Abstract
Bees are often exposed to pesticide residues during their foraging trips in agricultural landscapes. The analysis of in-hive stored pollen reflects the spectrum of visited plants and can be almost used to link the exposure to pesticides.In the current study, bee bread samples were collected in May and July from 17 sites located in southern Slovakia. Samples were analysed using a multi-residue pesticide analysis method for a broad spectrum of active substances and microscopic for pollen identification.Our results revealed a bee bread contamination with 19 different active substances, with fungicides being predominant. Sixteen of them are authorized in the EU, but chlorpyrifos, chlorpyrifos-methyl, and chloridazon are not. The highest concentrations for pendimethalin (1400 µg/kg), fluazifop-butyl (640 µg/kg), fenpropidin (520 µg/kg), fluopyram (130 µg/kg), and difenoconazole (95 µg/kg) were detected. The total residue load in bee bread sampled in the early season (May) was significantly higher than in the late season (July). The mean residue load of insecticides analysed in July comprised 46% of May’s load, which is alarming due to the importance of bee bread in the diet for winter-rearing bees. Moreover, results from both sampling periods showed that fungicides were positively associated with plant families Apiaceae and Papaveraceae and herbicides with Aceraceae, Salicaceae, and Brassica-type/Brassicaceae.Hence, bee bread can be considered a suitable matrix and a good bio-indicator reflecting honey bee exposure to pesticides over the season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Honey bees collect pollen and nectar from different plant species to ensure the colony development during the season. Plant pollen is bees’ main lipid source, and it is transferred to the hive, compressed in foragers’ corbiculae (pollen baskets), and stored in the hexagonal cells of combs. Roulston and Cane (2000) observed a range of lipid concentrations from 0.8 to 18.9% in pollen of different plant species. Honey bees do not directly consume “raw” pollen; after the biochemical changes, this is always turned into bee bread (perga), which is then an essential part of their diet. Honey bees prefer to consume bee bread stored for less than 4 days (Anderson et al. 2015; Carroll et al. 2017). The anaerobic lactic fermentation process is crucial to make the bee bread more digestible for adult bees, larvae, and humans as well (Aylanc et al. 2021). Due to the high concentration of simple sugars (40–50%) with their preservation properties like low pH (pH 4), low water activity, high oxidation–reduction potential, and the presence of lactic acid bacteria (Kwakman et al. 2010), bee bread is considered to have a unique composition (Anderson et al. 2015).
Several stressors contributed to the colony loss phenomenon reported from North America and Europe in the last decades, with pesticides being predominant (Goulson et al. 2015; Insolia et al. 2022). Generally, fat-soluble chemicals may leave residues in matrices such as wax, propolis, pollen, bee bread, and honey (Johnson et al. 2010; Mullin et al. 2010; Böhme et al. 2018; Martinello et al. 2020; Murcia-Morales et al. 2022; Kasiotis et al. 2023). Moreover, pollen and nectar are mostly subjected to pesticide residue analyses since the highest concentrations detected in these matrices may be considered in exposure refinement as the field realistic residue concentrations in risk assessment for honey bees (EFSA et al. 2023). Under some circumstances (e.g. overwintering), both the pollen and nectar are stored in a hive for several months, depending on geographic location (Döke et al. 2015).
Nowadays, the agriculture intensification linked with higher pesticide use means that pollinators are chronically exposed to an “agrochemical cocktail” during the season (Goulson et al. 2015). Therefore, monitoring of pesticide residues, including both the lipophilic and hydrophilic active substances, in bee bread can provide us with valuable information on the use of pesticides in the environment around the colony (Schaad et al. 2023) as well as on the exposure level within the colony. Nevertheless, the botanical origin of collected pollen plays an important role in the level of contamination. Some authors observed different seasonal residue contamination profile in bee bread or pollen pellets, which mostly correlated with the flowering periods of main agricultural plants (Friedle et al. 2021; Schaad et al. 2023).
Although the importance of bee bread in bees’ nourishment is essential, there are only a few authors who have focused on the pesticide residues analysed in bee bread to this time (Mullin et al. 2010; Orantes-Bermejo et al. 2010; Giroud et al. 2013; Daniele et al. 2018; Bergero et al. 2021; Kasiotis et al. 2023), while only two authors linked analysed levels to botanical origin (Favaro et al. 2019; Raimets et al. 2020). To this time, no residue study with pesticide level determination in relevant bee matrices has been conducted in Slovakia.
In light of these facts, the current study is aimed at the following: (1) to perform monitoring in 17 sites in Slovakia to investigate the pesticide contamination level in bee bread, (2) to compare the residue load of pesticides in bee bread in early and late season, and (3) to find potential relationship(s) between the botanical origin of collected pollen and detected pesticides.
2 Material and methods
2.1 Geographic information system (GIS) methodology
Matrices of bee bread were collected from 17 sites, mainly from southern Slovakia. The choice of study sites was based on the average pesticide consumption obtained from Slovakia’s harmonized registration—information system (Anonymous, 2022). The selected apiaries came from the districts with the highest reported yearly consumption of pesticides in Slovakia from the previous year (Figure 1).
In addition to the primary data collection and analysis, geographic information system (GIS) spatial analysis was employed to examine the relationship between land cover structure and pesticide contamination in bee bread. Utilizing QGIS software, a 2-km radius buffer zone was delineated around each hive site, which was geolocated based on their coordinates. This radius was chosen to cover the main foraging area of the honey bee colonies (Beekman and Ratnieks 2000). CORINE Land Cover data from 2018 (CLC 2018. Accessible from https://land.copernicus.eu/, 2023), obtained from the Copernicus Land Monitoring Service, was integrated into the QGIS software where we conducted several spatial geoprocessing steps. By employing the intersect tool within QGIS, the land cover types (artificial surfaces, agriculture, forests and semi-natural areas, wetlands, and water) within the 2-km radius of each hive site were extracted. These extracted data were then merged with the bee bread pesticide contamination data through a spatial join, facilitating a comprehensive analysis of the correlation between the land cover type and the pesticide exposure. The results of this GIS analysis contributed to the understanding of pesticide contamination in bee bread, supplementing the broader methodological framework of the study.
2.2 Sample collection
From each sampling apiary, a piece of a comb containing bee bread was taken twice per season (at the end of May and July 2022). At some sampling apiaries, varroa treatment was done before and/or between sampling periods using veterinary medicaments licensed for use in ecological apiculture in Slovakia (see Results and Discussion for details). Samples were immediately stored and transported in a portable freezer box (− 4 °C) to the Apiculture department in Liptovský Hrádok where they were kept under − 18 °C until the next laboratory processing. The bee bread was immediately extracted from the pieces of combs using a bee bread harvester (Wilara, Latvia) under − 4 °C. As two beekeepers could provide only the first samples, 17 samples from the first sampling period and 15 samples from the second period were analysed in this study.
2.3 Pesticide analyses
Each sample was split into two aliquots. One set of 32 samples was sent to FoodQS GmbH (Germany) and analysed by Bilacon Germany (Berlin), using multi-residue method (ASU L 00.00.115) searching for 807 pesticide residues; details can be found in supplementary data (Table S1). All the samples contained enough matrix with the range of 55–101 g. The samples were delivered to FoodQS GmbH under controlled thermoconditions (− 18 °C). The respective LOQs are summarized in supplementary material (supplementary data; Table S1). Multi-residue analysis is based on the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged, and Safe) and LC–MS/MS as well as GC–MS/MS methods (Anastassiades et al. 2003). The QuEChERS method is a two-step extraction in which water is usually added to the sample, and the residues are extracted with acetonitrile. A salt mixture is then added for phase separation. After centrifugation, an aliquot of the resulting supernatant is measured by GC–MS/MS and LC–MS/MS after additional purification. The second set of samples was used for a palynological analysis.
2.4 Palynological analysis (microscopic examination)
In our palynological analysis, we followed Favaro et al. (2019) with slight modifications. But briefly, an aliquot of 100 mg homogenized bee bread was transferred into a 50-ml tube containing 10 ml of demineralized water. After the sample was dissolved, using a Pasteur pipette, two drops (60 µl) of homogenous sediment were placed over an area of the microscope slide and spread with a microspatula. Then, the microscope slide was left on the hotplate for up to half an hour until the sample was completely dry. After drying, one drop of Kaiser’s glycerol gelatine was placed above and covered with a thin glass. In total, a thousand pollen grains were counted under a microscope for each slide.
When possible, the morphology at the family, genus, or species level was considered during pollen identification (Moore et al. 1991). Moreover, the palynological database, freely available at paldat.org, was used as an additional reference for pollen collection in our pollen identification. The palynological results in this study are expressed as a percentage of the individual plant species/genus to the total pollen content (relative pollen content).
2.5 Statistical analysis
All the graphical plots and statistical analyses were performed with R version 4.0.3 “Bunny-Wunnies Freak Out” (R Core Team 2020) at the significance level of 0.05. The ggplot2 package v. 3.3.3 (Wickham et al. 2016), the “vegan” package v. 2.6–2 (Oksanen et al. 2022), and package glmmTMB v.1.1.3 (Brooks et al. 2017) were used.
Redundancy analysis (RDA) was conducted to determine the relationship between the active substances (response variable) and botanical taxa in bee bread (dependent variable). RDA was performed separately for two sampling periods and for pooled data obtained from both sampling periods. Data were log + 1 transformed prior to analysis.
To explore how the agricultural, forest, and semi-natural areas affect the pesticide load in the samples, we fitted a generalized linear model (GLM) with gamma error distribution. The pesticide load (the sum of all detected residues in each sample) per site was log-transformed and set as a dependent variable, whereas the percentage of agricultural or forest and semi-natural area was set as an independent variable.
For data with repeated measures from the same sites during early and late season, we fitted a generalized linear model (GLMM) with gamma error for pesticide load or Poisson distribution for the number of active substances (Brooks et al. 2017). The pesticide load per site or number of detected active substances was set as a dependent variable and the sampling dates as the independent variable. The pesticide load per site during the early and late seasons was log + 1 transformed. Models were validated through visual inspection of residual plots.
3 Results and discussion
Observed colony losses over the world are driven by combined stressful factors, pesticides, parasites, and lack of flowers being dominant (Goulson et al. 2015). In this study, bee bread samples were collected from 17 apiaries/sites across Slovakia with a minimum distance of 7 km among them (12 km and more among all other apiaries). The agricultural land constituted 72.5% of the total sampling area (supplementary data; Table S2). The distance of 7 km is sufficient to avoid an overlapping bee foraging radius, supported by the finding of Beekman and Ratnieks (2000), who observed the mean bee foraging distance of 1 km in May in the UK. The choice of selected apiaries was based on the highest reported yearly utilization of pesticides in Slovakia from the previous year 2021 (Anonymous, 2022). May was chosen as the early season collection month because in this month, the peak of nectar and pollen in-hive inflow from agricultural plants is observed in Slovakia; July (late season) is considered an important month for colony preparation for the next season (rearing of winter generation of bees). In this study, the composition of active substances and plant taxa detected in May differed from that detected in July. Therefore, we present the grouped and separated data from individual sampling periods, where possible.
3.1 Residues and pollen profile in bee bread of different sites in Slovakia
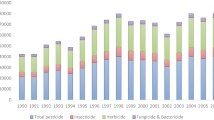
Overall, in this study, residues of 19 active substances (≥ LOQ; Table S1) of various pesticides were detected in bee bread, with fungicides being predominant. The most frequently analysed pesticide groups in this study were insecticides/acaricides (> 56%), followed by fungicides (> 31%) and herbicides (< 13%) (Table I). The results obtained are comparable with the literature. The seasonal sampling period (May–October 2018) of bee bread in Cordoba (Spain) showed the presence of 20 different residues (Morales et al. 2020). Vice versa to those findings, analysis of bee bread samples biweekly collected from five colonies located in an agricultural area in Switzerland throughout the active colony season (April–October 2022) revealed a total of 51 different residues of pesticides (Schaad et al. 2023).
Samples from the early season (May) contained 18 active substances, whereas ten active ones were detected in July samples (Figure 2). Of the 19 detected active substances, 16 are currently authorized in the EU (status to April 2023). Chlorpyrifos, chlorpyrifos-methyl, and chloridazon are not approved at the EU level; chloridazon is authorized nationally in Hungary and the Netherlands as herbicide. Schaad et al. (2023) revealed contaminated bee bread with thiacloprid despite its ban in Switzerland. Moreover, a recent study identified chlorpyrifos as one of the most important residues related to colony poisoning incidents in the Czech Republic (Kadlikova et al. 2021).
In addition, the results of this study showed that the number of compounds detected per positive sample varied from 1 to 13. In contrast, five samples were free of pesticide residues, predominantly from the second sampling period (Figure 2A). Similarly, the number of residues per sample ranged from 0 to 11 in the bee bread samples originating from Luxembourg (Beyer et al. 2018), while no sample free of pesticide residues was observed in pollen loads collected from apple orchards in the South Tyrol region (Italy), during and 2 weeks after blossom (Favaro et al. 2019). A mixture of 11 to 21 pesticide residues was observed in the larval pollen provisions of red mason bee species (Osmia bicornis) in commercial fruit orchards (Šlachta et al. 2020).
In this study, the maximum analysed concentrations ranged between 11 and 1400 µg/kg, with the highest detected concentrations for pendimethalin (1400 µg/kg), fluazifop-butyl (640 µg/kg), fenpropidin (520 µg/kg), fluopyram (130 µg/kg), and difenoconazole (95 µg/kg). Fluvalinate was the most frequently detected active substance (47%), followed by acetamiprid and flumethrin (31% each one substance), fluopyram (16%), azoxystrobin, boscalid, chlorpyrifos, and pendimethalin (all 9%). Dimoxystrobin, fluazifop-butyl, chloridazon, prosulfocarb, prothioconazole, and tebuconazole were quantified at the same frequency of 6%. The rest of the detected residues were found at a frequency of 3% (supplementary data; Table S3). Similarly to our findings, neonicotinoid acetamiprid together with thiacloprid were the most prevalent active substances detected in Swiss bee bread samples (Schaad et al. 2023).
The detection of pyrethroids, flumethrin, and fluvalinate, in bee bread collected during both sampling periods, is a specific case. Active substance fluvalinate is used in both agriculture, and apiculture as an insecticide and acaricide, respectively, while flumethrin is used in apiculture as an acaricide only. In total, six plant protection products containing the active substance fluvalinate are registered in Slovakia, including products for both professional and hobby users. Moreover, to that time (status to April 2024), there are four veterinary medicinal products (acaricides) containing flumethrin and fluvalinate (two products per each substance) licensed for the control of Varroa destructor infestation in apiculture in Slovakia. Only one of them is used at the hive entrance; remaining three are recommended to be used in the in-hive environment for long-term treatment (contact way of bee exposure). Strip use carries the risk of in-hive wax contamination and possible secondary honey/bee bread contamination (Morales et al. 2020; Alkassab et al. 2022), but to consider this way of bee bread contamination is difficult because the wax was not analysed in this study, and strip treatment should be done after the final honey extraction (in Slovakia, it is usually at the beginning of August). In our opinion, the observed positive association of flumethrin residues with the percentage of Fabaceae and Chenopodiaceae (Figure 5) can be explained by the use of veterinary medicaments licensed for the use in ecological apiculture during the flowering period of Fabaceae and Chenopodiaceae. These ecological medicaments contain essential oils and are proposed for spring, summer, and autumn treatment use, always after honey harvest in Slovakia. But unfortunately, several batches of these veterinary medicaments were repeatedly withdrawn from the Slovak market by the Institute for State Control of Veterinary Biologicals and Medicaments Nitra during last year (2022) due to their proven contamination with flumethrin. This fact was supported by the majority of individual beekeepers involved in this study, who confirmed the use of “ecological” veterinary medicaments at their apiaries before or between our sampling periods (personal communications).
3.2 Pollen origin and the relationships between the plant taxa and pesticide residues in bee bread samples
The updated EFSA manual recommends the use of the results of palynology, which may be involved in the risk assessment scheme to ensure that bees forage on the target crop as well as to evaluate the “dilution factor” of the exposure. Moreover, it is highly recommended to perform palynology from wildflowers or crops flowering in the surrounding area of the treated plots (EFSA 2023).
Bee foragers show certain loyalty to nectar/pollen sources during their flights, searching for a specific botanical species able to provide a sufficient amount of diet (Porrini et al. 2002). In this study, we observed some variation in plant taxa composition between bee bread samples collected in both sampling periods (Figure 2). Results of palynological analysis showed that Salicaceae and Aceraceae dominated in samples collected mainly during the early season (May), followed by Brassica-type/Brassicaceae and Rosaceae. Pollen grains from the plant taxa Helianthus annuus/Asteraceae and Asteraceae were detected only in samples collected in the late season (July). Pollen of Phacelia (Hydrophyllaceae) was found equally in bee bread samples collected during both sampling periods (suppl. data; Table S4).
While Schaad et al. (2023) hypothesized about potential bee bread contamination via drift during the spray treatment of the main agricultural crop, our analyses revealed a certain relationship between the plant taxa and residue levels analysed in bee bread samples. Assuming our results from both sampling periods, fungicides were positively associated with Apiaceae and Papaveraceae and herbicides with Aceraceae, Salicaceae, and Brassica-type/Brassicaceae (Figure 3). In contrary to our findings, Raimets et al. (2020) observed a significant positive correlation of the total amount of herbicides with Fabaceae pollen collected in May in Estonia.
Results of a redundancy analysis (RDA) performed on the concentrations of residues and the proportion of pollen taxa (%) in bee bread, collected in May and July in Slovakia. Positions of the vectors of response variables on the two first RDA axes are shown in red and those of the independent variables (proportion of pollen taxa) are shown in blue arrows.
Observed positive association of herbicides with Aceraceae, Salicaceae in this study may be explained that herbicides are usually applied before or during planting in the spring to maximize crop productivity by minimizing weed’s growth (Kraehmer et al. 2014). Moreover, the trees from Aceraceae and Salicaceae families, which offer pollen and nectar to honey bee colonies in the early season, are often found as a part of live hedgerows in landscape in Slovakia. Duque-Trujillo et al. (2023) observed that live hedgerows in agricultural landscape serve as natural barriers against the spread of insecticides.
Looking at the individual sampling data, a positive association between the levels of boscalid, fluazifop-p-butyl, and prosulfocarb residues and the percentage of Apiaceae and Phacelia/Hydrophyllaceae pollen can be seen in bee bread samples collected in early season (May). Furthermore, the levels of azoxystrobin and fluopyram were positively associated with the oilseed rape pollen (Brassica-type/Brassicaceae).
Moreover, the levels of difenconazole and fenpropidin were positively associated with the percentage of Asteraceae pollen (Figure 4). This observation is probably because several plant protection products (PPPs) contain both active substances registered in Slovakia. Raimets et al. (2020) observed a significant positive correlation between the percentage of oilseed rape and Apiaceae pollen (sampled in May) and the residues of dimethoate (an organophosphate insecticide) in Estonia. They also detected residues of thiacloprid (neonicotinoid insecticide), which were positively associated with Apiaceae pollen collected in both sampling periods (May and July), too.
Results of a RDA performed on the concentrations of residues and the proportion of pollen taxa (%) from bee bread samples collected in May in Slovakia. Positions of the vectors of response variables on the two first RDA axes are shown in red and those of the independent variables (proportion of pollen taxa) are shown in blue arrows (full names of detected residues are listed as suppl. data in Table S5).
Comparing analysed residue levels with the percentage of plant taxa in this study showed a high variation between sampling periods. Obtained results from the early season (May) give us an assumption that almost all residues found in different plant taxa are in accordance with the registered aim of plant protection products used, except for two active substances, chlorpyrifos and chloridazon, which are banned in EU. Detection of chlorpyrifos, which showed a positive association with the percentage of oilseed rape pollen and chloridazon positively associated with Apiaceae and Phacelia/Hydrophyllaceae pollens (Figure 4), gives us an assumption of their improper field application.
Further, looking at the individual sampling data obtained from the late sampling period (July), the number of detected active substances was much lower than in the early sampling period (Figure 2). Moreover, the levels of acetamiprid and fluopyram were positively associated with the percentage of Phacelia/Hydrophyllaceae pollen (Figure 5). The same pattern observed with these two residues may be explained by common praxis for using so-called tank mixes in agriculture when farmers simultaneously apply a mixture of registered plant protection products in the field. This practice carries a higher risk to bee colonies as fungicides appear more toxic to bees due to the common agricultural practice of using them in tank mixes with insecticides (Kadlikova et al. 2021; Rondeau and Raine 2022).
Results of a RDA performed on the concentrations of residues and the proportion of pollen taxa (%) from bee bread samples collected in July in Slovakia. Positions of the vectors of response variables (pesticide residues) on the two first RDA axes are shown in red and those of the independent variables (proportion of pollen taxa) are shown in blue arrows (full names of detected residues are listed as suppl. data in Table S5).
Residue analysis of the late-season bee bread samples (July) revealed three active substances banned in the EU: chlorpyrifos, chlorpyrifos-methyl, and chloridazon. The last two showed a similar picture, where the level of both pesticide residues was positively associated with the percentage of Fabaceae and Chenopodiaceae. Chlorpyrifos showed a positive association with the percentage of Plantaginaceae and Vitaceae. Detection of all three active substances in this study showed their improper field application in Slovakia. The source of PPPs containing these active substances banned in the EU is unknown at this time. We can only speculate about the illegal import and/or the use of the old stocks (the approval period for active substances chlorpyrifos and chlorpyrifos-methyl in the whole EU ended on 16/01/2020 and for chloridazon on 31/12/2018).
3.3 The impact of agricultural area on pesticide load
The effects of the agricultural, forest, and semi-natural areas surrounding the sampling sites (permanent apiaries) with a radius of 2 km on residue load in bee bread is shown in Figure 6.
Our results showed no statistically significant impact of the specific area surrounding the sampling sites (GLM, p = 0.101 and 0.213 for agricultural areas and forest and semi-natural areas, respectively) on the pesticide load, but an increasing trend with agricultural areas and a decreasing trend with forest and semi-natural areas were observed. This is in line with the study of Lambert et al. (2013), who reported similar results obtained in France.
3.4 Total residue load in bee bread samples in early and late season
Significantly lower residue load (GLM, p = 0.012) and the total number of active substances (GLM, p < 0.001) in the late season (Figure 7) can be explained by the year time of the second sampling period (July) when farmers are used to applying fewer pesticides per a hectare.
Comparison of the total residue load (A) and the number of active substances (B) detected in bee bread during early and late season. Boxplots represent the median and the edges of the box indicate the 25th and 75th percentiles. Red points show the mean. Asterisks indicate the significant differences (p < 0.05).
Generally, the increased spring pollen influx into the colony stimulates bee brood production. Maximum 4-day-old-stored bee bread is preferred by the bees preparing the diet within the colony during the season (Carroll et al. 2017). Regarding our results, this observation represents the potential risk of increased residue load in the spring. Because lipophilic pesticides/acaricides leave residues in in-hive matrices for a long time (Wallner 1999; Tsigouri et al. 2001; Johnson et al. 2010; Bonzini et al. 2011; Lambert et al. 2013; Erban et al. 2019), they are considered to cause hazardous environment for bees and larvae chronically exposed to their residues (Morales et al. 2020). Sublethal effects of pesticide residue exposure on bees, like impairment of learning abilities and memory, space orientation, impaired immune function, reduced longevity, and/or foraging and motor coordination, have been described in numerous studies (Thompson 2003; Ciarlo et al. 2012; Oruc et al. 2012; Garrido et al. 2013; Faita et al. 2018; Farina et al. 2019; Martinello et al. 2020; Sabová et al. 2022). Moreover, a combination of adverse toxic effects with dwindling natural pollen resources in July can potentially negatively influence the transition from summer bees to winter bees (Mattila and Otis 2007). Thus, we may expect that detected levels of residues in bee bread in this study may reflect a realistic in-hive exposure level, but this needs further investigation.
In addition, solitary bees and their developmental stages (larvae) are reported to be exposed via contaminated pollen and nectar, too. Delayed larval development and lowered larval and adult body weights were observed after long-term exposure of solitary bee larvae with three insecticides (acetamiprid, flonicamid, and sulfoxaflor) and a fungicide dodine (Phan et al. 2024). Moreover, several studies supported the assumption of a lower risk of pesticide exposure in urban sites than in arable land (Šlachta et al. 2020, 2023).
Finally, some authors reported potential risks from pollen and/or bee bread consummation (Zafeiraki et al. 2022; Végh et al. 2023), but this aspect was not considered in detail in this study. Maximum detected concentrations of eight active substances exceeded the maximum residue limits (MRLs) set by the EU for honey and other apiculture products (Table S3), e.g. fenpropidin and fluazifop-butyl 10 times and herbicide pendimethalin almost 30 times. It is also worth to mention that two banned active substances, chlorpyrifos and chlorpyrifos-methyl, exceeded also the MRL of 0.01 mg/kg set for those substances. In general, our study reflects the current situation of PPPs use in Slovakia during the early and late seasons. However, the reported residues should be interpreted with caution. This depends on the reporting limit of the contracted accredited laboratory (10 µg/kg) for the delivered results of residue analysis set by the laboratory, especially for several toxic substances like neonicotinoids (clothianidin and imidacloprid), which can occur mostly in trace concentrations than reported one. Therefore, we suggest a further refinement of the residue analysis methodology in bee matrices in the future to cover this issue.
4 Conclusion
Palynological analysis in this study revealed a broad spectrum of plant taxa in bee bread samples collected in Slovakia in two sampling periods (May and July). However, foragers prefer to gather pollen loads composed mainly of one plant species. Generally, Salicaceae and Aceraceae dominated (47% and 44%, respectively) in bee bread samples, followed by Asteraceae (31%), Brassica-type/Brassicaceae and Rosaceae (both 25%), and Phacelia/Hydrophyllaceae with Helianthus annuus/Asteraceae (both 22%).
Residue analysis showed that the most frequently analysed pesticide groups were insecticides/acaricides (56%), followed by fungicides (31%) and herbicides (13%). Moreover, it is alarming that the mean residue load of insecticides analysed in July comprised 46% of May’s load, mainly due to the importance of bee bread in the diet for winter-rearing bees and the adverse effects that can potentially arise. Moreover, pooled results from both sampling periods showed that fungicides were positively associated with Apiaceae and Papaveraceae and herbicides with Aceraceae, Salicaceae, and Brassica-type/Brassicaceae.
The obtained data indicate the need for future investigation of this matrix to better understand its possible involvement in the winter colony losses. Further bee matrices, like adult bees and larvae, should be included in residue analysis to achieve a more realistic exposure estimation of the honey bee colonies during the season and their related potentially harmful impact(s) on colony health.
Data availability
The data presented in this study are available in the article.
Code availability
Not applicable.
References
Alkassab AT, Bischoff G, Thorbahn D et al (2022) Transfer of xenobiotics from contaminated beeswax into different bee matrices under field conditions and the related exposure probability. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.135615
Anastassiades M, Lehotay ST, Stajnbaher D, Schenck J (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extration” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Anderson KE, Carroll MJ, Sheehan T et al (2015) Hive-stored pollen of honey bees: many lines of evidence are consistent with pollen preservation, not nutrient conversion. Mol Ecol. https://doi.org/10.1111/mec.12966
Anonymous (2022) UKSUP Spotreba prípravkov na ochranu rastlín na Slovensku (EN: UKSUP Consumtion of pesticides in Slovakia.) Available at: https://www.uksup.sk/spotreba-pripravkov-na-ochranu-rastlin
Aylanc V, Falcão SI, Ertosun S, Vilas-Boas M (2021) From the hive to the table: nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread. Trends Food Sci Technol 109:464–481
Beekman M, Ratnieks FLW (2000) Long-range foraging by the honey-bee. Apis Mellifera L Functional Ecology 14(4):490–496. https://doi.org/10.1046/j.1365-2435.2000.00443.x
Bergero M, Bosco L, Giacomelli A, Angelozzi G, Perugini M, Merola C (2021) Agrochemical contamination of honey and bee bread collected in the Piedmont Region, Italy. Environ 8:62. https://doi.org/10.3390/environments8070062
Beyer M, Lenouvel A, Guignard C, Eickermann M, Clermont A, Kraus F, Hoffmann L (2018) Pesticide residue profiles in bee bread and pollen samples and the survival of honeybee colonies-a case study from Luxembourg. Environ Sci Pollut Res Int 25(32):32163–32177. https://doi.org/10.1007/s11356-018-3187-4
Böhme F, Bischoff G, Zebitz CPW et al (2018) Pesticide residue survey of pollen loads collected by honeybees (Apis mellifera) in daily intervals at three agricultural sites in South Germany. PLoS ONE. https://doi.org/10.1371/journal.pone.0199995
Bonzini S, Tremolada P, Bernardinelli I et al (2011) Predicting pesticide fate in the hive (part 1): experimentally determined τ-fluvalinate residues in bees, honey and wax. Apidologie. https://doi.org/10.1007/s13592-011-0011-2
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2):378–400. https://doi.org/10.32614/RJ-2017-066
Carroll MJ, Brown N, Goodall C et al (2017) Honey bees preferentially consume freshly-stored pollen. PLoS ONE. https://doi.org/10.1371/journal.pone.0175933
Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7:e40848
Daniele G, Giroud B, Jabot C, Vulliet E (2018) Exposure assessment of honeybees through study of hive matrices: analysis of selected pesticide residues in honeybees, bee bread, and beeswax from French beehives by LC-MS/MS. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9227-7
Döke MA, Frazier M, Grozinger CM (2015) Overwintering honey bees: biology and management. Current Opinion in Insect Science, 10, art. No 147:185–193
Duque-Trujillo D, Hincapié CA, Osorio M et al (2023) Strategies for the attraction and conservation of natural pollinators in agroecosystems: a systematic review. Int J Environ Sci Technol 20:4499–4512. https://doi.org/10.1007/s13762-022-04634-6
EFSA (European Food Safety Authority), Ippolito A, del Aguila M, Aiassa E, Guajardo IM, Neri FM, Alvarez F, Mosbach- Schulz O, Szentes C (2020). Review of the evidence on bee backgroundmortality. EFSA Supporting Publications 17(7):1–76. https://doi.org/10.2903/j.efsa.2023.7989
Erban T, Vaclavikova M, Tomesova D et al (2019) Tau-fluvalinate and other pesticide residues in honey bees before overwintering. Pest Manag Sci. https://doi.org/10.1002/ps.5446
Faita MR, Oliveira EM, Júnior AVV, Orth AI, Nodari RO (2018) Changes in hypopharyngeal glands of nurse bees (Apis mellifera) induced by pollen-containing sublethal doses of the herbicide Roundup®. Chemosphere 211:566–572. https://doi.org/10.1016/j.chemosphere.2018.07.189
Farina WM, Balbuena MS, Herbert LT, Mengoni Goñalons C, Vázquez DE (2019) Effects of the herbicide glyphosate on honey bee sensory and cognitive abilities: individual impairments with implications for the hive. InSects 10(10):354. https://doi.org/10.3390/insects10100354
Favaro R, Bauer LM, Rossi M et al (2019) Botanical origin of pesticide residues in pollen loads collected by honeybees during and after apple bloom. Front Physiol. https://doi.org/10.3389/fphys.2019.01069
Friedle C, Wallner K, Rosenkranz P, Martens D, Vetter W (2021) Pesticide residues in daily bee pollen samples (April-July) from an intensive agricultural region in Southern Germany. Environ Sci Pollut Res Int 28(18):22789–22803. https://doi.org/10.1007/s11356-020-12318-2
Garrido PM, Antunez K, Martin M et al (2013) Immune-related gene expression in nurse honey bees (Apis mellifera) exposed to synthetic acaricides. J Insect Physiol 59:113–119
Giroud B, Vauchez A, Vulliet E et al (2013) Trace level determination of pyrethroid and neonicotinoid insecticides in bee bread using acetonitrile-based extraction followed by analysis with ultra-high-performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1316:53–61
Goulson D, Nicholls E, Botías C, Rotherayet EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. https://doi.org/10.1126/science.1255957
Insolia L, Molinari R, Rogers SR, Williams GR, Chiaromonte F, Calovi M (2022) Honey bee colony loss linked to parasites, pesticides and extreme weather across the United States. Sci Rep 12:20787. https://doi.org/10.1038/s41598-022-24946-4
Johnson RM, Ellis MD, Mullin CA, Frazier M (2010) Pesticides and honey bee toxicity - USA. Apidologie. https://doi.org/10.1051/apido/2010018
Kadlikova K, Vaclavikova M, Halesova T, Kamler M, Markovic M, Erban T (2021) The investigation of honey bee pesticide poisoning incidents in Czechia. Chemosphere 263:128056. https://doi.org/10.1016/j.chemosphere.2020.128056
Kasiotis KM, Zafeiraki E, Manea-Karga E, Anastasiadou P, Machera K (2023) Pesticide residues and metabolites in Greek Honey and Pollen: bees and human health risk assessment. Foods 6;12(4):706. https://doi.org/10.3390/foods12040706
Kraehmer H, Laber B, Rosinger C, Schulz A (2014) Herbicides as weed control agents state of the art I Weed control research and safener technology the path to modern agriculture. Plant Physiol. 166(3):1119–31. https://doi.org/10.1104/pp.114.241901
Kwakman PHS, te Velde AA, de Boer L et al (2010) How honey kills bacteria. Federation of American Societies for Experimental Biology 24:2576–2582
Lambert O, Piroux M, Puyo S et al (2013) Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of Western France. PLoS ONE. https://doi.org/10.1371/journal.pone.0067007
Martinello M, Manzinello C, Borin A et al (2020) A survey from 2015 to 2019 to investigate the occurrence of pesticide residues in dead honeybees and other matrices related to honeybee mortality incidents in Italy. Diversity. https://doi.org/10.3390/d12010015
Mattila HR, Otis GW (2007) Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn. Ecol Entomol 32:496–505
Moore PD, Webb JA, Collinson ME (1991) Pollen analysis. Blackwell Science, Oxford, p 216
Morales MM, Ramos MJG, Vázquez PP, Galiano FJD, Valverde MG, López VG, Flores JM, Fernández-Alba AR (2020) Distribution of chemical residues in the beehive compartments and their transfer to the honeybee brood. Sci Total Environ 710:136288. https://doi.org/10.1016/j.scitotenv.2019.136288
Mullin CA, Frazier M, Frazier JL et al (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5:e9754
Murcia-Morales M, Heinzen H, Parrilla-Vázquez P et al (2022) Presence and distribution of pesticides in apicultural products: a critical appraisal. TrAC - Trends Anal Chem. https://doi.org/10.1016/j.trac.2021.116506
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E et al (2022) Vegan: community ecology package. R Package Version 2.6–2. Available online https://CRAN.R-project.org/package=vegan
Orantes-Bermejo FJ, Pajuelo AG, Megias MM, Fernández-Píñar CT (2010) Pesticide residues in beeswax and bee bread samples collected from honey bee colonies (Apis mellifera L.) in Spain. Possible implications for bee losses. J Apic Res 48: 2432250.
Oruc HH, Hranitz JM, Sorucu A et al (2012) Determination of acute oral toxicity of flumethrin in honey bees. J Econ Entomol 105:1890–1894
Phan NT, Joshi NK, Rajotte EG, Zhu F, Peter KA, López-Uribe MM, Biddinger DJ (2024) Systemic pesticides in a solitary bee pollen food store affect larval development and increase pupal mortality. Sci Total Environ 10(915):170048. https://doi.org/10.1016/j.scitotenv.2024.170048
Porrini C, Ghini S, Girotti S et al (2002) Use of honey bees as bioindicators of environmental pollution in Italy. In: Devillers J, Pham-Delegue MH (eds) Honey bees: estimating the environmental impact of chemicals. CRC Press, London, p 62
R Core Team, 2020. R: a Language and Environment for Statistical Computing. R foundation for statistical computing, Vienna
Raimets R, Bontšutšnaja A, Bartkevics V et al (2020) Pesticide residues in beehive matrices are dependent on collection time and matrix type but independent of proportion of foraged oilseed rape and agricultural land in foraging territory. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.124555
Roulston TH, Cane JH (2000) Pollen nutritional content and digestibility for animals. Plant Syst Evol 222:187–209
Rondeau S, Raine NE (2022) Fungicides and bees: a review of exposure and risk. Environ Int. https://doi.org/10.1016/j.envint.2022.107311
Sabová L, Cingeľová Maruščáková I, Koleničová S, Mudroňová D, Holečková B, Sabo R, Sobeková A, Majchrák T, Ratvaj M (2022) The adverse effects of synthetic acaricide tau-fluvalinate (tech.) on winter adult honey bees. Environ Toxicol Pharmacol. 92:103861. https://doi.org/10.1016/j.etap.2022.103861
Schaad E, Fracheboud M, Droz B et al (2023) Quantitation of pesticides in bee bread collected from honey bee colonies in an agricultural environment in Switzerland. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-26268-y
Šlachta M, Erban T, Votavová A, Bešta T, Skalský M, Václavíková M, Halešová T, Edwards-Jonášová M, Včeláková R, Cudlín P (2020) Domestic gardens mitigate risk of exposure of pollinators to pesticides—an urban-rural case study using a red mason bee species for biomonitoring. Sustainability 12(22):9427. https://doi.org/10.3390/su12229427
Šlachta M, Erban T, Votavová A, Tomešová D, Václavíková M, Halešová T, Shcherbachenko E, Bešta T, Edwards-Jonášová M, Včeláková R, Procházková T, Cudlín P (2023) Moderate use of pesticides in allotment gardens as indicated by their residues in larval food provisions of Osmia solitary bees. J Apic Res. https://doi.org/10.1080/00218839.2023.2285143
Thompson HM (2003) Behavioural effects of pesticides in bees – their potential for use in risk assessment. Ecotoxicology 12:317–330
Tsigouri AD, Menkissoglu-Spiroudi U, Thrasyvoulou A (2001) Study of taufluvalinate persistence in honey. Pest Manag Sci. https://doi.org/10.1002/ps.303
Végh R, Csóka M, Mednyánszky Z, Sipos L (2023) Pesticide residues in bee bread, propolis, beeswax and royal jelly - a review of the literature and dietary risk assessment. Food Chem Toxicol 176:113806. https://doi.org/10.1016/j.fct.2023.113806
Wallner K (1999) Varroacides and their residues in bee products. Apidologie 30, 235–248
Wickham H, Chang W, Henry L et al (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York. ISBN 978–3–319–24277–4. Available at: https://ggplot2.tidyverse.org
Zafeiraki E, Kasiotis KM, Nisianakis P, Manea-Karga E, Machera K (2022) Occurence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem Toxicol 161:112826
Acknowledgements
The authors would like to thank the beekeepers for providing samples of bee bread for this study.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic This research was funded by the Slovak ministry of Agriculture with the project “Comparison of the toxicological exposure of bees between agriculturally exposed areas of the Slovak Republic and Germany” (project number 1092/2022/MPRVSR-930). This research was partly supported by the Slovak Grant Agency APVV-21–0185, VEGA 1/0161/23, and VEGA 1/0166/21.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The experimental design and sample collection were performed by Martin Staroň and Rastislav Sabo; material preparation and botanical pollen origin analysis: Martin Staroň and Alexandra Valenčáková. Statistical analysis was performed by Abdulrahim T. Allkassab and Lenka Demková. Landscape structure analysis was done by Miloslav Michalko. Jaroslav Legáth and Jens Pistorius supervised the study.
Literature search was performed by Lucia Sabová and Abdulrahim T. Allkassab; the first draft of the manuscript was written by Rastislav Sabo, Lucia Sabová, and Abdulrahim T. Allkassab. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors agreed with the content, all authors gave explicit consent to submit, and we obtained consent from the responsible authorities at the institute/organization where the work was carried out, before the work was submitted.
Consent for publication
All authors gave the publisher the permission to publish the work.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: Peter Rosenkranz
Alexandra Valencáková died before publication of this work was completed.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staroň, M., Allkassab, A.T., Sabo, R. et al. Higher early than late-season residue load of pesticides in honey bee bread in Slovakia. Apidologie 55, 41 (2024). https://doi.org/10.1007/s13592-024-01079-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01079-3