Abstract
The western honey bee is highly valued for its important role as natural pollinator. In Morocco, pollination services of Apis mellifera along with other insect pollinators were estimated at 1.2 billion US$. Regrettably, biological stressors including pathogens are leading factors contributing to colony losses and hampering pollination services. The aim of this study was to investigate the occurrence and prevalence of four agents responsible for nosemosis and foulbrood diseases in the oases of southeastern Morocco. Using polymerase chain reaction (PCR), we have analyzed 87 beehives sampled during two successive years from four provinces and representing two different beekeeping operation types, namely migratory (N = 51) versus stationary (N = 36). The results obtained revealed the absence of Nosema apis and Paenibacillus larvae. However, Nosema ceranae and Melissococcus plutonius were present at prevalence rates of 16.09% and 62.07% respectively. During the winter of 2020, there was higher prevalence rates of both pathogens compared to the winter of 2019. The province of Errachidia was free of N. ceranae, while the other three provinces were infected with different prevalence rates. On the other hand, M. plutonius was more prevalent in all four sampling areas. In addition, infection and co-infection patterns of N. ceranae and M. plutonius were found to be more prevalent in migratory beehives as opposed to stationary colonies. Results of this work constitute the first contribution to study infection patterns of nosemosis and foulbrood in Morocco, while more inquiries are needed in order to elaborate sustainable strategies for the beekeeping industry in Morocco.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The western honey bee is a globally distributed pollinator with more than 31 subspecies, disseminated naturally in Africa, Europe, and western Asia (Ruttner 1988; Ilyasov et al. 2020). This species is largely appreciated for its important role as a pollinator of natural ecosystems and industrial crops all around the world. In fact, the economic value of insect pollination (mainly honey bees) was estimated at 4.5 billion US$ in North Africa (Gallai et al. 2009), and at 1.2 billion US$ in Morocco, which accounts for 8.52% of the total value of the agricultural GDP (Sabbahi 2022). Furthermore, 68.57% of Morocco’s major crops are directly affected by insect pollination including honey bees (Sabbahi 2022).
In recent decades, beekeeping in Morocco has become one of the main agricultural activities that contribute to improving the income of local populations. According to the Ministry of Agriculture, there were about 910,100 beehives that have been registered in Morocco during the year 2019 with an increase of 482% compared with the year 2009. In addition, the overall honey production for the same year has reached 7960 tonnes with an added value of 0.08 billion US$ (Ministry of Agriculture 2019). Furthermore, during the year 2020, the overall production has reached 8334 tonnes of honey and 59 tonnes of beeswax (FAOSTAT 2020).
Regrettably, the human food security is currently at high risk as there are many reports of registered honey bee colony loses all over the world (Gray et al. 2019, 2020, 2022). Morocco is no exception, as the year of 2021/2022 has registered high and unprecedent beehives mortality reaching a mean rate of 30% and ranged between 4 and 80% depending on region (Ministry of Agriculture 2022). The reasons behind these losses have been deeply investigated by researchers and blamed on a variety of factors including climate change, pesticides, beekeeping practices, land use intensification, and habitat loss, fragmentation, and degradation (Potts et al. 2010). However, parasites and pathogens remain one of the leading factors responsible for honey bee mortality (Potts et al. 2010). Nosemosis and foulbrood are two well-known diseases found to be infecting the western honey bees Apis mellifera. Nosema apis and Nosema ceranae — recently renamed as Vairimorpha ceranae (Tokarev et al. 2019) — are the two microsporidian species responsible for the Nosema infection (Fries et al. 1996, 2013; Higes et al. 2006). On the other hand, Paenibacillus larvae and Melissococcus plutonius are the two bacteria responsible for American and European foulbrood respectively (de Graaf et al. 2013; Forsgren et al. 2013). Both diseases are listed in the world organization for animal health (WOAH Terrestrial Animal Health Code 2022) and are widely known to cause substantial economic losses to beehives and beekeepers worldwide. Nosemosis is a common infection of brood and adult honey bees (Fries et al. 2013), while both foulbrood types are recognized to infect honey bee brood (de Graaf et al. 2013; Forsgren et al. 2013). Nosema ceranae, Paenibacillus larvae, and Melissococcus plutonius have already been detected in Moroccan apiaries (Matheson 1993; Roudel et al. 2013); however, no information is available regarding the occurrence of Nosema apis (Boncristiani et al. 2020). To our knowledge, none of the four pathogenic agents have been investigated in Moroccan beehives in terms of their prevalence, intensity, and geographic distribution. Thus, the goal of this study is to investigate with molecular analyses the occurrence, prevalence, infection levels, and geographic distribution of the four agents responsible for nosemosis and foulbrood diseases.
2 Materials and methods
2.1 Study area and sampling
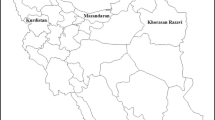
Honey bee samples consisting of 30 worker bees per colony, when possible, were obtained from four provinces of southeastern oases of Morocco (Figure 1). Samples were taken from a total number of 87 clinically healthy beehives distributed along the Darâa-Tafilalet region as follows: Ouarzazate “N = 29”, Zagora “N = 19”, Tinghir “N = 28”, and Errachidia “N = 11”. The colonies were chosen based on two beekeeping operation types: stationary beehives “N = 36” and migratory beehives “N = 51”. Sampling was carried out during winter season (December through February) of two consecutive years. During winter 2019/2020, all four provinces were sampled “N = 64”; however, during winter 2020/2021, only Tinghir and Zagora were sampled “N = 23”.
2.2 Nosema spore count
Detection and quantification of Nosema spp. was traditionally performed using light microscopy, and the infection level was estimated using a hemocytometer to count spores according to the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2022). Positive samples were then submitted to species identification with a PCR end-point method. All samples with a concentration of < 3300 spores/bee, which is a very low level of infection, according to the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2022) were not used for molecular diagnosis.
2.3 Homogenate preparation
Each honey bee sample was washed three times with PBS ISO solution (IZSLER, Brescia). Samples were mechanically homogenized in a stomacher bag by keeping the ratio of 3 honey bee/1 mL physiological saline (max. 30 honey bee/10 mL). The filtered homogenate was collected and stored at − 70 °C for further investigation.
2.4 DNA extraction
Two milliliters of each bee filtered homogenate was centrifuged at 21,000 × g for 10 min. The obtained pellet was washed with 1 mL of a solution containing 10 mM Tris–HCl, 1 mM EDTA, and NaCl 0.9%, centrifuged at 21,000 × g for 10 min, resuspended in 400 µL of lysozyme solution, and incubated for 1 h at 37 °C according to the protocol previously described by Bakonyi et al. (2003). Then, DNA was purified according to QIA symphony DSP Virus/Pathogen kit (Qiagen, Hilden, Germany) with the addition of 10 µL of Internal Control (IC) High Concentration (Qiagen, Hilden, Germany). At the end, DNA was eluted in 60 µL of elution buffer according to the manufacturer’s instructions.
2.5 End-point PCR for the detection of nosemosis
DNA was amplified using different sets of primers depending on the pathogen investigated (Table I). For N. ceranae and N. apis, two different PCR were performed in a total volume of 25 µL containing a final concentration of 1X GoTaq Hot Start Master Mix 2x (Promega, Madison, WI, USA), 1 mM MgCl2, 0.1 µM of each set of primers, and 5 µL of DNA. PCR was carried out according to the listed thermal cycling conditions: denaturation step at 95 °C for 2 min and by 40 cycles of PCR, each consisting of 30 s at 95 °C, 30 s at 55 °C (N. apis) and at 62 °C (N. ceranae), and 1 min at 73 °C. Reactions were completed by a final elongation step for 5 min at 73 °C. The PCR products were electrophoresed in a 2% Tris–acetate-EDTA-agarose gel and stained with GelRed Nucleic Acid Stain (BIOTIUM). A UV trans-illumination was used to visualize the DNA bands. The size of DNA fragments was estimated by comparison with the 100 bp DNA Ladder (Invitrogen, Waltham, MA, USA).
2.6 Real-time PCR assays for the detection of foulbrood agents
Two distinct real-time PCR assays were set up for each sample: one for determining the presence/absence of Paenibacillus larvae DNA and one for the presence/absence of Melissococcus plutonius DNA. Both real-time PCR assays were performed with the QuantiFast Pathogen PCR + (Internal Control) IC kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For Paenibacillus larvae, the amplification reactions were carried out in a final volume of 25 µL containing 5 µL of DNA extract, 1 X QuantiFast Pathogen PCR Master Mix with ROX, 350 nM of forward primer, 700 nM of reverse primer, 200 nM of probe (Table I), 1 X Internal Control Assay (Qiagen, Hilden, Germany), and RNase-Free water according to Carra et al. (2022). For Melissococcus plutonius, the amplification reactions were set up as for P. larvae using 700 nM of both primers and 200 nM of the probe according to Roetschi et al. (2008) and listed in Table I. For both targets, the thermal profile was composed of an initial denaturation step at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s, as recommended by the manufacturer’s instructions of the Master Mix. A fluorescent signal was detected during the annealing/extension step at each cycle. All of the assays were performed on a StepOnePlus™ Real-Time PCR System, Thermo Fisher Scientific (formerly Applied Biosystems™) (Waltham, MA, USA). At the end of each run, all of the samples tested were evaluated for the presence of an amplification curve of the IC, and then evaluated for the presence/absence of the specific target curve. In the case of the absence of the IC curve, the samples were retested and diluted 1/10 in order to reduce the PCR inhibition factor.
2.7 Statistical analysis
Chi-square test was used to determine possible association between infection prevalence and beekeeping operation type. Likewise, Mann–Whitney test was used to compare results of infection levels (based on cycle threshold (CT) values) between the two beekeeping operation types. A 5% level of significance was considered in all analyses. All statistical analyses were performed using ggstatsplot package (Patil 2021) under R software ver. 4.2.2.
3 Results
3.1 Prevalence rates of nosemosis and foulbrood infections
All investigated colonies across both years have resulted in a negative detection of either N. apis and P. larvae. Conversely, infections were detected in 14 (16.09%) and 54 (62.07%) out of the 87 beehives sampled for N. ceranae and M. plutonius, respectively. Both of the detected pathogens showed an overall higher prevalence rate during the winter of 2020 (47.83% and 69.57% respectively for N. ceranae and M. plutonius) in comparison to the winter of 2019 (4.69% and 59.38% respectively for N. ceranae and M. plutonius) (Figure 3). It is worth mentioning that for both pathogens, the prevalence rate decreases in stationary beehives during the second year, while the opposite was observed for migratory beehives (Figure 3). All analyzed samples from Errachidia province showed no infection with N. ceranae, while the three other provinces showed varying infection rates (Figure 1). In contrast, honey bee samples from all four provinces were positively infected with M. plutonius. The highest prevalence rate was found in Ouarzazate (68.97%), followed by Errachidia (63.64%), Zagora (63.16%), and finally Tinghir (53.57%) (Figure 1).
Interestingly, the analysis of pathogens’ infection based on the beekeeping operation type followed by beekeepers has revealed that stationary beehives showed lower prevalence rates (8% and 53% for N. ceranae and M. plutonius, respectively) against higher rates in migratory beehives (22% and 69% respectively for the same pathogens) (Figure 2a and b). However, statistical analysis based on chi-square test revealed no significant difference for both pathogens (χ2 = 2.74, p = 0.10 and χ2 = 2.25, p = 0.13 respectively for N. ceranae and M. plutonius).
Out of the 87 analyzed beehives, 30 were found free of any of the investigated pathogens (34.48%). Conversely, 46 colonies were found to be infected with a single pathogen (52.87%), while only 11 of them revealed double infection (12.64%) (Figure 2c). The comparison of co-infection patterns shows that stationary beehives with no infection were more abundant (44%) than their migratory relatives (27%). The same trend was also observed when comparing single and double infection between the two beekeeping operation types, where stationary colonies were less infected than migratory ones. However, chi-square test also shows no association between co-infection patterns and beekeeping operation type (χ2 = 4.30, p = 0.12).
3.2 Evaluation of M. plutonius infection levels
In the case of samples resulted positive by real-time PCR for the presence of M. plutonius DNA, it was possible to do a semi-quantitative assessment of the infection level on the basis of the cycle threshold (CT) values obtained. Higher and lower CT values indicate lower and higher infection levels respectively. In particular, cycle thresholds below 26 were categorized as severe infection, between 26 and 33 as moderate infection, and above 33 as low infection. The results obtained indicated that 33 (61.11%) colonies were characterized as having low infection, while 6 (11.11%) colonies were designated as moderately infected, and finally, 15 (27.78%) colonies were classified as heavily infected.
Our survey included determination of beekeeping operation type, which enabled us to differentiated between migratory versus stationary colonies. We initially hypothesized that higher pathogen loads would occur within colonies of the former operation type. Surprisingly, results of the analysis have revealed that migratory beehives showed slightly lower infection levels with M. plutonius over stationary beehives; however, this difference was not statistically significant (Wmann-Whitney = 396, p = 0.25) (Figure 4).
Distribution of M. plutonius real-time PCR cycle threshold (Ct) values between migratory and stationary beehives, a semi-quantitative assessment of the infection level was done on the basis of the Ct values obtained. On Y-axis, high Ct values correspond to low positivity and vice versa, low Ct values correspond to high positivity. The horizontal lines corresponding to the Ct values 26 and 33 specifying the three infection levels: below 26 high infection, between 26 and 33 as moderate infection, and above 33 as low infection.
4 Discussion
The epidemiological survey conducted in this study to investigate nosemosis and foulbrood infecting agents has revealed the absence of N. apis and P. larvae from honey bee populations in the oases of southeastern Morocco. The American Foulbrood has already been detected in Morocco (Matheson 1993). However, to our knowledge, there are no studies investigating the prevalence and geographic distribution of this pathogen in Moroccan beehives. The AFB is present widely throughout northern Africa (Boncristiani et al. 2020). To date, there is no report of N. apis being present in Moroccan apiaries (Boncristiani et al. 2020). Nevertheless, this pathogen has been detected in honey bee colonies from other north African countries such as Algeria (Higes et al. 2009) and Tunisia (Abdi et al. 2018).
N. ceranae was detected in honey bee colonies of southeastern Morocco with a prevalence rate of 16.09%. The infection rate in our study was lower than that found in northern (> 90%) and southern (> 80%) Algeria (Adjlane et al. 2015; Menail et al. 2016) as well as in other Mediterranean countries: Italy (Bordin et al. 2022 (> 60%); Cilia et al. 2022 (42.9%)), Turkey (> 26%) (Özkırım et al. 2019), and Spain (> 39%) (Buendía et al. 2018). However, this low infection could be related to the sampling period of our study (winter season), as the majority of the aforementioned studies were carried out during spring/autumn season. Several studies have reported that N. ceranae is now displacing N. apis in honey bee colonies (Traver and Fell 2011; Martín-Hernández et al. 2018). This might be the reason behind the absence of this latter in our study; however, this cannot be confirmed since there are no historical reports of neither its occurrence nor its prevalence in Moroccan beehives.
Although the AFB and EFB are brood diseases, we carried out the analysis on asymptomatic adult bees and not brood samples. During cleaning activities, adult bees become contaminated by P. larvae spores which makes them one of the possible diagnosis matrices suitable for foulbrood diseases. In fact, reports show that larval mortality was closely linked to the spore load of adult bees (Lindström et al. 2008). In addition, a recent study has shown by mean of a comparison between different matrices for the detection of AFB that adult bees are well-suited as sample materials for the early detection of P. larvae spores (Ebeling et al. 2023).
Our survey has identified the occurrence of bees infected by M. plutonius, the causative agent of European Foulbrood, with an average prevalence rate of 62.07%. The investigation carried out in the extreme of north-east Algeria during two consecutive years has resulted in a negative detection of M. plutonius (Menail et al. 2016). Prevalence rate in our study was higher than the one reported from Turkey (Mayack and Hakanoğlu 2022). Although our enquiry revealed high prevalence rates of the EFB’s agent and the molecular analysis performed did not allow for exact quantification, the real-time PCR threshold cycle (Ct) of positivity can serve as an estimate of pathogen load (Roetschi et al. 2008). On the basis of the semi-quantitative assessment done, it was possible to infer that the infection severity was low with the majority of bee samples showing real-time PCR cycle thresholds above 33.
To study the effect of beekeeping management on the infection level and/or prevalence of N. ceranae and M. plutonius, we compared infection and co-infection patterns between migratory and stationary beehives. Results of the infection patterns have revealed that both pathogens were more prevalent in migratory colonies than in stationary ones. Similarly, co-infection patterns indicate higher prevalence of single and double infections in migratory beehives as well as a higher number of pathogen-free colonies in stationary colonies. Nevertheless, statistical analyses have ruled out any association between infection/co-infection patterns and beekeeping operation type. Unexpectedly, M. plutonius infection level was higher in stationary beehives; however, this difference was also not statistically significant. The lack of statistical support could be attributed to the low number of tested bee colonies or simply to the fact that honey bee samples were obtained from small scale beekeepers with generally low intensive migratory beekeeping. It is worth mentioning that there are other factors underlying pathogens’ prevalence such as operation size, land use, time of year, and ecosystem type which makes it difficult to interpret differences between the two beekeeping operation types (Pfeiffer and Crowder 2022; Simone-Finstrom et al. 2022). There is a variety of research investigations that have highlighted the negative effect of migratory beekeeping as one of the factors inducing susceptibility to various diseases and acting as pathogen spillover both in honey bees and wild pollinators (Alger et al. 2018; Simone-Finstrom et al. 2022; Martínez-López et al. 2022). For instance, prevalence analysis of several pathogens including viruses, bacteria, and microsporidia occurring in honey bee colonies implicated in almond pollination revealed increased positive tests ranging from 155 bee samples to 223 respectively before and after almond pollination (Cavigli et al. 2016). Specifically, infection levels of N. ceranae have amplified from 14 to 66.5% before and after almond pollination respectively (Cavigli et al. 2016). Likewise, infestation rates of chalkbrood disease Ascosphaera apis in migratory beehives were higher and significantly different than those found in stationary colonies (Jara et al. 2018). The same trend was also observed for viral infections, where migratory colonies show a higher number of infecting viruses than stationary colonies (Aglagane et al. unpublished). The differences found in infection levels of pathogens between the two beekeeping operation types could be explained by the stress associated with migratory management (Jara et al. 2020). In fact, several studies have pointed out that long distance transportation of beehives can interfere with honey bee glands development (Ahn et al. 2012), decreases life span, and affects oxidative stress levels (Simone-Finstrom et al. 2016), which may lead to a higher vulnerability to pathogens.
5 Conclusion
The primary purpose of our survey was to determine the occurrence, prevalence, and geographic distribution of four agents responsible for nosemosis and foulbrood diseases in the oases of southeastern Morocco. Results of this investigation were negative for N. apis and P. larvae, but positive for N. ceranae and M. plutonius. Both of the latter pathogens were present at infection levels of 16.09% and 62.07% respectively. Nevertheless, the level of infection of M. plutonius was categorized as generally low. To our knowledge, information regarding the prevalence, infection level, and geographic distribution of nosemosis and foulbrood infecting agents is lacking not only for Morocco but also for the majority of north African countries, and more inquiries are needed to fill the gap. In a second time, we were interested in investigating the effect of migratory beekeeping on the intensity and/or prevalence of N. ceranae and M. plutonius as well as on their co-infection. Both pathogens along with their co-infection were found to occur with higher prevalence levels in migratory beehives when compared to stationary colonies. However, we found no statistical significance linking the prevalence rates of those diseases to the beekeeping operation type chosen by beekeepers. Additional work is needed to inquire the prevalence and infection level of these pathogens in other regions of Morocco. In addition, the effect of migratory beekeeping as a possible factor of pathogen spillover needs further investigations in order to promote sustainable beekeeping.
Availability of data and material
Data will be made available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Abdi K, Belguith K, Hamdi C, Souissi Y, Essanaa J, Hajji T, Mosbah A, Hamida TB, Cherif A et al (2018) Parasites-Iflavirus association and emergence of three master variants of DWV affecting Apis mellifera intermissa in Tunisian apiaries. Bull Insectology 71(2):273–282
Adjlane N, Dainat B, Gauthier L, Dietemann V et al (2015) Atypical viral and parasitic pattern in Algerian honey bee subspecies Apis mellifera intermissa and A. m. sahariensis. Apidologie 47:631–641. https://doi.org/10.1007/s13592-015-0410-x
Ahn K, Xie X, Riddle J, Pettis J, Huang ZY et al (2012) Effects of long-distance transportation on honey bee physiology. J Entomol 2012:1–9. https://doi.org/10.1155/2012/193029
Alger SA, Burnham PA, Lamas ZS, Brody AK, Richardson LL et al (2018) Home sick: impacts of migratory beekeeping on honey bee (Apis mellifera) pests, pathogens, and colony size. PeerJ 6:e5812. https://doi.org/10.7717/peerj.5812
Bakonyi T, Derakhshifar I, Grabensteiner E, Nowotny N et al (2003) Development and evaluation of PCR assays for the detection of Paenibacillus larvae in honey samples: comparison with isolation and biochemical characterization. Appl Environ Microbiol 69:1504–1510. https://doi.org/10.1128/AEM.69.3.1504-1510.2003
Boncristiani H, Ellis JD, Bustamante T, Graham J, Jack C, Kimmel CB, Mortensen A, Schmehl DR et al (2020) World honey bee health: the global distribution of Western honey bee (Apis mellifera L.) pests and pathogens. Bee World 98(1):2–6. https://doi.org/10.1080/0005772X.2020.1800330
Bordin F, Zulian L, Granato A, Caldon M, Colamonico R, Toson M, Trevisan L, Biasion L, Mutinelli F et al (2022) Presence of known and emerging honey bee pathogens in apiaries of Veneto region (Northeast of Italy) during spring 2020 and 2021. Appl Sci 12:2134. https://doi.org/10.3390/app12042134
Buendía M, Martín-Hernández R, Ornosa C, Barrios L, Bartolomé C, Higes M et al (2018) Epidemiological study of honeybee pathogens in Europe: the results of Castilla-La Mancha (Spain). Span J Agric Res 16:e0502. https://doi.org/10.5424/sjar/2018162-11474
Carra E, Galletti G, Carpana E, Bergamini F, Loglio G, Bosi F, Palminteri S, Bassi S et al (2022) A probe-based qPCR method, targeting 16S rRNA gene, for the quantification of Paenibacillus larvae spores in powdered sugar samples. Appl Sci 12:9895. https://doi.org/10.3390/app12199895
Cavigli I, Daughenbaugh KF, Martin M, Lerch M, Banner K, Garcia E, Brutscher LM, Flenniken ML et al (2016) Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 47:251–266. https://doi.org/10.1007/s13592-015-0395-5
Cilia G, Tafi E, Zavatta L, Caringi V, Nanetti A et al (2022) The epidemiological situation of the managed honey bee (Apis mellifera) colonies in the Italian Region Emilia-Romagna. Vet Sci 9:437. https://doi.org/10.3390/vetsci9080437
de Graaf DC, Alippi AM, Antúnez K, Aronstein KA, Budge G, De Koker D, De Smet L, Dingman DW, Evans JD, Foster LJ, Fünfhaus A, Garcia-Gonzalez E, Gregore A, Human H, Murray KD, Nguyen BK, Poppinga L, Spivak M, van Engelsdorp D, Wilkins S, Genersch E et al (2013) Standard methods for American foulbrood research. J Apic Res 52:1–28. https://doi.org/10.3896/IBRA.1.52.1.11
Ebeling J, Reinecke A, Sibum N, Fünfhaus A, Aumeier P, Otten C, Genersch E et al (2023) A comparison of different matrices for the laboratory diagnosis of the epizootic American foulbrood of honey bees. Vet Sci 10:103. https://doi.org/10.3390/vetsci10020103
FAOSTAT (2020) Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. [online] https://www.fao.org/faostat/en/#data/QCL. Accessed 10 January 2023
Forsgren E, Budge GE, Charrière JD, Hornitzky MAZ et al (2013) Standard methods for European foulbrood research. J Apic Res 52:1–14. https://doi.org/10.3896/IBRA.1.52.1.12
Fries I, Chauzat MP, Chen YP, Doublet V, Genersch E, Gisder S, Higes M, McMahon DP, Martín-Hernández R, Natsopoulou M, Paxton RJ, Tanner G, Webster TC, Williams GR et al (2013) Standard methods for Nosema research. J Apic Res 52:1–28. https://doi.org/10.3896/IBRA.1.52.1.14
Fries I, Feng F, da Silva A, Slemenda SB, Pieniazek NJ et al (1996) Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur J Protistol 32:356–365. https://doi.org/10.1016/S0932-4739(96)80059-9
Gallai N, Salles JM, Settele J, Vaissière BE et al (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Gray A, Adjlane N, Arab A et al (2020) Honey bee colony winter loss rates for 35 countries participating in the COLOSS survey for winter 2018–2019, and the effects of a new queen on the risk of colony winter loss. J Apic Res 59:744–751. https://doi.org/10.1080/00218839.2020.1797272
Gray A, Adjlane N, Arab A et al (2022) Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: the combined effects of operation size, migration and queen replacement. J Apic Res. https://doi.org/10.1080/00218839.2022.2113329
Gray A, Brodschneider R, Adjlane N et al (2019) Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J Apic Res 58:479–485. https://doi.org/10.1080/00218839.2019.1615661
Higes M, Martín R, Meana A et al (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol 92:93–95. https://doi.org/10.1016/j.jip.2006.02.005
Higes M, Martín-Hernández R, Garrido-Bailón E, Botías C, Meana A et al (2009) The presence of Nosema ceranae (Microsporidia) in North African honey bees (Apis mellifera intermissa). J Apic Res 48:217–219. https://doi.org/10.3896/IBRA.1.48.3.12
Ilyasov RA, Lee M, Takahashi J, Kwon HW, Nikolenko AG et al (2020) A revision of subspecies structure of western honey bee Apis mellifera. Saudi J Biol Sci 27:3615–3621. https://doi.org/10.1016/j.sjbs.2020.08.001
Jara L, Martínez-López D, Muñoz I, De la Rua P et al (2018) Epidemiological survey of Ascosphaera apis in small-scale migratory Apis mellifera iberiensis colonies. Sociobiology 65:285. https://doi.org/10.13102/sociobiology.v65i2.2685
Jara L, Ruiz C, Martín-Hernández R, Muñoz I, Higes M, Serrano J, De la Rúa P et al (2020) The effect of migratory beekeeping on the infestation rate of parasites in honey bee (Apis mellifera) colonies and on their genetic variability. Microorganisms 9:22. https://doi.org/10.3390/microorganisms9010022
Lindström A, Korpela S, Fries I et al (2008) The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J Invertebr Pathol 99:82–86. https://doi.org/10.1016/j.jip.2008.06.010
Martín-Hernández R, Bartolomé C, Chejanovsky N, Le Conte Y, Dalmon A, Dussaubat C, García-Palencia P, Meana A, Pinto MA, Soroker V, Higes M et al (2018) Nosema ceranae in Apis mellifera: a 12 years post detection perspective. Environ Microbiol 20:1302–1329. https://doi.org/10.1111/1462-2920.14103
Martínez-López V, Ruiz C, De la Rúa P et al (2022) Migratory beekeeping and its influence on the prevalence and dispersal of pathogens to managed and wild bees. Int J Parasitol 18:184–193. https://doi.org/10.1016/j.ijppaw.2022.05.004
Matheson A (1993) World bee health report. Bee World 74:176–212. https://doi.org/10.1080/0005772X.1993.11099183
Mayack C, Hakanoğlu H (2022) Honey bee pathogen prevalence and interactions within the Marmara region of Turkey. Vet Sci 9:573. https://doi.org/10.3390/vetsci9100573
Menail AH, Piot N, Meeus I, Smagghe G, Loucif-Ayad W et al (2016) Large pathogen screening reveals first report of Megaselia scalaris (Diptera: Phoridae) parasitizing Apis mellifera intermissa (Hymenoptera: Apidae). J Invertebr Pathol 137:33–37. https://doi.org/10.1016/j.jip.2016.04.007
Ministry of Agriculture (2019) Filière Apicole. https://www.agriculture.gov.ma/fr/filiere/apicole. Accessed 10 January 2023
Ministry of Agriculture (2022) Colloque scientifique sur “le syndrome d’effondrement des colonies d’abeilles”. https://iav.ac.ma/fr/colloque_apiculture. Accessed 14 March 2022
Özkırım A, Schiesser A, Keskin N et al (2019) Dynamics of Nosema apis and Nosema ceranae co-infection seasonally in honey bee (Apis mellifera L.) colonies. J Apic Sci 63:41–48. https://doi.org/10.2478/jas-2019-0001
Patil I (2021) Visualizations with statistical details: the “ggstatsplot” approach. JOSS 6:3167. https://doi.org/10.21105/joss.03167
Pfeiffer VW, Crowder DW (2022) Factors affecting virus prevalence in honey bees in the Pacific-Northwest, USA. J Invertebr Pathol 187:107703. https://doi.org/10.1016/j.jip.2021.107703.
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE et al (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Roetschi A, Berthoud H, Kuhn R, Imdorf A et al (2008) Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 39:362–371. https://doi.org/10.1051/apido:200819
Roudel M, Aufauvre J, Corbara B, Delbac F, Blot N et al (2013) New insights on the genetic diversity of the honeybee parasite Nosema ceranae based on multilocus sequence analysis. Parasitology 140:1346–1356. https://doi.org/10.1017/S0031182013001133
Ruttner F (1988) Biogeography and taxonomy of honeybees. Springer, Berlin Heidelberg, Germany
Sabbahi R (2022) Economic value of insect pollination of major crops in Morocco. Int J Trop Insect Sci 42:1275–1284. https://doi.org/10.1007/s42690-021-00645-x
Simone-Finstrom M, Li-Byarlay H, Huang MH, Strand MK, Rueppell O, Tarpy DR et al (2016) Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci Rep 6:32023. https://doi.org/10.1038/srep32023
Simone-Finstrom M, Strand MK, Tarpy DR, Rueppell O et al (2022) Impact of honey bee migratory management on pathogen loads and immune gene expression is affected by complex interactions with environment, worker life history, and season. J Insect Sci 22:17. https://doi.org/10.1093/jisesa/ieab096
Tokarev YS, Huang WF, Solter LF, Malysh JM, Becnel JJ, Vossbrinck CR et al (2019) A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J Invertebr Pathol. https://doi.org/10.1016/j.jip.2019.107279
Traver BE, Fell RD (2011) Prevalence and infection intensity of Nosema in honey bee (Apis mellifera L.) colonies in Virginia. J Invertebr Pathol 107:43–49. https://doi.org/10.1016/j.jip.2011.02.003
Webster TC, Pomper KW, Hunt G, Thacker EM, Jones SC (2004) Nosema apis infection in worker and queen Apis mellifera. Apidologie 35(1):49–54. https://doi.org/10.1051/apido:2003063
World Organization for Animal Health (WOAH). Manual of diagnostic tests and vaccines for terrestrial animals., 2022. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/. Accessed 02 February 2023
World Organization for Animal Health (WOAH). Terrestrial Animal Health Code (2022) Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/. Accessed 02 February 2023
Acknowledgements
We would like to express our sincere gratitude to “Association Albisher pour le Développement et l’Environnement” for facilitating meetings with beekeepers. We are also thankful for beekeepers of the Darâa-Tafilalet region for providing us with honey bee samples.
Funding
The study was performed thanks to the scientific collaboration agreement between the Istituto Zooprofilattico Sperimentale della Lombardia e dell’ Emilia Romagna “Bruno Ubertini” (IZSLER) and the Faculty of Science Semlalia, Cadi Ayyad University (FSSM) (Prot. IZSLER n.11993/2022, rep.1002).
Author information
Authors and Affiliations
Contributions
Conceptualization: AA, MF, and MA. Funding acquisition: EE and MF. Fieldwork: AA, OE, and EE. Laboratory experiments: EC, VR, and ES. Data analysis: AA, EC, MF, and MA. The first draft of the manuscript was written by AA and EC, and MF commented on a previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: David Tarpy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abdessamad Aglagane and Elena Carra contributed equally to this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aglagane, A., Carra, E., Ravaioli, V. et al. Molecular examination of nosemosis and foulbrood pathogens in honey bee populations from southeastern Morocco. Apidologie 54, 42 (2023). https://doi.org/10.1007/s13592-023-01022-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01022-y