoomba, Queensland, Australia
Jenkins L, Brill R (2012) The effect of time of sowing on phenology and yield of chickpeas at Trangie Central West, NSW, 2011. In: Capturing opportunities and overcoming obstacles in Australian agronomy. Proceedings of 16th Australian Agronomy Conference 2012, 14-18 October 2012, Armidale, New South Wales, Australia, Australian Society of Agronomy Inc. 2012
Johansen C, Krishnamurthy L, Saxena N, Sethi S (1994) Genotypic variation in moisture response of chickpea grown under line-source sprinklers in a semi-arid tropical environment. Field Crop Res 37(2):103–112
Joshi P, Rao PP (2017) Global pulses scenario: status and outlook. Ann N Y Acad Sci 1392(1):6–17
Jumrani K, Bhatia VS (2014) Impact of elevated temperatures on growth and yield of chickpea (Cicer arietinum L.). Field Crop Res 164:90–97
Khanna-Chopra R, Sinha S (1987) Chickpea: Physiological Aspects of Growth and Yield. In: Saxena M, Singh KB (eds). The Chickpea. CAB International, Wallingford, Oxon, pp 163–189
Krishnamurthy L, Gaur P, Basu P, Chaturvedi S, Tripathi S, Vadez V, Rathore A, Varshney R, Gowda C (2011) Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genetic Resources 9(1):59–69
Kumar J, Abbo S (2001) Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments. Adv Agron 72:107–138
Lake L, Sadras VO (2014) The critical period for yield determination in chickpea (Cicer arietinum L.). Field Crop Res 168:1–7
Lake L, Sadras VO (2016) Screening chickpea for adaptation to water stress: associations between yield and crop growth rate. Eur J Agron 81:86–91
Lake L, Sadras V (2017) Associations between yield, intercepted radiation and radiation-use efficiency in chickpea. Crop Pasture Sci 68(2):140–147
Lake L, Chauhan YS, Ojeda J, Cossani C, Thomas D, Hayman P, Sadras V (2021) Modelling phenology to probe for trade-offs between frost and heat risk in lentil and faba bean. Eur J Agron 122:126154
Li Y, Lake L, Chauhan Y, Taylor J, Sadras V (2022) Genetic basis and adaptive implications of temperature dependent and temperature-independent effects of drought on chickpea reproductive phenology. J Exp Bot. https://doi.org/10.1093/jxb/erac195
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 255–268
Maphosa L, Richards MF, Norton SL, Nguyen GN (2020) Breeding for abiotic stress adaptation in chickpea (Cicer arietinum L.): A comprehensive review. Crop Breeding, Genetics and Genomics 2(4):e200015. https://doi.org/10.20900/cbgg20200015
McBride G (2005) A proposal for strength-of-agreement criteria for Lin’s concordance correlation coefficient. NIWA Client Report: HAM2005-062. Available at: www.medcalc.org/download/pdf/McBride2005.pdf
Mir AH, Bhat MA, Fayaz H, Dar SA, Maqbool S, Bhat NA, Thudi M, Mir RR (2019) Assessment of cold tolerance in chickpea accessions in North-Western Himalayas of Jammu and Kashmir India. J Pharm Phytochem 8(4):2268–2274
Naderi R, Bijanzadeh E, Egan TP (2021) Short-term response of chickpea yield, total soil carbon, and soil nitrogen to different tillage and organic amendment regimes. Commun Soil Sci Plant Anal 52(9):998–1007
Or E, Hovav R, Abbo S (1999) A major gene for flowering time in chickpea. Crop Sci 39(2):315–322
Palmero F, Fernandez JA, Garcia FO, Haro RJ, Prasad PV, Salvagiotti F, Ciampitti IA (2022) A quantitative review into the contributions of biological nitrogen fixation to agricultural systems by grain legumes. Eur J Agron 136:126514
Peake A, Meier E, Bell K, Whish J, Dreccer MF, Swan T, Sands D, Agius P, Moodie M, Gardner M (2021) Optimising chickpea sowing and flowering dates for maximum yield. Grains Research Update: 44. Accessed at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2021/03/optimising-chickpea-sowing-and-flowering-dates-for-maximum-yield
Pyett S, de Vet E, Trindade L, van Zanten H, Fresco L (2019) Chickpeas, crickets and chlorella: Our future proteins. Wageningen Food & Biobased Research. Accessed at https://edepot.wur.nl/496402
R Core Team (2021) R: A language and environment for statistical computing. Published online 2021. https://www.r-project.org/
Ramamoorthy P, Lakshmanan K, Upadhyaya HD, Vadez V, Varshney RK (2016) Shoot traits and their relevance in terminal drought tolerance of chickpea (Cicer arietinum L.). Field Crop Res 197:10–27
Rani BS, Krishna TG (2016) Response of chickpea (Cicer arietinum L.) varieties to nitrogen on a calcareous vertisols. Indian J Agric Res 50(3):278–281
Rani A, Devi P, Jha UC, Sharma KD, Siddique KH, Nayyar H (2020) Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front Plant Sci 10:1759
Richards MF, Preston AL, Napier T, Jenkins L, Maphosa L (2020) Sowing date affects the timing and duration of key chickpea (Cicer arietinum L.) growth phases. Plants 9(10):1257
Richards MF, Maphosa L, Preston AL (2022) Impact of sowing time on chickpea (Cicer arietinum L.) biomass accumulation and yield. Agronomy 12(1):160
Richards M, Maphosa L, Preston A, Napier T, Hume I (2019) Pulse adaptation–optimising grain yield of chickpea and lentils
Roberts E, Summerfield R, Minchin F, Hadley P (1980) Phenology of chickpeas (Cicer arietinum) in contrasting aerial environments. Exp Agric 16(4):343–360
Roberts E, Hadley P, Summerfield R (1985) Effects of temperature and photoperiod on flowering in chickpeas (Cicer arietinum L.). Ann Bot 55(6):881–892
Robertson M, Carberry P, Huth N, Turpin J, Probert ME, Poulton P, Bell M, Wright G, Yeates S, Brinsmead R (2002) Simulation of growth and development of diverse legume species in APSIM. Aust J Agric Res 53(4):429–446
Saget S, Costa M, Barilli E, de Vasconcelos MW, Santos CS, Styles D, Williams M (2020) Substituting wheat with chickpea flour in pasta production delivers more nutrition at a lower environmental cost. Sustain Prod Consum 24:26–38
Saini R, Das R, Adhikary A, Kumar R, Singh I, Nayyar H, Kumar S (2022) Drought priming induces chilling tolerance and improves reproductive functioning in chickpea (Cicer arietinum L.). Plant Cell Rep 41(10):2005–2022
Sihag R, Kumar P, Singh K, Kumar J, Kumar A (2019) Growth and productivity of chickpea genotypes under different soil moisture environment. Indian J Agric Res 53(6):708–712
Singh P (1991) Influence of water-deficits on phenology, growth and dry-matter allocation in chickpea (Cicer arietinum). Field Crop Res 28(1–2):1–15
Singh P, Virmani S (1996) Modeling growth and yield of chickpea (Cicer arietinum L.). Field Crops Res 46(1–3):41–59
Smithson J, Thompson J, Summerfield R, Roberts E (1985) Grain legume crops. Summerfield, RJ and Roberts, EH (eds):312
Soltani A, Hammer G, Torabi B, Robertson M, Zeinali E (2006) Modeling chickpea growth and development: phenological development. Field Crop Res 99(1):1–13
Vadez V, Soltani A, Sinclair TR (2013) Crop simulation analysis of phenological adaptation of chickpea to different latitudes of India. Field Crop Res 146:1–9
Willmott CJ, Robeson SM, Matsuura K (2012) A refined index of model performance. Int J Climatol 32(13):2088–2094
Acknowledgements
YSC and SK thank the Department of Agriculture and Fisheries, Queensland, for providing an innovation grant to model soil water’s effect on chickpea phenology. NG, MRA, MFR, and RR thank the NSW Department of Primary Industries (NSWDPI) and the Grains Research and Development Corporation (GRDC) for providing the funding under the projects BLG111, BLG112, and BLG118. GRDC project UOT1909-002RTX funded VOS and LL work on chickpea phenology. The authors also would like to acknowledge the APSIM Initiative for providing APSIM software used in this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Part of this work was supported by the Department of Agriculture and Fisheries Innovation Project Grant through YSC. The field work to collect data collection on phenology in New South Wales and Queensland locations was funded by the Grains Agronomy & Pathology Partnership (GAPP), a co-funded agreement between NSWDPI through NG, MRA, MFR, and RR and the Grains Research and Development Corporation (GRDC) under the projects BLG111, BLG112, and BLG118. GRDC project UOT1909-002RTX funded VOS and LL work on phenology in South Australia.
Author information
Authors and Affiliations
Contributions
YSC, MRA, MFR, RR, NG, DJL, SK, VOS, and LL designed the research, YSC conceptualised the model analysis, and all authors wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Field data were shared under a GRDC generic material transfer agreement between NSWDPI and DAF, Queensland.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Photoperiod and temperature-based models were incomplete predictors of chickpea phenology.
• Including soil water in the APSIM model improved the prediction of timing of flowering and pod-set.
• Improved prediction of flowering and pod-set led to improved computation of the frequency of frost and heat-stress events.
• The matching of phenology and environments needed to account for the effect of water on development.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chauhan, Y.S., Anwar, M.R., Richards, M.F. et al. Effect of soil water on flowering and pod-set in chickpea: implications for modelling and managing frost and heat stress. Agron. Sustain. Dev. 43, 49 (2023). https://doi.org/10.1007/s13593-023-00903-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-023-00903-x