Abstract
How beekeepers can propagate the Varroa-resistant traits they have in their colonies depends on how varroa resistance, i.e. the key hygienic behavioural traits, is passed onto the next generation. This study investigates if the key hygienic traits are passed between workers via learning as is known to happen in bumble bees, or are the resistant traits encoded into the queens and thus her offspring. To test this, we re-queened known mite-resistant colonies with mite-naïve (susceptible) queens in both Hawaii and the UK. We also placed resistant queens in susceptible colonies in the UK. After 5 months in Hawaii and 12 months in the UK, mite levels in adults and brood were measured. In Hawaii, mite removal and cell recapping levels were also assessed. In both locations, the mite levels in colonies headed by suspectable (mite-naïve) queens or their daughters significantly exceeded that found in colonies headed by resistant queens or their daughters. The initial presence of resistant or suspectable workers did not affect the result. Therefore, to propagate mite-resistant traits, beekeepers only need to re-queen a colony with a locally mated queen from an established resistant population, as some UK and Hawaiian beekeepers are already doing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, the ectoparasitic mite Varroa destructor remains the main pest that beekeepers must regularly deal with. In Northern Hemisphere countries, mite populations are usually controlled using a range of miticides that are administered by beekeepers at least annually. In the UK, oxalic acid, thymol, amitraz, and formic acid account for 80% of the miticides used (Valentine and Martin 2023). In many Southern Hemisphere countries, mite treatments are either too expensive, not available, or their honey bees have just been left to adapt to the mite naturally. For example, both South Africa (Allsopp 2006) and Cuba (Luis et al. 2022) never used miticides but instead allowed V. destructor resistance to develop, and after several years of losses, colonies developed mite resistance. Most Northern Hemisphere-managed colonies are now locked into long-term treatments to control their in-hive mite populations. Thus, beekeepers need a more controlled approach to transition towards managing mite-resistant honey bees.
Recent studies in Europe (Oddie et al. 2018), the UK (Hawkins and Martin 2021), South Africa (Allsopp 2006), the Caribbean (Luis et al. 2022), and Latin America (Martin et al. 2019) all found that mite-resistant populations have developed resistance to V. destructor using the same mechanism (Grindrod and Martin 2021). Briefly, resistant honey bee workers can perceive that a group of unique compounds (ketones and acetates) found in cells are associated with the presence of mite offspring (Mondet et al. 2021). This leads to increased targeted removal via cannibalism of the infested pupae by the worker bees. Although the mother mite escapes, her immature offspring all perish since the detection and removal of mite-infested cells typically occurs many days before bee eclosion. V. destructor mites are only able to perform two to three reproductive cycles (Fries and Rosenkranz 1996; Martin and Kemp 1997) due to the limited number of eggs they can produce (Akimov and Yastrebtsov 1984; Mikityuk 1979). The persistent loss of offspring leads to increased rates of infertility in the mites (although some virgin females can mate with their own son, this is rare [Häußermann et al. 2020]), which leads to lower mite population growth and therefore lower viral loads (de Souza et al. 2020; Mendoza et al. 2020). The increased detection of mite-infested brood also leads to increased recapping rates since recapping is spatially associated with mite-infested cells (Grindrod and Martin 2021).
A survey of 2897 UK beekeepers in 2020 found at least 6% of beekeepers had been managing their honey bees without any mite treatments for over a decade (Valentine and Martin 2023). In addition, around 25% of the respondents were trying to become treatment-free beekeepers. To help the beekeepers transition to treatment-free beekeeping, it is important to understand how mite resistance is passed on successfully between each generation of honey bees. Beekeepers can buy newly mated queens or packages of bees (workers plus the newly mated queen). Learning by observation of a new behaviour between workers has been shown in bumblebees (Loukola et al. 2017) and several other insects (Adam et al. 2022; Leadbeater and Chittka 2007). Therefore, if the detection and removal of mite-infested worker cells is a worker-worker learnt trait, then packages of bees would need to be purchased, whereas if the key hygienic traits have become fixed in the queen and thus passed onto her workers, then the cheaper and easier option of selling locally mated queens derived from resistant colonies would be possible. This is important since the raising and selling of new queens is an already well-established worldwide industry.
The aim of this study was to investigate the role of workers versus the queen in passing on resistant behaviours to the next generation of honey bees. This was achieved by re-queening resistant colonies with susceptible mite-naïve queens in both the UK and Hawaii, USA. In addition, in the UK, we also were able to re-queen mite-naïve colonies with resistant queens.
2 Material and methods
2.1 Study sites setup
Due to coronavirus and legal restrictions, a fully bi-directional experiment exchanging resistant and mite-naïve (susceptible) queens was not possible since it is illegal or irresponsible to introduce V. destructor into any established mite-naïve honeybee population. Therefore, the studies were conducted within well-established mite-resistant populations that had not been treated for over 10 (UK) or 8 (USA) years. Once re-queened, the existing worker population would be gradually replaced with the genetics of the new queen; thus, during that period, there would be an opportunity for the old workers to teach the new workers how to detect and remove infested cells. Since the environmental conditions at each study site were very different (temperate vs sub-tropical), the study periods were of different duration.
2.2 Hawaii
In January 2022, 11 colonies were randomly selected from over 100 colonies in a single apiary on the Hawaiian Island of Oahu that had not been treated for at least 8 years. The 11 study colonies each had their levels of recapping, mite brood infestation, number of phoretic mites, and their ability to remove mite-infested worker cells evaluated at the start of the study, all of which confirmed their resistant status. Then, six mite-naïve colonies from Kauai Island had their recapping levels measured. Due to importation restrictions, we moved six mated queens (without attendant workers) from the Kauai apiary to the study apiary under license. The 11 resistant study colonies were ranked on brood infestation levels, and the six odd-ranked colonies were de-queened before introducing the six Kauai queens. Five of the queens were accepted and continued egg-laying. Over the next 4 months, the five colonies headed by Kauai queens and five resistant colonies were left to develop. On 7 May 2022, the recapping levels, brood infestation, number of phoretic mites, and removal of mite-infested worker pupae were all re-evaluated in the remaining ten study colonies. Although one colony with a Kauai queen died in early May 2022 due to V. destructor, as evidenced by bees with deformed wings, lack of bees, and high mite levels, despite this, we were still able to measure recapping levels in this colony.
2.3 England
The UK study was conducted in Worcestershire, England, where a previous study (Hawkins and Martin 2021) had found both high recapping rates and low mite infestation in this resistant population. The beekeeper had around 70 colonies in 10 different apiaries that had not been treated for over 10 years, confirming their resistance. The apiaries covered an area of approximately 75 square miles (195 km2), but an additional survey of recapping rates in a further five neighbouring apiaries indicated that they were also very likely to be mite-resistant with recapping rates of infested worker brood greater than 55%. This extends the area of the resistant population to around 200 square miles (500 km2).
On 2 September 2020, four mite-naïve queen-right five-framed nuclei colonies from the V. destructor-free Isle of Colonsay, Scotland, plus two spare Colonsay queens were transported overnight to the Worcestershire study apiary that already contained six resistant queen-right five-framed nuclei. In early September, the six marked mite-naïve Colonsay queens were introduced into the six nuclei containing resistant workers, while four marked resistant queens were placed into the queen-less colonies containing mite-naïve workers (Figure 1). During the winter, one colony headed by a Colonsay queen died with another Colonsay queen dying during summer. In spring, a Colonsay queen was split for swarm control and its daughter was mated locally, i.e. most likely with a resistant drone, and kept in the trial. The four nuclei containing Colonsay workers and a resistant queen all swarmed in early April 2021. One swarm was caught and re-hived. The remaining three colonies raised daughter queens that were mated locally, i.e. with resistant drones. On 13 July 2021, two more mite-naïve queen-right colonies from Colonsay were brought to Worcestershire. These were re-queened with resistant queens, and two Colonsay queens were introduced into the two resistant colonies that had been de-queened, but one Colonsay queen was rejected and removed from the study. On 8 September 2021, sealed brood samples (200–300 cells) were collected from the five colonies (four queens plus one daughter) headed by Colonsay queens (Q-N) and four resistant daughters (Q-R) that had sufficient sealed brood to be sampled to calculate its infestation rate. After 1 year on 14 October 2021, all experiment colonies (five naive queens plus one daughter and one resistant queen plus six daughters) had their phoretic mite populations calculated since all the colonies had no or very little worker brood present at that time.
A general summary of the experimental setup of the queen swap study in both the UK and Hawaii. Green represents resistant workers or queens/daughters and red V. destructor-naïve workers or queens/daughters. Q = queen, W = worker, N = V. destructor-naïve, and R = V. destructor-resistant. The numbers in brackets indicate the number of colonies.
2.4 Measuring rates of recapping, removal, and phoretic mites
The sugar shake method (Dietemann et al. 2013) was used to estimate the number of phoretic mites using approximately 700 bees (UK) and 300 bees (Hawaii). Samples were weighed to determine the number of bees sampled.
The mite removal and recapping experiments were only performed in Hawaii due to covid logistical reasons and measured in January 2022 and again in May 2022. Measuring recapping followed the well-established method (Oddie et al. 2018; Martin et al. 2019). Briefly, the cell cap of a worker brood aged yellow thorax or older was carefully removed and inverted. This typically revealed the shiny silk cocoon covering the entire underside of the cap, indicating the normal untouched state of the cell cap. However, if the cell cap had at some point been opened and recapped, the central part of the silk cocoon would be missing and have been replaced by darker coloured wax during the recapping process. The size of the recapped area was estimated on a scale of 1 to 5 mm, with 5 mm indicating the entire cell cap had been removed in the past. In Hawaii, 107–158 cells were sampled per colony, due to consistently high recapping levels in this population. However, due to the low infestation rate and small number of cells sampled (around 120 per colony), we combined the number of infested and non-infested cells in each group before data analysis.
The mite removal experiment was conducted by inserting 15 individual mites into a frame containing a recently sealed worker brood, along with 15 sham ‘control’ openings. The mites were obtained from a recently sealed drone brood that was still in the larvae or white-eyed pupae stage, i.e. mites actively laying eggs. The hope was that by inserting reproductive mites rather than phoretic mites, the removal rates would be more realistic since we know that honey bees detect compounds produced by the mite offspring and potentially not the mother mite (Mondet et al. 2021). Eight days later, the cells were assessed for removal of pupae. Due to the small number of mites inserted (n = 15) per colony, all the data was combined per group and the total number of cells not removed was analysed.
2.5 Statistical analysis
As the data were not normally distributed a Kruskal–Wallis One-way ANOVA with Post Hoc Mann–Whitney U tests were used to compare the infestation level of adult bees. A One-way ANOVA with Tukey’s post hoc test was used to compare the recapped hole size between the infested and non-infested cells, since this data were normally distributed. To compare the size of the recapped hole between infested and non-infested cells within each colony t-tests were used. Chi-squared tests were used to compare the number of infested versus non-infested worker cells, levels of recapping and mite removal rates between colonies with resistant or varroa-naïve queens.
3 Results
3.1 Mite infestation studies
3.1.1 Hawaii
There was an overall significant difference between the number of phoretic mites per 100 workers in colonies headed by resistant queens and mite-naïve queens in May (H(2,17) = 7.01, p = .03). The post hoc Mann–Whitney U test showed no significance change in phoretic mite numbers between January and May in the resistant colonies, but a significant increase in phoretic mites (p = .016) between the six resistant colonies (January and May) and the four remaining colonies headed by mite-naïve queens (Figure 2a).
Significant increase in the number of phoretic mites in the colonies headed by naïve queens/daughters (red bars) compared to colonies headed by resistant queens (green bars) both in a Hawaii and b the UK. The same pattern was also seen in the V. destructor infestation levels of worker brood both in c Hawaii and d the UK over the study period. The numbers in the parenthesis indicate the number of colonies used to calculate the value.
The mite-infested worker cells showed the same pattern as the phoretic mite numbers (Figure 2c). When comparing the total number of infested versus non-infested worker cells in each group, overall, there was a significant difference between the four groups (X2[1, n=2664] = 27.26, p = .00001). Further comparisons showed no significant difference (X2[1, n=2043] = .676, p = .41) between the January and May resistant groups, but a significant increase (X2[1, n=1327] = 12.98, p = .0003) in the naïve queen group in May’s infested worker brood infestation levels (Figure 2c).
3.1.2 UK
Since the removal of the mite-naïve daughter queen or the three additional July 2021 colonies had no significant effect (p < .03) on either the adult or brood mite infestation data (supplemental data), all data was used. A similar pattern was observed in the UK since, at the end of the study, significantly higher (H(1,12) = 9, p = .0027) mite infestation levels of adult bees were found in the six colonies headed by mite-naïve queens, or their daughter, compared to the seven colonies headed by a mite-resistant queen or six daughters (Figure 2b).
For the infestation level of worker brood (Figure 2d), there were significant differences between the four groups (X2[3, n=5309] = 1171.31, p < .00001); further comparisons showed no significant difference in the worker brood infestation level in the seven colonies headed by resistant queens/daughters after 1 year, whereas there was a significant increase (p < .00001) in the worker brood infestation level in the six colonies headed by mite-naïve queens.
3.2 Recapping: Hawaii (only)
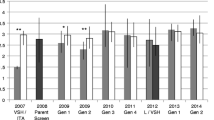
The level of recapping in the five Kauai colonies was determined by inspecting 3090 worker cell caps. The median and mean recapping levels were 1.3% and 3.3% respectively due to a single colony having a recapping level of 11%. Total recapping levels remained consistent in the resistant colonies (59% in January and 60% in May) (Figure 3a). Although there was a large increase from 3% in recapping from the mite-free colonies to 34% in mite-infested colonies, it was still significantly below (X2[1, n=199] = 12.1, p < .0005) that found in resistant colonies (Figure 3a). Within every colony (n = 22), the percentage of infested cells recapped was always greater than that found in non-infested cells (Figure 3b).
a The total recapping activity (yellow and red strips) in the resistant colonies remained stable between January and May. Whereas the workers of naïve queens increased recapping from V. destructor-free colonies (yellow) to those that were infested (yellow and red strips) but still significantly below the level of the resistant queens. b In all groups, infested cells (red) were recapped significantly more than non-infested cells (yellow). c The sizes of the recapped holes of infested worker cells were always significantly larger in colonies headed by resistant queens, but not in colonies headed by mite-naïve queens.
The mean diameter of the recapped hole was consistently greater in infested brood than in non-infested brood (Figure 3c). Analysis of all the data indicated a significant difference between the means (f(6, 1408) = 35.9, p < .00001) of the seven groups. Tukey’s post hoc tests showed significantly larger holes in the infested cells of the resistant colonies in both January (Q = 6.65, p < .0001) and May (Q = 12.15, p < .00001). However, in the mite-naïve group, there was no significant difference in diameter between the infested and non-infested cells (Q = 3.98, p = .07), although there was a significant increase in hole size from January to May (Q = 7.21, p < .00001) (Figure 3c).
3.3 Mite removal study: Hawaii (only)
There was no significant difference between the high removal rates of artificially infested cells in the resistant colonies between January 2022 and May 2022 (X2[1, n=246] = 0.188, p = .66). However, the percentage of artificially mite-infested cells removed in May was significantly lower in the mite-naïve colonies (X2[1, n=152] = 10.33, p = .001) compared to resistant queen colonies. The number of control cells removed, dropped significantly (X2[1, n=239] = 4.7, p = .03) in the resistant colonies between January 2022 and May 2022. However, there was no significant difference (X2[1, n=152] = 0.59, p = 0.44) in the removal of control cells between the resistant and naïve colonies in May (Fig. 4).
4 Discussion
Despite the environmental differences between the UK and Hawaii, the results from both locations confirmed that V. destructor levels on the adult bees and in the worker brood increased significantly in colonies headed by mite-naïve queens or their daughters. In contrast, mite levels remained constant in colonies headed by a resistant queen or their daughters. All the colonies headed by mite-naïve queens/daughters died, in Hawaii within 6–8 months or the start of the study and in the UK during the following winter. The percentage of mites in both adults and worker brood in the UK naïve queen colonies was almost double that of found in Hawaii. This likely reflects the lack of worker brood towards the end of the UK season, whereas, in Hawaii, worker brood was produced continuously and increased throughout the experiment. The key trait of ‘removal of infested cells’ in Hawaii showed a consistently high (70%) level in the resistant colonies, whereas it was significantly lower (45%) in the colonies headed by mite-naïve queens. This lower level of mite removal was similar to that found by Hawkins and Martin (2021) where V. destructor-naïve colonies from the Isle of Man removed 39%, 37%, 33%, and 7% of the live mites used to artificially infest worker cells.
All colonies headed by resistant queens survived; however, the colony dynamics differed between the two sites. In the UK, colonies headed by resistant queens swarmed, and the resulting six daughter colonies all survived. In Hawaii, the colonies headed by a resistant queen were healthy but had not swarmed when the study ended. This difference in colony dynamics was likely due to the length of the study periods and to a very unusual UK season in which several of the colonies swarm very early in April; nonetheless, all the new queens mated successfully. The colonies headed by the naïve (Colonsay) queens in the UK had approximately half the amount of brood and were not as strong as those headed by the resistant queens at their first inspection in April 2021.
The percentage of recapping in the Hawaii resistant control colonies remained higher than in the colonies headed by naïve queens. However, those colonies headed by naïve queens, and hence later naïve workers, showed an unexpected increase in the recapping of the worker-infested cells compared to the original levels of recapping in their mite-free populations. Nevertheless, the comparatively lower level of infested brood removal by naïve workers led to significant increases in the mite population and ultimately the demise of their colony. This may suggest that they first developed the ability to investigate cells with unusual odours but had yet to fully develop the key behaviour of hygienic ‘removal via cannibalisation’ response.
Mondet et al. (2021) showed that although many bees can detect the ketones and acetates coming from an infested cell, only colonies with workers that can link the perceived odour with the presence of V. destructor have become resistant. One possible mechanism is that this trait could be first perceived or ‘learnt’ by workers and then passed onto the queen before becoming hardwired into her genetics. There are several epigenetic processes including transcription factor binding, histone post-translational modifications, DNA methylation, and regulation by non-coding RNAs that all function in concert to stabilise phenotypic responses to transient environmental cues by inducing and maintaining associated gene expression patterns (Yan et al. 2014). Whether such epigenetic modifications (e.g. DNA methylation) are heritable across generations in eusocial insects is still been debated (Sieber et al. 2021). The other option is that the offspring of some queens have a higher ability to perceive new odours and adapt their behaviour accordingly; further studies are needed to shed light on the precise mechanism.
It is unknown if some colonies or populations are ‘spring-loaded’, i.e. they already have a high level of perception among some of their workers so can adapt quickly to new pests. The appearance of feral/wild surviving populations despite V. destructor across Europe and the USA (Moro et al. 2021), the UK (www.varroaresistant.uk), and Ireland (Browne et al. 2021) all demonstrate that many wild and managed populations can become resistant once the initial devastating wave of mites has passed through the population. Currently, it is unknown if some populations of colonies are pre-adapted to deal with V. destructor, i.e. already possess workers with a high level of perception, or does each colony/population need to develop resistance to the mite before spreading through the population. Unlike in managed colonies, feral colonies are free to swarm, and this is known to temporarily reduce the mite population and may help with the development of resistance as shown by Seeley (2017).
The role the drones play in resistance was not measured in this study directly. However, despite being locally mated, the daughter of the queen-naïve still had a high level of brood infestation (39% vs. overall mean for the naïve queens = 40%), and 25 phoretic mites per 100 bees which was the same as two other naïve queens from the study. For the colony headed by the original resistant queen, the number of phoretic mites was five per 100 bees while the average of the six daughters was seven per 100 bees. Despite the swarming in April, the average worker brood infestation level in autumn was 7%, which is higher than the 3% measured in this population in 2019 (Hawkins and Martin 2021).
The key finding that a locally mated resistant queen can be used to re-queen any colony and that the colony will become resistant is supported by the observations of beekeepers. It is now well established that beekeepers in the UK and the Hawaiian island of Oahu manage collected swarms from long-lived free-living colonies that have developed V. destructor resistance. Queens raised from this stock have then been used to re-queen susceptible colonies to allow beekeepers to manage their bees without the need for miticide treatments. There are several case studies of this given on www.varroaresistant.uk.
Data availability
The raw data is available from the lead author upon request.
Code availability
Not applicable.
References
Adam E, Hansson BS, Knaden M (2022) Fast learners: one trial olfactory learning in insects. Front Ecol Evol. https://doi.org/10.3389/fevo.2022.876596
Allsopp MH (2006) Analysis of Varroa destructor infestation of southern African honeybee populations. (MRes thesis). University of Pretoria, Pretoria, South Africa
Akimov IA, Yastrebtsov AV (1984) Reproductive system of Varroa jacobsoni I. Female reproductive system and oogenesis. Vestnik Zoologii 6:61–68
Browne KA, Hassett J, Geary M, Moore E, Henriques D, Soland-Reckeweg G et al (2021) Investigation of free-living honey bee colonies in Ireland. J Apic Res 60(2):229–240. https://doi.org/10.1080/00218839.2020.1837530
de Souza FS, Allsopp M, Martin SJ (2020) Deformed wing virus prevalence and load in honeybees in South Africa. Arch Virol 166:237–241
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS et al (2013) Standard methods for varroa research. In V. Dietemann, J. D. Ellis & P. Neumann (Eds), The COLOSS BEEBOOK, Volume II: Standard methods for Apis mellifera pest and pathogen research. J Apic Res 52(1):1–54
Fries I, Rosenkranz P (1996) Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp Appl Acarol 20:103–112
Grindrod I, Martin SJ (2021) Parallel evolution of Varroa resistance in honey bees; a common mechanism across continents? Proc R Soc B 288:20211375
Hawkins GP, Martin SJ (2021) Elevated recapping behaviour and reduced Varroa destructor reproduction in mite-resistant Apis mellifera honey bees from the UK. Apidologie 52:647–657
Häußermann CK, Giacobino A, Munz R et al (2020) Reproductive parameters of female Varroa destructor and the impact of mating in worker brood of Apis mellifera. Apidologie 51:342–355
Leadbeater E, Chittka L (2007) Social learning in insects- from miniature brains to consensus building. Curr Biol 21:17(16):R703–R713
Loukola OJ, Solvi C, Coscos L, Chittka L (2017) Bumblebees show cognitive flexibility by improving on an observed complex behavior. Science 355:833–836
Luis AR, Grindrod I, Webb G, Piñeiro AP, Martin SJ (2022) Recapping behaviour in Cuba: home to the world’s largest population of Varroa-resistant European honeybees. Sci Rep 12:15597
Martin SJ, Kemp D (1997) Average number of reproductive cycles performed by the parasitic mite Varroa jacobsoni in Apis mellifera colonies. J Apic Res 36:113–123
Martin SJ, Hawkins GP, Brettell LE, Reece N, Correia-Oliveira ME, Allsopp MH (2019) Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 51(3):369–381. https://doi.org/10.1007/s13592-019-00721-9
Mendoza Y et al (2020) Unravelling honey bee–Varroa destructor interaction: multiple factors involved in differential resistance between two Uruguayan populations. Vet Sci 7:116
Mikityuk M (1979) Reproductive ability of Varroa females. Pchelovodstvo 9:2
Mondet F et al (2021) Chemical detection triggers honey bee defense against a destructive parasitic threat. Nat Chem Biol 17:524–530
Moro A, Beaurepaire A, Dall’Olio R, Rogenstein S, Blacquière T, Dahle B, et al (2021) Using citizen science to scout honey bee colonies that naturally survive Varroa destructor infestations. InSects 12(6):536. https://doi.org/10.3390/insects12060536
Oddie MAY, Buchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8(1):7704
Seeley TD (2017) Life-history traits of wild honey bee colonies living in forests around Ithaca, NY, USA. Apidologie 48:743–754
Sieber KR, Dorman T, Newell N, Yan H (2021) (Epi)Genetic mechanisms underlying the evolutionary success of eusocial insects. InSects 12:498
Valentine A, Martin SJ (2023) A survey of UK beekeeper’s treatment habits. PLosOne 18(2):e0281130
Yan H et al (2014) Eusocial insects as emerging models for behavioural epigenetics. Nat Rev Genet 15:677–688
Funding
Funding for Isobel Grindrod and Georgina Webb was provided by Bee Diseases Insurance Ltd and British Beekeepers Association respectively. Funding for Stephen Martin and Ethel Villalobos was provided by awards from Project Apis-m and Western Sustainable Agriculture Research and Education (SARE) respectively, both based in the USA.
Author information
Authors and Affiliations
Contributions
The authors Stephen Martin, Ethel Villalobos, and Rhona Toft contributed to the study conception and design. Material preparation and data collection were performed by all authors, and data analysis was performed by Georgina Webb and Stephen Martin. The first draft of the manuscript was written by Stephen Martin and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for this study as the experimental work was conducted with unregulated invertebrate species.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: Zachary Huang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin, S.J., Grindrod, I., Webb, G. et al. Resistance to Varroa destructor is a trait mainly transmitted by the queen and not via worker learning. Apidologie 55, 40 (2024). https://doi.org/10.1007/s13592-024-01084-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01084-6