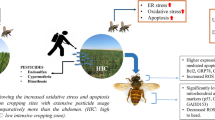
Abstract
tau–Fluvalinate (fluvalinate) is a commonly used miticide (Varroa destructor), the major driver of colony collapse disorder (CCD), in the apicultural industry. Despite the relatively high tolerance of honey bees to this miticide, recent studies showed several adverse effects. The side effect on cognitive abilities, however, remains still elusive. Here, we aimed to investigate the effects of fluvalinate on the cognitive abilities of honey bees, especially associative learning, and memory. We tested the proboscis extension response (PER) to sugar taste and Pavlovian conditioning in forager bees that received a sub-lethal dose of fluvalinate on the abdomen. The current study demonstrated that sub-lethal fluvalinate induced cognitive impairment in bees. Furthermore, the comparison of gene expression patterns showed that this disorder was caused by changes in the energy metabolism associated with the subsets of specific neuropeptides that are indirectly involved in detoxification processes. Taken together, our findings are strong evidence that xenobiotics affect sensory cognition through indirect effects as well as direct damage. It might be applicable as a novel approach to exploring the mechanisms underlying the side effects of xenobiotics in various organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The western honey bee, Apis mellifera L. (Hymenoptera: Apidae), performs sophisticated social behaviors based on cognitive functions, such as foraging, waggle dance, and hygienic behaviors (Frisch 1967; Seeley 2009). Surprisingly, these behaviors are controlled by the tiny brain of honey bees, consisting of about a million neurons in size of less than 1 mm3 (Witthöft 1967). Moreover, the genome size of this eusocial animal is only a tenth that of human (Weinstock et al. 2006). Thus, the honey bee is a good model organism for understanding the relationship between behavior and cognition through a variety of approaches (Menzel 2012).
Honey bees inside or outside the hive can be exposed to a variety of xenobiotics that do not target honey bees but are still potentially toxic to bees (Johnson 2015). Among them, τ-fluvalinate (hereafter, fluvalinate), widely used in Varroa mites control, belongs to pyrethroid neurotoxins that interfere with the recovery of action potentials through binding to voltage-gated sodium channels (Gupta and Crissman 2013; Soderlund et al. 2002). Even though the voltage-gated sodium channel of the honey bee is as vulnerable to fluvalinate as that of Varroa (Gosselin-Badaroudine and Chahine 2017), the honey bee has a relatively high tolerance against fluvalinate due to rapid detoxification by cytochrome P450 monooxygenase (Johnson et al. 2006; Mao et al. 2011). Albeit fluvalinate can lead to death or several adverse effects on honey bees (Dai et al. 2017; Lim et al. 2020; Rangel and Tarpy 2015; Rinderer et al. 1999). Since efficient cognitive quantifying assays for honey bees in the laboratory conditions are well known (Giurfa and Sandoz 2012), cognitive impairments have been typically studied as a side effect of various xenobiotics (Belzunces et al. 2012; Hesselbach and Scheiner 2018; Williamson and Wright 2013). Previous research showed that the intake of fluvalinate affects gustation, learning, and memory (Frost et al. 2013). In addition, another study reported that fluvalinate and other miticides affected gene expression related to memory (Gashout et al. 2020). Furthermore, we recently demonstrated topical external contact with fluvalinate induces olfactory deficit in foragers (Lim et al. 2020). However, little is known about the mechanism of cognitive impairment caused by xenobiotics, including the relatively innocuous fluvalinate. The aim of the present study delves into investigating whether sub-lethal fluvalinate abdominal contact affects overall cognitive behavior as well as olfactory sensitivity (Lim et al. 2020). We hypothesize that the fluvalinate-induced negative effects on cognition may be an indirect side effect of detoxification, not directly acting as a neurotoxin. In addition, we examined the molecular mechanism underlying the physiological changes induced by xenobiotics. Our findings would be applied to neuroscience research for uncovering insufficient knowledge about cognition.
2 Materials and methods
2.1 Insects
The experiments were performed in April and May 2019 with honey bees (Apis mellifera) from three colonies raised in the Incheon National University Apiary, Republic of Korea (37°22′25″, 126°37′40″). Foragers, who exhibit the most consistent performance in the proboscis extension response (PER) assay and have the highest learning ability among the different tasks, were used for the test (Pham-Delegue et al. 1990; Ray and Ferneyhough 1999). Returning foragers with pollen were caught directly at the entrance of the hive.
2.2 Exposure to fluvalinate
The τ-fluvalinate (#46,294; Sigma–Aldrich, St Louis, MO) was diluted in acetone (#270,725; Sigma-Aldrich) at a concentration of 2 μg/μl, which was identified as a sub-lethal dose in our previous study (Lim et al. 2020). After bees were anesthetized on ice, prepared fluvalinate solution was topically on the ventral side of the abdomen. Then, they were kept in an incubator (darkness/30 ± 2 ˚C/RH 60 ± 5%) and fed sugar candy ad libitum. After 21 h, each bee was harnessed on the trimmed blue tip of a 1-ml pipette for behavioral experiments. Then, the harnessed bees were fed with 50% sucrose (#S0389; Sigma-Aldrich) and returned to the incubator for 3 h without feeding. As described in Figure 1a, the foreleg of harnessed bees was freed to allow self-grooming. For molecular experiments, the survivors were stored in a deep freezer (− 80 ˚C) immediately after 24 h of post-treatment. In entire experiments, the bees exposed to solvent (acetone) were used as a control group.
Comparison of sucrose responsiveness between control (white) and fluvalinate (blue) groups. a Schematic of harnessed bee on trimmed blue tip and its PER. b Sucrose concentration-PER ratio graph. PER rate increases with increasing sucrose concentration in both groups. c Box plot of gustatory response scores. Note that the markedly low scores in the fluvalinate group. Thick lines and whiskers represent median and min/max values, respectively. Statistical significance was determined by Mann–Whitney U test (***P < 0.001, ncontrol = 54, nfluvalinate = 61).
2.3 PER: quantifying gustatory responsiveness
To quantify the gustatory responsiveness, water and a series of sucrose concentrations (10−2.5, −2, −1.5, −1, −0.5, 0 M) were sequentially contacted with the antennae of each bee using a toothpick (Figure 1a). The inter-series interval was 3 min. For each series, individuals with PER were checked. The bees that showed a PER in the water trial were discarded. The sum of PER to the stimulation with six different sucrose concentrations constitutes the gustatory response score (GRS; 0 to 6), which is an excellent measure of a honey bee’s gustatory responsiveness (Scheiner et al. 2015).
2.4 PER: quantifying associative learning and memory
After 3 h of starvation, each harnessed bee was transferred to the arena to test associative olfactory learning and memory (Figure 2a). The arena consists of 24 rooms, each of which has a vacuum flow (0.05 L/min). We used 1 M linalool diluted in mineral oil as a conditioned stimulus (CS) and 1 M sucrose as the unconditioned stimulus (US) (#L2602 linalool, #330,760 mineral oil; Sigma–Aldrich). Twenty microliters of odorant was absorbed onto a filter paper, which was then inserted into a glass Pasteur pipette (#Z310727; Volac, 150 mm; Poulten & Graf Ltd, Barking, UK). The detailed procedure is as follows (Figure 2b).
Comparison of learning and memory between control and fluvalinate groups. a, b Overall schematic diagram of experiment method for proboscis extension response (PER). c Olfactory associative acquisition curve. The acquisition curve of bees treated with the fluvalinate differed significantly from the control (P < 0.0001, ncontrol = 100, nfluvalinate = 100). d Percentage of bees that passed the MTM = 1 h and LTM = 24-h memory test. The fluvalinate group showed a significant decrease in MTM = 1-h memory (Pearson chi-square test, ***P < 0.0001, ncontrol = 72, nfluvalinate = 41).
3 Pre-training
The tested bees were stimulated by constant airflow (0.04 L/min) with linalool for 6 s, which was automatically controlled by a stimulus controller (CS-55, Syntech, Hilversum, Netherlands). In this step, the bees showing spontaneous PER were discarded. After 15 min, the following steps were performed on individuals who passed pre-training.
4 Acquisition
For the six acquisition trials, the conditioned odorant was delivered for 6 s. Immediately after 3 s out of six, the sucrose solution was delivered using a toothpick to the antennae to derive a PER. Then, the bee was allowed to lick sucrose freely while the odorant was still applied. In each trial, the bee was recorded whether it showed PER during the first 3 s of odorant application. The inter-trial interval was 8 min. Each tested bee was given an acquisition score (0 to 5) as described in the previous paper (Scheiner et al. 1999). On the first trial, the bees that displayed PER without the US or did not respond to the US were discarded. After the last trial, the bees were placed in the incubator until the 1-h memory test.
5 Memory test
The memory test was conducted only on the bees that had learned during the acquisition phase. One hour after the acquisition step, the bees were re-transferred to the arena. Then, the bees were presented with the constant flow with CS for 6 s without the US. Of the three trials, individuals that performed at least two PERs were recorded. The inter-trial interval was 5 min. After a 1-h memory test, the bees were fed to satiation with 50% sucrose solution and placed in the incubator for a 24-h memory test. Twenty-four-hour memory test was performed the same way as the 1-h memory test.
5.1 Comparative analysis of gene expression patterns of neuropeptides in the bee brain
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to analyze gene expression patterns. For this, total RNA was isolated from the bee brains using a Qiagen RNeasy Mini Kit (#74,104; Qiagen, Valencia, CA, USA). Three brains were used in each replicate. Using 500 ng of total RNA, cDNA was synthesized with oligo-dT with Invitrogen Superscript III enzyme following the manufacturer’s instructions (#18,080,044; Invitrogen, Carlsbad, CA). qRT-PCR was performed on the AriaMx Real-Time PCR System (#G8830A; Agilent Technologies, Santa Clara, CA) using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (#600,882; Agilent Technologies). All primer sets were synthesized in Macrogen Inc. (Seoul, Korea). For all performed analyses, ribosomal protein 49 (RP49) was used as a housekeeping gene for normalization. Thermal cycling was set at 95 °C for 1 min, followed by 40 cycles of 95 °C for 5 s and 60–63 °C for 10 s. Three technical replicates were performed for each biological replicate, and relative mRNA expression levels were calculated using the 2−△△CT method as described by Livak and Schmittgen (2001).
5.2 Statistical analysis
To determine the effect of fluvalinate, the sucrose responsiveness and the olfactory acquisition curves were compared by generalized linear model (GLM) using binary (PER or not) logistic regression analysis. Mann–Whitney U tests were conducted to analyze the differences in gustatory response scores and acquisition scores between both groups. The number of bees showing PER in the pre-training step was tested using Pearson chi-square test (χ2). The number of individuals that passed the memory test was applied in the same statistical method. Differences in mRNA expression patterns were examined by Student’s t test. All statistical analyses were performed with SPSS® Statics 25 (IBM, Armonk, NY), and graphs were prepared in GraphPad® Prism 8 (GraphPad Software, Inc., La Jolla, CA). Final arrangements were carried out using CorelDRAW Graphics Suite 2020 (Corel Corporation, Ottawa, Canada).
6 Results
6.1 Sub-lethal effect on taste behavior
For forager bees exposed to fluvalinate and control solution, the PER rate increased with increasing sugar concentrations, demonstrating a preference for higher sucrose concentrations regardless of treatment. The comparison between the two groups, however, showed that abdominal contact with fluvalinate has a negative effect (GLM, Wald = 44.673, P < 0.0001; Figure 1b). Even at the highest concentration of sucrose, the response rate of the control group was 96.3% while that of fluvalinate group was 70.5%. Moreover, GRS was significantly reduced in foragers exposed to fluvalinate (Mann–Whitney U tests, U = 869, P < 0.0001, ncontrol = 54, nfluvalinate = 61; Figure 1c).
6.2 Sub-lethal effect on olfactory associative learning and memory
We investigated the effect of fluvalinate on the cognitive abilities of forager bees using classical conditioning of the PER. First, there was no significant difference between groups in the spontaneous response to odor (chi-square test, χ2 = 0.014, P = 0.9070, ncontrol = 129, nfluvalinate = 128; Figure S1). Acquisition tests of six trials demonstrated that fluvalinate abdominal contact reduced olfactory-associated learning ability (GLM, Wald = 78.228w, P < 0.0001, ncontrol = 100, nfluvalinate = 100; Fig. 2c). In the last trial, the acquisition rate of the fluvalinate group was about half of the control group (control = 72.0%, fluvalinate = 37.0%).
Then, we asked whether fluvalinate affected the memory retrieval of learned bees (Figure 2d). In 1-h memory test, the fluvalinate group showed significantly lower memory retrieval than the control (control = 86.1%, fluvalinate = 58.5%; chi-square test, χ2 = 10.923, P < 0.0001, ncontrol = 72, nfluvalinate = 41). In 24-h memory test, however, there is no significant difference between groups (control = 63.0%, fluvalinate = 50.0%; chi-square test, χ2 = 1.488, P = 0.223, ncontrol = 54, nfluvalinate = 36). The honey bee’s memory stages are largely divided into three stages: short-term memory (STM), mid-term memory (MTM), and long-term memory (LTM) (Reviewed in Menzel 2012). Based on this knowledge, our results showed that fluvalinate exposure to the abdomen of forager bees significantly affects MTM but not LTM.
6.3 Sub-lethal effect on gene expression of neuropeptides in the bee brain
Based on previous papers on several neuropeptides that might affect learning and memory (Hergarden et al. 2012; Knapek et al. 2013; Takeuchi et al. 2004), we considered that some neuropeptides might be involved in the mechanisms underlying the negative effects of fluvalinate topically applied to the abdomen of forager bees. Among the candidates, our results demonstrated that short neuropeptide F (sNPF) and tachykinin (TK) in the brain are not involved in the cognitive impairment induced by fluvalinate (t test, tsNPF = 1.869, PsNPF = 0.121; tTK = 0.716, PTK = 0.506; ncontrol = 6, nfluvalinte = 6; Figure S2). Then, we focused on the other candidates that are also associated with juvenile hormone (JH), which is known to affect cognition in the honey bee (Corona et al. 2007). qRT-PCR showed that fluvalinate significantly increases ILP1 and decreases ILP2 in forager’s brain (t test, tILP1 = 3.320, PILP1 = 0.021; tILP2 = 3.421, PILP2 = 0.019; ncontrol = 6, nfluvalinte = 6; Figure 3). In the case of allatostatic factors, only AstCCC was observed to increase significantly in fluvalinate treated bees (t test, tAstA = 1.201, PAstA = 0.283; tAstCC = 1.323, PAstCC = 0.243; tAstCCC = 5.171, PAstCCC = 0.004; ncontrol = 6, nfluvalinte = 6; Figure 4a). On the contrary, allatotropin (AT) was significantly reduced (tAT = 3.000, PAT = 0.030; ncontrol = 6, nfluvalinte = 6; Fig. 4b). C-type allatostatin receptor, the receptor of AstCC and CCC, also increased significantly in fluvalinate abdominal contact (t test, tAstC-R = 4.913, PAstC-R = 0.004; ncontrol = 6, nfluvalinte = 6; Figure S2). Thus, our qRT-PCR data demonstrated that fluvalinate abdominal contact affects the ILP-associated JH pathway in the honey bee brain.
The gene expression patterns of ILPs. Relative mRNA expression levels of ILPs in brain from control (white bar) and sub-lethal dose treated (grey bar) foragers. Each bar represents the mean ± SE. Data points represent values from biological replicates. Statistical significance was determined by student’s t test (*P < 0.05, ncontrol = 6, nfluvalinate = 6).
The gene expression patterns of allatostatic factors. Relative mRNA expression levels of a AstA, CC, CCC, and b AstC-R in brain from control (white bar) and sub-lethal dose treated (grey bar) foragers. Each bar represents the mean ± SE. Data points represent values from biological replicates. Statistical significance was determined by student’s t-test (n.s. = non-significant, *P < 0.05, ncontrol = 6, nfluvalinate = 6).
7 Discussion
Unperceived effects of fluvalinate on honey bee health have led beekeepers to abuse fluvalinate to control Varroa (Johnson et al. 2006). However, fluvalinate intake or injection was reported to have adverse effects on the various physiological activities of honey bees (Dai et al. 2017; Rangel and Tarpy 2015; Rinderer et al. 1999). Recently, we proved that fluvalinate is highly toxic enough to kill bees by abdominal contact alone (Lim et al. 2020). In the present study, we also identified that more bees in the fluvalinate group died between acquisition and memory testing than in the control group. It was also demonstrated that abdominal contact with fluvalinate induces a significant decrease in taste responsiveness. It was revealed that exposure to sub-lethal doses causes a reduction of olfactory sensitivity in foragers (Lim et al. 2020). In the current study, moreover, we demonstrated another cognitive impairment caused by fluvalinate abdominal contact. Along with the results of a previous study on intake experiments (Frost et al. 2013), these findings provide strong evidence that fluvalinate, which is known to be relatively safe for honey bees, may impair cognitive function. The study found negative effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival, particularly at high oral doses. However, the high dose (1.25 μg) used in the study was lower than the dose used in our experiments (2 μg). Yet, little is known about molecular and neural mechanisms underlying the effects of fluvalinate on the cognitive function of honey bees.
7.1 Cognitive impairment indirectly induced by xenobiotics
Cognitive function is involved in various types of behavior and is essential to maintain the honey bee colony. In the insect brain, the main region of cognitive function, which plays the role of the hippocampus in mammals, is the mushroom body (MB) (Menzel 2012). MB might be affected by xenobiotics, as the hippocampus is affected (Nasuti et al. 2014). The cognitive function of honey bees can be impaired by the direct impact of xenobiotics on synaptic receptors, neuronal ion channels, and cellular signaling. Pyrethroids like fluvalinate can directly impair the olfactory-cognitive neurons of the bees by modifying the voltage-gated sodium channel (Kadala et al. 2014). In nature, however, due to the efficient detoxification mechanism of honey bees on pyrethroid, especially fluvalinate (Johnson et al. 2006), this xenobiotic may not be a direct factor in the cognitive impairment of honey bees. Interestingly, however, cognitive impairment was observed 1 h after acute contact with fluvalinate in our previous and current studies. Within this period, fluvalinate was sufficiently degraded by CYP450 systems in honey bees (Hillier et al. 2013). Interestingly, some metabolites converted from xenobiotics by detoxification are as toxic as their parent compounds (Guez et al. 2003; Nauen et al. 2001; Suchail et al. 2001). Fluvalinate is also transformed into metabolite (4′-hydroxylated subfragment) by CYP450s (Mao et al. 2011), which may cause severe toxic or indirect effects on neurophysiological impairment or mental disorders.
Another possibility is that there might be changes in energy demand from detoxification processes which may have an effect. The xenobiotic detoxification process, in general, sequentially involves the degradation of toxins (Phase I: functionalization), and these products are conjugated (Phase II: conjugation) and excreted from the cells (Phase III: excretion) (Reviewed in Berenbaum and Johnson 2015). Although the number of enzyme genes involved in the detoxification mechanism is relatively low (Claudianos et al. 2006), honey bees are no more susceptible to xenobiotics than other insects (Hardstone and Scott 2010). Similar to some insects that require a fitness cost for tolerance to insecticides, detoxification processes in honey bees increase energy demand (Kliot and Ghanim 2012). Furthermore, increased energy demand by detoxification upregulates energy metabolism which leads to increased production of reactive oxygen species (ROS) (Rand et al. 2015). ROS production, typically, is known to be a major factor in neurodegenerative diseases that accompany cognitive impairment, such as Parkinson’s and Alzheimer’s disease in mammals (Smith et al. 2002; Tabner et al. 2001). Surprisingly, ROS production not only affects the cognitive function of vertebrates (Liu et al. 2003) but can also cause cognitive impairment in honeybee and Drosophila models (Farooqui 2008; Haddadi et al. 2014). In the current study, we identified that fluvalinate significantly increases ILP1 and decreases ILP2; thus, fluvalinate abdominal contact affects the ILP-associated JH pathway in the honey bee brain (Shpigler et al. 2021). Thus, cognitive impairment may be caused by changes in energy metabolism induced by detoxification. Our study indicates that another pathway in neuropeptide expression has been downregulated by the detoxification processes.
7.2 Molecular mechanism underlying cognitive impairment
The cognitive ability of honey bees is highly dependent on behavioral development. Young honey bees have poor cognitive abilities, but they gradually improve with age and peak when they become foragers (Ray and Ferneyhough 1999). Juvenile hormone (JH), the key regulator of behavior development, might be involved in the mechanism of cognition by organizing the neuroanatomy of MB in the honey bee brain (Withers et al. 1995). Indeed, JH-injected bees showed significantly enhanced both appetitive and aversive olfactory learning and 1-h memory recall (MTM) (McQuillan et al. 2014).
JH of insects, generally, is synthesized in the corpora allata by regulation of neuropeptides. These regulatory peptides consist of three types of inhibitory Ast and one stimulatory AT, depending on the species (Weaver and Audsley 2009). In the A. mellifera genome, AT and one A-type (FGLamide) and two C-type (PISCF) AST (ASTCC and CCC) are present, but the B-type (W(X6)Wamide) AST is absent (Veenstra 2016; Veenstra et al. 2012). Although it is not clear which honeybee ASTs are JH synthesis inhibitors, previous reports imply that C-type AST (in particular AST-CCC) plays a role in inhibitory effects on JH synthesis in honey bees (Urlacher et al. 2016). In our study, qRT-PCR of Ast-CCC significantly increased, and it might also affect as an inhibitor of JH synthesis. Furthermore, the artificial increase of two C-type ASTs reduces the olfactory learning ability of foragers, but only AST-CC affects young bees (Urlacher et al. 2019). In the current study, we tested only forager bees, and qRT-PCR of Ast-CC was increased, but it was not significant. It might be due to the significant reduction of JH synthesis by C-type AST in the forager that was more affected by JH than young bees (Elekonich et al. 2001). Interestingly, C-type AST in Aedes aegypti acts as the JH synthesis inhibitor by blocking the transport of citrate from mitochondria to cytosol (Nouzova et al. 2015). Blocked citrate can be used in the mitochondrial TCA cycle rather than cytoplasmic JH synthesis to increase energy metabolism. In addition, JH synthesis relies on the balance of AST and AT, which is usually regulated by nutritional status (Noriega 2004). Therefore, our findings demonstrated that xenobiotic detoxification could increase energy demand and consequent reduction of JH (Shpigler et al. 2021). Upstream of allatostatic or -tropic mechanism may include ILPs that are highly related to nutrient status (Colombani et al. 2003; Corona et al. 2007). Although many lines of research on the interaction between JH and two types of ILP in honeybees have been conducted, its complex relationships with neural and molecular mechanisms underlying memory modulation are still unclear (Ament et al. 2008; Ihle et al. 2014; Nilsen et al. 2011). In our present study, the change in the ILPs genes induced by fluvalinate suggests a correlation between AST, AT, and JH, which may be downstream of ILPs. Thus, we propose a novel hypothetical model in which cognitive impairment can be induced by ILPs-JH interaction (Figure 5).
Hypothetical model of cognitive impairment caused by fluvalinate exposure. Fluvalinate causes the need for detoxification, thus increasing the energy demand. This in turn changes ILP expression in brain, which influences the expression of downstream Ast-CCC and AT. As a result, JH expression in corpora cardiaca (CC) and corpora allata (CA) is reduced, followed by cognitive impairment.
In summary, our findings suggest that cognitive impairment induced by abdominal contact with fluvalinate is an indirect side effect rather than direct damage to neurons associated with learning and memory. The initiation of this indirect mechanism is the change in energy demand by detoxification. Subsequently, neuropeptide functions in cellular and molecular cascades may lead to the regulation of JH synthesis, leading to physiological behavioral changes. Our research suggests a new approach to understanding the side effects caused by xenobiotics. More studies are needed to investigate further mechanisms underlying the interplay of neuropeptides and toxification processes in detail.
Data availability
All the relevant experimental data are included in the manuscript.
Code availability
Not applicable.
References
Ament SA, Corona M, Pollock HS, Robinson GE (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci 105:4226–4231
Belzunces LP, Tchamitchian S, Brunet J-L (2012) Neural effects of insecticides in the honey bee. Apidologie 43:348–370
Berenbaum MR, Johnson RM (2015) Xenobiotic detoxification pathways in honey bees. Curr Opin Insect Sci 10:51–58
Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Feyereisen R, Oakeshott JG (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15:615–636
Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P (2003) A nutrient sensor mechanism controls Drosophila growth. Cell 114:739–749
Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE (2007) Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci 104:7128–7133
Dai P, Jack CJ, Mortensen AN, Ellis JD (2017) Acute toxicity of five pesticides to Apis mellifera larvae reared in vitro. Pest Manag Sci 73:2282–2286
Elekonich MM, Schulz DJ, Bloch G, Robinson GE (2001) Juvenile hormone levels in honey bee (Apis mellifera L.) foragers: foraging experience and diurnal variation. J Insect Physiol 47:1119–1125
Farooqui T (2008) Iron-induced oxidative stress modulates olfactory learning and memory in honeybees. Behav Neurosci 122:433–447
Frisch KV (1967) The dance language and orientation of bees. Belknap Press of Harvard University Press, Cambridge, Mass
Frost EH, Shutler D, Hillier NK (2013) Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J Exp Biol 216:2931–2938
Gashout HA, Guzman-Novoa E, Goodwin PH, Correa-Benítez A (2020) Impact of sublethal exposure to synthetic and natural acaricides on honey bee (Apis mellifera) memory and expression of genes related to memory. J Insect Physiol 121:104014
Giurfa M, Sandoz JC (2012) Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem 19:54–66
Gosselin-Badaroudine P, Chahine M (2017) Biophysical characterization of the Varroa destructor NaV1 sodium channel and its affinity for τ-fluvalinate insecticide. FASEB J 31:3066–3071
Guez D, Belzunces LP, Maleszka R (2003) Effects of imidacloprid metabolites on habituation in honeybees suggest the existence of two subtypes of nicotinic receptors differentially expressed during adult development. Pharmacol Biochem Behav 75:217–222
Gupta RC, Crissman JW (2013) Chapter 42 - agricultural chemicals. In: Third Edition)" (W. M. Haschek, C. G. Rousseaux and M. A. Wallig, (ed) "Haschek and Rousseaux’s Handbook of Toxicologic Pathology. Academic Press, Boston, pp 1349–1372
Haddadi M, Jahromi SR, Sagar BK, Patil RK, Shivanandappa T, Ramesh SR (2014) Brain aging, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav Brain Res 259:60–69
Hardstone MC, Scott JG (2010) Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci 66:1171–1180
Hergarden AC, Tayler TD, Anderson DJ (2012) Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A 109:3967–3972
Hesselbach H, Scheiner R (2018) Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci Rep 8:4954
Hillier NK, Frost EH, Shutler D (2013) Fate of dermally applied miticides fluvalinate and amitraz within honey bee (Hymenoptera: Apidae) bodies. J Econ Entomol 106:558–565
Ihle KE, Baker NA, Amdam GV (2014) Insulin-like peptide response to nutritional input in honey bee workers. J Insect Physiol 69:49–55
Johnson RM (2015) Honey bee toxicology. Annu Rev Entomol 60:415–434
Johnson RM, Wen Z, Schuler MA, Berenbaum MR (2006) Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J Econ Entomol 99:1046–1050
Kadala A, Charreton M, Jakob I, Cens T, Rousset M, Chahine M, Le Conte Y, Charnet P, Collet C (2014) Pyrethroids differentially alter voltage-gated sodium channels from the honeybee central olfactory neurons. PLoS ONE 9:e112194
Kliot A, Ghanim M (2012) Fitness costs associated with insecticide resistance. Pest Manag Sci 68:1431–1437
Knapek S, Kahsai L, Winther AM, Tanimoto H, Nassel DR (2013) Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. J Neurosci 33:5340–5345
Lim S, Yunusbaev U, Ilyasov R, Lee HS, Kwon HW (2020) Abdominal contact of fluvalinate induces olfactory deficit in Apis mellifera. Pestic Biochem Physiol 164:221–227
Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M (2003) Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci 100:8526–8531
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Mao W, Schuler MA, Berenbaum MR (2011) CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc Natl Acad Sci U S A 108:12657–12662
McQuillan HJ, Nakagawa S, Mercer AR (2014) Juvenile hormone enhances aversive learning performance in 2-day old worker honey bees while reducing their attraction to queen mandibular pheromone. PLoS ONE 9:e112740
Menzel R (2012) The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci 13:758–768
Nasuti C, Fattoretti P, Carloni M, Fedeli D, Ubaldi M, Ciccocioppo R, Gabbianelli R (2014) Neonatal exposure to permethrin pesticide causes lifelong fear and spatial learning deficits and alters hippocampal morphology of synapses. J Neurodev Disord 6:7
Nauen R, Ebbinghaus-Kintscher U, Schmuck R (2001) Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manag Sci 57:577–586
Nilsen K-A, Ihle KE, Frederick K, Fondrk MK, Smedal B, Hartfelder K, Amdam GV (2011) Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J Exp Biol 214:1488–1497
Noriega FG (2004) Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochem Mol Biol 34:687–693
Nouzova M, Rivera-Perez C, Noriega FG (2015) Allatostatin-C reversibly blocks the transport of citrate out of the mitochondria and inhibits juvenile hormone synthesis in mosquitoes. Insect Biochem Mol Biol 57:20–26
Pham-Delegue MH, De Jong R, Masson C (1990) Age-dependency of the conditioned proboscis extension response in honeybees. Comptes Rendus De L’academie Des Sciences - Serie III 310:527–532
Rand EED, Smit S, Beukes M, Apostolides Z, Pirk CWW, Nicolson SW (2015) Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci Rep 5:11779
Rangel J, Tarpy DR (2015) The combined effects of miticides on the mating health of honey bee (Apis melliferaL.) queens. J Apic Res 54:275–283
Ray S, Ferneyhough B (1999) Behavioral development and olfactory learning in the honeybee (Apis mellifera). Dev Psychobiol 34:21–27
Rinderer TE, Guzman LID, Lancaster VA, Delatte GT, Stelzer JA (1999) Varroa in the mating yard. I. The effects of Varroa jacobsoni and apistan on drone honey bees. Am Bee J 139
Scheiner R, Abramson CI, Brodschneider R, Crailsheim K, Farina WM, Fuchs S, Grünewald B, Hahshold S, Karrer M, Koeniger G, Koeniger N, Menzel R, Mujagic S, Radspieler G, Schmickl T, Schneider C, Siegel AJ, Szopek M, Thenius R (2015) Standard methods for behavioural studies ofApis mellifera. J Apic Res 52:1–58
Scheiner R, Erber J, Page RE Jr (1999) Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J Comp Physiol A 185:1–10
Seeley TD (2009) The Wisdom of the Hive: the social physiology of honey bee colonies. Harvard University Press
Shpigler HY, Magory Cohen T, Ben-Shimol E, Ben-Betzalel R, Levin E (2021) Juvenile hormone functions as a metabolic rate accelerator in bumble bees (Bombus terrestris). Horm Behav 136:105073
Smith MA, Perry G, Pryor WA (2002) Causes and consequences of oxidative stress in Alzheimer’s disease1, 2 1Guest Editors: Mark A. Smith and George Perry 2This article is part of a series of reviews on “Causes and consequences of oxidative stress in Alzheimer’s disease.” The full list of papers may be found on the homepage of the journal. Free Rad Biol Med 32:1049
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171:3–59
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20:2482–2486
Tabner BJ, Turnbull S, El-Agnaf O, Allsop D (2001) Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr Top Med Chem 1:507–517
Takeuchi H, Yasuda A, Yasuda-Kamatani Y, Sawata M, Matsuo Y, Kato A, Tsujimoto A, Nakajima T, Kubo T (2004) Prepro-tachykinin gene expression in the brain of the honeybee Apis mellifera. Cell Tissue Res 316:281–293
Urlacher E, Devaud JM, Mercer AR (2019) Changes in responsiveness to allatostatin treatment accompany shifts in stress reactivity in young worker honey bees. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 205:51–59
Urlacher E, Soustelle L, Parmentier ML, Verlinden H, Gherardi MJ, Fourmy D, Mercer AR, Devaud JM, Massou I (2016) Honey bee allatostatins target galanin/somatostatin-like receptors and modulate learning: a conserved function? PLoS ONE 11:e0146248
Veenstra JA (2016) Allatostatins C, double C and triple C, the result of a local gene triplication in an ancestral arthropod. Gen Comp Endocrinol 230–231:153–157
Veenstra JA, Rodriguez L, Weaver RJ (2012) Allatotropin, leucokinin and AKH in honey bees and other Hymenoptera. Peptides 35:122–130
Weaver RJ, Audsley N (2009) Neuropeptide regulators of juvenile hormone synthesis: structures, functions, distribution, and unanswered questions. Ann N Y Acad Sci 1163:316–329
Weinstock GM, Robinson GE, Gibbs RA, Weinstock GM, Weinstock GM, Robinson GE, Worley KC, Evans JD, Maleszka R, Robertson HM, Weaver DB, Beye M, Bork P, Elsik CG, Evans JD, Hartfelder K, Hunt GJ, Robertson HM, Robinson GE, Maleszka R, Weinstock GM, Worley KC, Zdobnov EM, Hartfelder K, Amdam GV, Bitondi MMG, Collins AM, Cristino AS, Evans JD, Michael H, Lattorff G, Lobo CH, Moritz RFA, Nunes FMF, Page RE, Simões ZLP, Wheeler D, Carninci P, Fukuda S, Hayashizaki Y, Kai C, Kawai J, Sakazume N, Sasaki D, Tagami M, Maleszka R, Amdam GV, Albert S, Baggerman G, Beggs KT, Bloch G, Cazzamali G, Cohen M, Drapeau MD, Eisenhardt D, Emore C, Ewing MA, Fahrbach SE, Forêt S, Grimmelikhuijzen CJP, Hauser F, Hummon AB, Hunt GJ, Huybrechts J, Jones AK, Kadowaki T, Kaplan N, Kucharski R, Leboulle G, Linial M, Littleton JT, Mercer AR, Page RE, Robertson HM, Robinson GE, Richmond TA, RodriguezZas SL, Rubin EB, Sattelle DB, Schlipalius D, Schoofs L, Shemesh Y, Sweedler JV, Velarde R, Verleyen P, Vierstraete E, Williamson MR, Beye M, Ament SA, Brown SJ, Corona M, Dearden PK, Dunn WA, Elekonich MM, Elsik CG, Forêt S, Fujiyuki T, Gattermeier I, Gempe T, Hasselmann M et al (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443:931–949
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807
Withers GS, Fahrbach SE, Robinson GE (1995) Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. J Neurobiol 26:130–144
Witthöft W (1967) Absolute anzahl und verteilung der zellen im him der honigbiene. Zeitschrift Für Morphologie Der Tiere 61:160–184
Funding
This research was supported by “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ014761012022)” from Rural Development Administration, Republic of Korea and also by Incheon National University Research Grant (2018) to HWK.
Author information
Authors and Affiliations
Contributions
YY, HJK, SL, JL, and HWK conceived this research and designed the experiments. YY, HJK, SL, and JL performed the experiments, analysis, and art work. YY, HJK, SL, JL, and HWK wrote the manuscript. HWK supervised all the research, experiments, and project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participant
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Zachary Huang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yun, Y., Kim, H.J., Lim, SH. et al. Cognitive impairment caused by abdominal exposure with fluvalinate in the Western honey bee, Apis mellifera. Apidologie 54, 48 (2023). https://doi.org/10.1007/s13592-023-01026-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01026-8