Abstract
The tiny population of the endemic Maltese honey bee (A. m. ruttneri) is threatened by anthropogenic influences, such as urbanisation, habitat loss, and unsustainable agricultural practices, but most prominently by the importation of commercially important non-native stock. To obtain data on the colony life cycle parameters of A. m. ruttneri and to measure its apicultural performance in relation to imported A. m. ligustica under Maltese conditions, we conducted a comparative study between mid-2017 and early 2020. Over one full season, colonies of both subspecies (A. m. ruttneri (n = 15) vs. A. m. ligustica (n = 18)) were regularly assessed for survival, colony size, behaviour, and presence of diseases. The comparative assessments were completed in September 2018, but monitoring and sampling of the surviving colonies of A. m. ruttneri continued until March 2020. Our results clearly indicate that the tested group of sister queens of A. m. ruttneri is well adapted to the prevailing environmental conditions in Malta. The colonies survived significantly longer compared to the tested group of sister queens of A. m. ligustica and performed better in several parameters measured, their colony development, and health being well in tune with the environment. A. m. ruttneri received acceptable scores for behavioural traits (gentleness and calmness on the comb), showing potential for improvement by breeding. The results from this pioneering study clearly indicate that A. m. ruttneri, with its superior adaptation to Maltese conditions and the potential to improve by breeding, represents a prime option towards economically sound beekeeping on the Maltese archipelago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The subspecific diversity of the western honey bee (Apis mellifera L.) is particularly high around the Mediterranean basin and in adjacent regions. More than 12 of the currently recognised 30 subspecies worldwide occur in this region, representing the species’ four major lineages A (African), M (West and North Europe), C (South-Eastern Europe), and O (Oriental and Western Asia) (Ruttner 1988; Sheppard et al. 1997). Several distinct subspecies have been described from Mediterranean islands, such as A. m. cypria from Cyprus, A. m. adami from Crete, or A. m. siciliana from Sicily. The subspecies native to the Maltese archipelago is A. m. ruttneri, a representative of the A (African) lineage, with A. m. siciliana and A. m. intermissa from North Africa as its closest relatives (Sheppard et al. 1997).
The native honey bee population of Malta is very small, encompassing between 4,000 and 6,000 colonies, with 5,459 reported in 2021 (NAP 2019; National Livestock Database 2022; Gemma 2022, Alexander, personal communication). Like numerous indigenous honey bee populations occurring in southern Europe, it is threatened by anthropogenic influences, such as habitat loss and unsustainable agricultural practices, including the excessive use of plant protection products (De la Rúa et al. 2009). In addition, honey bees of commercially important foreign stock, predominantly A. m. ligustica, Buckfast and, probably to a lesser extent, A. m. carnica, have been imported for decades in significant numbers, by individual beekeepers, or by large-scale operations that produce queens for the European market (Sheppard et al. 1997; Zammit-Mangion et al. 2017; Mifsud, unpublished data). One key motivation for importing queens of non-native origin is the beekeepers’ perception of their superiority in regard to economically important apicultural traits such as docility and honey production. The lack of extension services in Malta, such as beekeeper training and support, may also play a role. In consequence, introgression of foreign genotypes presents a particularly severe threat for A. m. ruttneri (Uzunov et al. 2022) and not only may lead to loss of specific adaptations but could result in the loss of the entire gene pool.
However, recent research showed that, although signatures of introgression, such as mitochondrial DNA patterns typical for Italian and Carniolan stock, can be detected in the gene pool of present-day A. m. ruttneri (Zammit-Mangion et al. 2017), a significant proportion of the native honey bee population can still be addressed as comparatively unhybridised. Genomic (Momeni et al. 2021; Chen et al. 2022) and geometric morphometric (Janczyk et al. 2020) analyses confirmed the presence of a distinct Maltese population of honey bees that still resembles the original description of Sheppard et al. (1997). Amongst the reasons hypothesised to limit introgression are a lack of adaptation of imported genotypes to the seasonally extremely harsh environmental conditions of Malta, with summer temperatures often exceeding 40 °C, windy conditions, and nectar dearth between May and July caused by drought (Supplementary Material). There are observations of disparity in the availability of sexuals of both subspecies, which, together with potential assortative mating (Oleksa et al. 2013), may contribute to restraining hybridisation.
Recently, the native Maltese honey bee has been regaining popularity amongst beekeepers (Uzunov et al. 2018), and efforts have been initiated for its conservation, propagation, and sustainable management. Amongst other measures, local breeding programmes for the improvement of its apicultural performance have been established to promote its acceptance amongst local beekeepers. As a consequence of these efforts, A. m. ruttneri queens are now increasingly available for beekeepers on the islands, which may contribute to reducing beekeepers’ motivation to import foreign genotypes (Galea, unpublished data).
Nonetheless, apart from anecdotal observations, there is currently no data available on colony life cycle parameters and other traits of A. m. ruttneri with adaptive or apicultural relevance. At the same time, driven by the demand for docile stock with satisfying economic performance, Maltese beekeepers continue to import honey bee queens, mostly from Italy. Yet, evidence that would substantiate the perception of superior performance of A. m. ligustica under the environmental conditions of Malta is lacking.
To fill this knowledge gap, we conducted an experiment to study colony life cycle parameters and performance of native A. m. ruttneri and imported A. m. ligustica under Maltese conditions. Over one full season, colonies of both subspecies were regularly assessed for survival, colony size, behaviour (gentleness, calmness, and swarming), and presence of diseases (Varroa, viruses, and Nosema). In this paper, we present the results of this experiment that may serve as a valuable basis for strategies in regard to breeding and conservation of A. m. ruttneri.
2 Material and methods
2.1 Experimental set-up and colony management
The study was initiated in May 2017 on the island of Malta. Colonies headed by queens of the native subspecies A. m. ruttneri (n = 15) and the introduced A. m. ligustica (n = 18) were comparatively assessed for traits of apicultural and economic relevance. The comparative assessments were completed in September 2018, but monitoring and sampling of the surviving colonies of A. m. ruttneri continued until March 2020.
The A. m. ruttneri queens consisted of a sister group, descending from a breeding apiary of the SMARTBEES project on Malta (www.smartbees.eu) that was confirmed to consist of pure A. m. ruttneri (Momeni et al. 2021). The A. m. ligustica queens also consisted of a sister group, obtained from a registered breeder in Bologna (region of Emilia-Romagna, Italy) maintaining a well-selected population for the main economically relevant traits. They were imported to Malta with valid documentation. Both groups of queens were open mated in their respective area of origin.
The queens were introduced into nucleus colonies of equal size in regard to bees as well as brood combs (two brood combs fully occupied with bees) and food. They were established in newly constructed British standard wooden hives, and sugar syrup was frequently supplemented to the colonies to induce colony development. The colonies were evenly distributed between two locations, Msida (Wied Gћollieqa–University of Malta grounds) and Siġġiewi (Wied Qirda), with an in-between distance of about 7 km (Table I). The colonies were arranged in line and in a randomised order.
The colonies were managed according to a standardised protocol (Table II) and in accordance with the local beekeeping practice, in particular regarding the aspects of swarming prevention, food supply for the period with limited food sources, and disturbance by ants. Our main interest was to follow the fate of the colonies and not that of the queens. Thus, we allowed a single supersedure after queen loss and continued to assess the colony after queen change.
To minimise the initial Varroa mite (Varroa destructor) infestation and to standardise it, all colonies were subject to a single treatment with Apitraz (active substance Amitraz) in September 2017. No further treatments against any pathogen or parasite were applied until the end of the experiment in March 2020.
The main forage sources at both locations consisted mainly of wild flora including Carob Trees (Ceratonia siliqua), Eucalyptus (Eucalyptus camaldulensis and E. gamphocephala), Boar thistle (Galactites tomentosa), Common Borage (Borago officinalis), Summer Asphodel (Asphodelus ramosus), Cape sorrel (Oxalis pes-caprea), and Sulla (Hedysarum coronarium).
2.2 Colony assessment
The colony monitoring started in August 2017, when the majority of the workers in the colonies were descendants of the newly introduced queen. Colony assessments for the main traits of interest (Table II) were performed at regular intervals of two months (± 2.5 days) and concluded in September 2018. They included a complete assessment of colony size (number of bees and brood cells) following the Liebefeld method (Imdorf et al. 1987, 2019) as well as the traits of gentleness and calmness on the comb (Costa et al. 2012; Büchler et al. 2013). Honey yield was assessed once, at the honey harvest on the 26th of May 2018. A colony was recorded as lost either when it had died or when it was considered too weak for further measurements.
2.3 Sampling and analysis for identification of honey bee pathogens
Samples for V. destructor analysis consisted of about 50 g of adult bees and were collected monthly, starting in December 2017. The last samples from A. m. ligustica colonies were collected in July 2018, but the sampling of A. m. ruttneri colonies was continued until March 2020. Samples for the analysis of the gut parasite Nosema spp. and for the analysis of honey bee viruses consisted of about 40 g of bees and were collected once before the winter period (October 2017) and twice during the spring of 2018 (April and May). These samples were collected from the outer frames of the brood chamber or from a honey super (when present) (Meixner et al. 2014). All samples were stored in 96% ethanol at − 20 °C, until analysis.
2.4 Determination of Varroa mite infestation levels
The mite infestation in relation to the adult bees was assessed using the powdered sugar method described in Dietemann et al. (2013). Briefly, the bee sample was weighed and then placed in a wide opened jar that was covered with mesh. About 35 g of dry powdered sugar was applied through the mesh, and the jar was rolled for three minutes to ensure that all bees were covered with sugar. Subsequently, the jar was left to settle for an additional minute, then turned mesh-side down and shaken thoroughly over an additional mesh and white surface to detach the mites from the bees. Mites fallen onto the white surface were counted, and the mite proportion per 10 g bees was calculated.
2.5 Determination of infection levels with Nosema spp.
Following the OIE guidelines (Fries et al. 2013; OIE 2018), the abdomens of 60 bees per sample were separated, homogenised in water, and subsequently filtered through fabric with a mesh size of 10 µm. The final volume of the macerate was then adjusted to 1 ml per bee with water. A drop of the suspension was examined in a counting chamber (Bürker), and the spores were counted.
2.6 Analysis of honey bee viruses
Ten bees per sample were analysed for the presence of four common honey bee viruses; deformed wing virus (DWV), acute bee paralysis virus (ABPV), sacbrood virus (SBV), and chronic bee paralysis virus (CBPV). Following procedures described in Genersch et al. (2010) and de Miranda et al. (2013), the bees’ heads were homogenised in RTL buffer (Mixer Mill, Retsch MM300), and total RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). A one-step RT-PCR protocol was used to detect viral RNA (Qiagen one step RT-PCR kit, MultiGene Optimax Thermocycler). Primers and PCR conditions followed those described by Genersch et al. (2010).
2.7 Hygienic behaviour
The assessment of hygienic behaviour was carried out following the procedure described in Büchler et al. (2013). Fifty sealed worker brood cells, containing pupae with pink eyes, were pierced with an insect pin (No. 2). After 9 to 16 h, depending on the daylight duration and weather conditions, the number of cleaned cells was counted and the percentage in relation to all pierced cells was calculated.
2.8 Swarming tendency
The incidence of swarming (presence of swarming cells or occurred swarming) was monitored during the regular inspections, as well as by additional colony check-ups throughout the swarming season.
2.9 Data analysis and statistical analysis
An overview of all traits and parameters measured is given in Table II.
Due to environmental conditions, including extreme heat and lack of forage, connected with a higher risk of robbing and attacks from hornets (Vespa orientalis), it was not always possible to assess, test, or sample all colonies at all scheduled inspections.
The data was collected using tailor-made record-keeping cards and introduced into an online database with logical and conditional functions. The characteristics of adult bee population, worker and drone brood, gentleness, and calmness were analysed with GLM (Generalised Linear Model) with date of colony inspection, location, and origin as fixed factors. A combination of relevant co-variables, such as adult bee population, worker and drone brood and pollen stores, were applied as appropriate. A One-Way ANOVA (Analysis of Variance) was used to detect differences between the groups (ruttneri vs. ligustica) at each inspection for adult bee population, worker and drone brood. Varroa mite infestation was analysed by One-Way ANOVA (Analysis of Variance). For the analysis of infections with DWV and ABPV, the effect of date of sampling, location, and origin were considered in the GLM analysis. In addition, each virus was used as a co-variable for each other in a vice-versa approach. The Varroa mite infestation for the last two relevant samplings (April and May 2018) was also used as a co-variable in the virus analysis. The effect of the month of test, origin, and location on hygienic behaviour was analysed with GLM. The effect of the honey bee origin on honey production was analysed by ANOVA. The correlation between gentleness and calmness was estimated by Pearson correlation. The survival analysis was performed with a Kaplan–Meier model. All statistical analyses were performed in SPSS 20.
3 Results
The comparative assessment of the colonies from both origins was possible until September 2018. Complete assessments of the remaining A. m. ruttneri colonies continued until March 2019, and monitoring of Varroa mite infestation on adult bees continued until March 2020.
3.1 Colony survival
Under our experimental regime without any treatments, 12 of the initial 15 A. m. ruttneri colonies were still alive by September 2018, but only three of the initial 18 A. m. ligustica colonies survived by then, and none of the A. m. ligustica colonies survived after January 2019. The average survival duration for untreated A. m. ligustica was 11.3 ± 0.9 months, whereas untreated A. m. ruttneri colonies lived an average of 25.4 ± 2.7 months, with some colonies surviving past the end of the field observations (Figure 1). The difference in survival time between colonies of the two origins was highly significant (p < 0.01).
3.2 Colony development
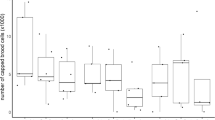
The size of A. m. ruttneri colonies increased faster when compared to A. m. ligustica, and they were significantly stronger between December 2017 and March 2018 (December: ANOVA, p < 0.05; January, March: ANOVA, p < 0.01). The average values of the number of adult bees, including workers and drones, are reported in Figure 2. The peak colony size of both subspecies was observed in March 2018, with an average number of 23,127 and 16,400 adult bees in colonies of A. m. ruttneri and A. m. ligustica, respectively. The colony size of both groups decreased rapidly after March, reaching a minimum in July (6,400 bees for A. m. ruttneri) and September (3,540 bees for A. m. ligustica). The developmental curve of A. m. ruttneri shows a bimodal shape, with a minor second peak in late autumn. Besides origin and month of colony inspection, colony size was also significantly influenced by the amount of worker and drone brood and pollen stores in the colonies (GLM, p < 0.01, S1).
Similar to the number of adult bees, the amount of worker brood cells in the colonies reached a peak in March 2018, with a mean of 35,010 worker brood cells for A. m. ruttneri and 25,997 cells for A. m. ligustica (Figure 3). From December 2017 onwards, A. m. ruttneri colonies had significantly more worker brood cells than A. m. ligustica (ANOVA, p < 0.01). Similar to the adult bee population, the A. m. ruttneri brood curve followed a bimodal pattern, with the main peak in March, and a minor second peak from September to November. In both populations, the lowest amount of worker brood was observed in mid-summer and late autumn. Month of colony inspection, adult bee population, and drone brood significantly influenced the amount of worker brood (GLM, p < 0.01, S1).
The highest number of drone brood cells was observed in March, with an average of 2,484 and 1,282 cells for A. m. ruttneri and A. m. ligustica, respectively (Figure 4). In A. m. ruttneri colonies, a minor second peak was observed in mid-autumn (November 2018). In total, A. m. ruttneri colonies reared significantly more drone brood (ANOVA, p < 0.05). Month of colony inspection and location were fixed factors that affected the amount of drone brood, whilst adult bee population, worker brood, and pollen stores as co-variables significantly influenced the amount of drone brood (GLM, p < 0.01, S1).
3.3 Colony behaviour
Gentleness and calmness on the comb differed significantly between the colonies of the two populations (gentleness: GLM, p < 0.05; calmness: GLM, p < 0.01, S2). The average colony scores of A. m. ligustica were higher for both traits (gentleness 3.51; calmness 3.65) than those of A. m. ruttneri (gentleness 3.25; calmness 3.17). However, the scores were, to a significant extent, also influenced (GLM, p < 0.01, S2) by time of inspection (seasonality) and, for the calmness score, by the presence/absence of pollen stores (GLM, p < 0.05, S2). Scores of the two traits were significantly correlated with each other (Pearson, r = 0.584 and p < 0.01).
No swarming drive was observed during the comparative assessment of the colonies from either origin.
3.4 Hygienic behaviour
The A. m. ruttneri colonies were significantly more hygienic (pairwise comparison, mean difference 14.3%, GLM, p < 0.01, S3) than the colonies of A. m. ligustica (Figure 5). However, the trait was variable over time, with months of testing significantly (GLM, p < 0.01, S3) affecting the scores. There was no significant effect of the location on the trait (GLM, p > 0.05, S3).
3.5 Honey yield
Colonies with A. m. ruttneri queens produced significantly (ANOVA, p < 0.05) more honey than A. m. ligustica, with an average of 6.2 kg and 2.0 kg respectively. Excluding the origin as a factor, the difference between the locations was not significant (ANOVA, p > 0.05).
3.6 Parameters of colony health
3.6.1 Infestation with Varroa destructor
During the first few months of the experiment, the mean Varroa mite infestation level in both groups remained below 10 mites/10 g bees. A sharp increase in Varroa mite infestation was observed in the A. m. ligustica colonies after April 2018, with maximum values of 57 mites/10 g bees in May 2018 and 36 mites/10 g bees in June 2018. As a consequence, a rapid collapse of these colonies was observed, with most of them perishing by September 2018, and only a single one surviving until January 2019. In contrast, the increase of Varroa mites in A. m. ruttneri colonies was comparatively small, and between May and July 2018, mite infestation remained significantly lower than in A. m. ligustica colonies (May 2018: ANOVA, p < 0.01; June 2018: ANOVA, p < 0.03; July 2018: ANOVA, p < 0.014). The maximum infestation level observed in A. m. ruttneri colonies never exceeded 22.3 mites/10 g bees. By January 2019, 11 colonies of this group survived. The peak of the mean infestation level for both groups occurred in May 2018 (Figure 6).
Following the end of the comparative assessment, the A. m. ruttneri colonies were maintained without any acaricide treatments until March 2020, and monthly assessments of their mite infestation were continued. The mean infestation levels during this time continued to follow an annual pattern, with the lowest values observed in October and the highest ones in May/June, but they never exceeded 7.1 mites/10 g bees. In March 2020, eight of the initial 15 A. m. ruttneri colonies were still alive. The remaining seven colonies collapsed due to high Varroa mite infestation.
3.6.2 Viruses and Nosema spp. infections
The infection rate of the two viruses known to be associated with Varroa mites, deformed wing virus (DWV) and acute bee paralysis virus (ABPV), increased with time in both populations; this was, however, only significant for DWV (GLM, p < 0.01, S4). In May 2018, significantly more colonies of the A. m. ligustica group were infected with DWV (ANOVA, p < 0.01) (Figure 7). We could not verify a significant contribution (GLM, p > 0.05, S4) of the V. destructor infestation level to the occurrence of either virus. However, the presence of one of the two viruses significantly influenced the detection of the other one (GLM, p < 0.05).
Sacbrood virus was not detected in any of the samples. Chronic bee paralysis virus was only observed in a total of five samples collected in April and May 2018 (two A. m. ligustica and three A. m. ruttneri samples). These positive cases were too few to enable statistical analysis.
In colonies of either group, spores of the gut parasite Nosema spp. were only sporadically found in very low quantities (< < 1 million spores/bee).
4 Discussion
In this paper, we provide the first systematic data collection on development, behaviour, productivity, and vitality of the endemic honey bee subspecies of Malta, A. m. ruttneri. We describe and compare the performance and Varroa mite infestation of native A. m. ruttneri and imported A. m. ligustica genotypes over one full apicultural season under Maltese environmental conditions. The two groups of colonies were managed with standardised methods, but without the application of chemical disease treatments.
Colonies of the native A. m. ruttneri survived substantially and significantly longer than those of imported A. m. ligustica, confirming results of previous research in that native genotypes survive longer than foreign ones in a given environment (Büchler et al. 2014).
The peak size of the A. m. ligustica colonies in our experiment was about 16,400 adult bees, which is slightly higher than the summer values of about 11,000 to 14,000 bees previously reported by Hatjina et al. (2014). Colonies of A. m. ruttneri developed faster and reached a significantly larger average size of more than 23,000 bees at the developmental peak in spring. Whilst there is no data for A. m. ruttneri available from the literature, Hatjina et al. (2014) report a much lower maximum colony size for the closely related A. m. siciliana, with about 11,500 to 14,800 bees on Sicily and in Italy, respectively. In our experiment, the average A. m. ruttneri colony size was unexpectedly high and reached values known from the C-lineage subspecies A. m. carnica and A. m. macedonica (Hatjina et al. 2014). Thus, our results contradict the general description in the literature that honey bee colonies in Mediterranean environments, and especially in island populations, are of smaller size than those in northern European or continental subspecies (Ruttner 1988). We consider our figures reliable and think the possibility of grave measurement errors unlikely, as the measurements reported here and the data cited above were obtained using the same standardised methods and, at least partially, by the same experimenters. Nevertheless, as our study was limited to testing one sister group each, they may not be representative of the entire subspecies and should be interpreted with caution.
At the time of the developmental peak, the population size of native colonies outnumbered the introduced ones by 30% or almost 7,000 bees. Not surprisingly, this difference in colony strength is also reflected in a significant difference of honey production, with colonies of A. m. ruttneri producing significantly more honey than those of the introduced A. m. ligustica.
A similar result was observed for the number of worker and drone brood cells, where A. m. ruttneri colonies also developed considerably faster and, at the peak of development, had significantly more worker brood cells than A. m. ligustica. When compared to the only comparative studies available in the literature, previously reported for A. m. siciliana (about 24,000 brood cells; Hatjina et al. 2014), the mean number of A. m. ruttneri worker brood cells in March, with more than 35,000 cells, was considerably higher.
Brood rearing in A. m. ligustica started later in the season, maybe precluding the colonies from reaching their maximum potential to exploit the main nectar flow in late spring. In particular, the rearing of drones appeared delayed in comparison to A. m. ruttneri, and in total, significantly less drones were produced per colony. Potentially, this could provide an explanation for the limited signs of introgression of foreign genotypes found in the Maltese honey bee population (Zammit-Mangion et al. 2017; Momeni et al. 2021), maybe together with potential assortative mating (Oleksa et al. 2013).
Whilst A. m. ligustica colonies mostly perished by July (eight months after establishment), colony development data, number of bees and number of brood cells of A. m. ruttneri followed a clear bimodal distribution. In close adaptation to the Maltese environmental conditions defined by annual cycles of rainfall and vegetation, colonies of both genotypes showed a clear major peak in number of adult bees, drone and worker brood cells in March. Moreover, the surviving colonies of A. m. ruttneri showed a second developmental peak in November, indicating a typical Mediterranean annual display (Ruttner 1988; Hatjina et al. 2014). The lowest values of colony size and number of worker and drone brood cells were observed in July, during the period of drought and nectar dearth. During our study, we did not observe periods without brood in summer, although there are anecdotal reports of such summer brood breaks.
From the point of view of apiculturists, behavioural traits such as gentleness, calmness, and swarming (categorised from 1—worst, to 4—best scores) play an essential role in beekeeping operations (Ruttner 1972). Although A. m. ruttneri is generally perceived as “aggressive” by Maltese beekeepers, and there have never been any attempts to select for docility, our experimental colonies reached very acceptable gentleness scores, with a mean value of 3.25. On the other hand, queen breeders in Italy place a high value on the gentle behaviour of their bees and consistently select for gentleness; therefore, it is not surprising that the A. m. ligustica colonies reached a higher mean score of 3.51. Interestingly, the gentleness scores for A. m. ligustica genotypes reported by Uzunov et al. (2014) were much lower (3.07 and 3.15), whilst a mean score of 3.7 was documented for A. m. siciliana, the close relative of A. m. ruttneri. Our results, thus, may indicate that the Maltese honey bees do not deserve their negative reputation amongst local beekeepers and the general public. One explanation for this low esteem may lie in the very active and noisy behaviour that Maltese bees display during manipulations, as demonstrated by their lower mean score for calmness (3.17). The bees tend to fly off when the hives are opened, creating an intensive humming sound, particularly during the reproductive season, with the presence of airborne drones (personal observations). The lower calmness score of A. m. ruttneri also indicates that colonies are harder to manage, an aspect that significantly affects beekeepers’ perception as well as the economics of beekeeping. In addition, it could be possible that, similar to the case of A. m. siciliana, A. m. ruttneri’s reputation of aggressiveness may predominantly stem from beekeepers’ observations with hybridised colonies (Uzunov et al. 2014). As also known from previous research with A. m. mellifera (Fresnaye and Lavie 1976; Ruttner 1988), hybridised colonies may show significantly increased defensiveness in comparison to their parental generation of pure origin.
We did not observe any swarming drive during the first year, which is not surprising as the colonies in both groups were headed by young queens (Winston 1987), and the season was particularly dry (Supplementary Material) with scarce nectar flow. Also later, in the continuing monitoring of A. m. ruttneri, no significant swarming drive was observed, contradicting the observations of Ruttner (1988) and Tiemann and Brückner (1993) of high swarming tendency in the neighbouring and closely related A. m. siciliana. Our results may, however, not be representative as we only tested one sister group, and they could also be explained by improved colony management.
Varroa mite infestation levels in both groups increased sharply after April 2018. From May to July 2018, they were significantly higher in A. m. ligustica colonies than in A. m. ruttneri ones. Whilst infestation levels in A. m. ruttneri mostly remained below 10 mites/10 g of bees, maximum infestation levels in A. m. ligustica reached almost 60 mites/10 g of bees. Such high levels were particularly observed during the period of low brood rearing between May and July, when most of the mites were present on the adult bees. Similar extreme infestation levels were reported previously in untreated colonies in hot climates that partially even survived the following winter (Meixner et al. 2014).
The relative mite infestation curve followed a similar pattern as known from bee populations in temperate regions, where relative infestation also increases after the developmental peak around midsummer (Rosenkranz et al. 2010; Traynor et al. 2020). In A. m. ruttneri, the Varroa mite infestation levels also followed the bimodal curve of the adult bee population and brood, showing a second minor peak in late autumn.
In contrast to previous studies (Meixner et al. 2014), we observed a strong influence of the genotype on Varroa mite infestation, with levels in the introduced A. m. ligustica by far exceeding those of the native A. m. ruttneri. This observation is conspicuous; especially since A. m. ruttneri colonies were much stronger and had significantly more brood, particularly more drone brood, during late winter and early spring, thus offering optimum opportunities and conditions for mite reproduction. Similar observations were, however, reported by Francis et al. (2014) in a case study in Greece with native A. m. macedonica. There, in an experimental apiary with four different honey bee genotypes, significantly lower mite infestation levels were observed in colonies of the native genotype compared to the three foreign ones. Most of the native colonies survived more than two years without treatment, whilst the great majority of the foreign colonies developed extreme mite infestation levels and perished after one year.
The consistently and significantly higher scores for hygienic behaviour of A. m. ruttneri colonies, measured with the pin test, offer a ready explanation for their comparatively low Varroa mite infestation rates that, in contrast to expectations, also did not show a notable increase over time.
In a further study (Galea 2020), 10 of the surviving A. m. ruttneri colonies were screened for other behavioural traits of Varroa mite resistance, such as suppression of mite reproduction (SMR; Harbo and Harris 2005; Mondet et al. 2020) and opening and recapping of infested cells (REC; Oddie et al. 2018). Although A. m. ruttneri had never been subject to any selection and breeding attempts at all, the colonies from A. m. ruttneri reached scores (SMR: 0.57; REC: 0.9) that are well in the range of colonies originating from resistant “survivor” populations (Grindrod and Martin 2021), or from long-term selection programmes (Büchler et al. 2022). Varroa reached Malta in the 1990s, followed by devastating colony losses (Zammit-Mangion et al. 2017). Given the lack of extension services and governmental support for the Maltese beekeeping community even today, we can assume that such kind of support, including advice on Varroa control strategies, did not exist in the 1990s. In consequence, A. m. ruttneri’s strong behavioural response to parasite reproduction might indeed result from natural selection. Unfortunately, however, no data from earlier years on mite population development or reproduction are available that would allow further exploration or testing of this hypothesis.
The prevalence of DWV and ABPV and mite infestation levels increased in parallel during the comparative colony assessment. Against expectations, the relationship between both parameters was not significant, maybe due to high variation of mite numbers, particularly in A. m. ligustica colonies. According to our data, other pathogens, such as Nosema spp. or other viruses, occurred only sparsely and do not seem to play a major role for colony health in Malta.
In conclusion, our results clearly indicate that A. m. ruttneri is well adapted to the prevailing environmental conditions in Malta. The colonies survived significantly longer compared to the A. m. ligustica group and performed better in several parameters measured, their colony development and health being well in tune with the environment. They received acceptable scores for behavioural traits, showing potential for improvement by selection. Large bee population numbers of the A. m. ruttneri colonies at the developmental peak promise potential for productive honey harvests, and superior performance in Varroa mite resistance traits has been found to limit mite population growth. In contrast, our results show that in the absence of Varroa treatments, A. m. ligustica colonies perform poorly under Maltese conditions, produce little honey, and mostly perish within one year. Nonetheless, A. m. ligustica is highly popular amongst Maltese beekeepers, so our results seemingly present an open discrepancy. Several reasons could explain this. First, it is important to stress that our results originate from an extreme experimental scenario that should not be compared to the real-life beekeeping situation on Malta. Whilst reliable data on chemical Varroa treatments in Malta do not exist, we can safely assume that Maltese beekeepers frequently treat imported A. m. ligustica colonies. Therefore, colonies in the care of beekeepers are highly unlikely to accumulate Varroa infestation levels comparable to the colonies in our experiment that were left untreated for several months. Consequently, their colony strength and performance including honey production in the course of the season will not suffer, at least not in dimensions comparable to our experimental colonies. Secondly, due to insufficient extension services and beekeeper education in Malta, few Maltese beekeepers possess the skills to restock their colonies with queens raised by themselves and consequently are forced to purchase queens. Many of them may resolve to purchase queens of Italian stock, not primarily because they favour Italian queens over Maltese ones, but simply because they are easily available whereas production of native queens is low and hard to come by.
Until recent years, the native A. m. ruttneri has not been too popular amongst local beekeepers, and selective breeding efforts were more or less absent. Recently initiated breeding efforts, although still in their infancy, appear promising and could make a significant contribution to the preservation of the Maltese honey bee as part of the natural heritage. In addition, the development and implementation of beekeeper education and extension efforts will promote sustainable beekeeping on the Maltese archipelago. Better knowledge and experience in colony management, promoting the use of biotechnical Varroa mite management methods and basic queen production skills, will help local beekeepers to obtain better beekeeping results and honey harvests. The results of local breeding efforts will increase the appreciation of the native Maltese honey bee and may contribute to reducing importations of foreign genotypes, one of the main threats to the native gene pool.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Alexander, AL (Personal Commun) Animal Health Unit, Ministry for Agriculture, Fisheries and Animal Rights, Malta
Büchler R, Andonov S, Bienefeld K, Costa C, Hatjina F, Kezic N, Kryger P, Spivak M, Uzunov A, Wilde J (2013) Standard methods for rearing and selection of Apis mellifera queens. J Apic Res 52:1–30
Büchler R, Costa C, Hatjina F, Andonov S, Meixner M, Le Conte Y, Uzunov A, Berg S, Bienkowska M, Bouga M, Dražić M, Dyrba W, Kryger P, Panasiuk B, Pechhacker H, Petrov P, Kezić N, Korpela S, Wilde J (2014) The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J Apic Res 53:230–232
Büchler R, Uzunov A, Costa C, Meixner M, Le Conte Y, Mondet F, Kovacic M, Andonov S, Carreck NL, Dimitrov L, Basso B, Bienkowska M, Dall’Olio R, Hatjina F, Wirtz U (2022) EurBeST — a pilot study testing Varroa-resistant bees under commercial beekeeping conditions. Am Bee J 162(2):213.
Chen C, Parejo M, Momeni J, Langa J, Nielsen RO, Shi W, SMARTBEES WP3 DIVERSITY CONTRIBUTORS, Vingborg R, Kryger P, Bouga M, Estonba A, Meixner MD (2022) Population structure and diversity in European honey bees (Apis mellifera L.)—an empirical comparison of pool and individual whole-genome sequencing. Genes 13:182.
Costa C, Lodesani M, Bienefeld K (2012) Differences in colony phenotypes across different origins and locations: evidence for genotype by environment interactions in the Italian honeybee (Apis mellifera ligustica)? Apidologie 43:634–642
De la Rua P, Jaffé R, Dall’Olio R, Muñoz I, Serrano J (2009) Biodiversity, conservation and current threats to European honeybees. Apidologie 40:263–284
de Miranda JR, Bailey L, Ball BV, Blanchard P, Budge GE, Chejanovsky N, Chen Y-P, Gauthier L, Genersch E, de Graaf DC, Ribière M, Ryabov E, De Smet L, van der Steen JJM (2013) Standard methods for virus research in Apis mellifera. J Apic Res 2013(52):1–56
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis JD (2013) Standard methods for varroa research. J Apic Res 52:1–54
Francis RM, Amiri E, Meixner MD, Kryger P, Gajda A, Andonov S, Uzunov A, Topolska G, Charistos L, Costa C, Berg S, Bienkowska M, Bouga M, Büchler R, Dyrba W, Hatjina F, Ivanova E, Kezic N, Korpela S, Le Conte Y, Panasiuk B, Pechhacker H, Tsoktouridis G, Wilde J (2014) Effect of genotype and environment on parasite and pathogen levels in one apiary - a case study. J Apic Res 53(2):230–232. https://doi.org/10.3896/IBRA.1.53.2.14
Fresnaye J, Lavie P (1976) Selection et hybridation de 1’abeille en France. Symp. Génétique, selection et reproduction de 1’abeille. Bull Tech Apic OPIDA 3:15–20.
Fries I, Chauzat M-P, Chen Y-P, Doublet V, Genersch E, Gisder S, Higes M, Mcmahon D, Martín-Hernández R, Natsopoulou M, Paxton R, Retschnig G, Webster T, Williams G (2013) Standard methods for Nosema research. J Apic Res 52.
Galea T (2020) The Development of Varroa destructor in native Apis mellifera ruttneri and in introduced Apis mellifera ligustica colonies on the island of Malta. B.Sc. dissertation University of Malta
Gemma P (2022) Animal Health Unit, Ministry for Agriculture, Fisheries and Animal Rights, Malta
Genersch E, von der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R, Berg S, Ritter W, Mühlen W, Gisder S, Meixner M, Liebig G, Rosenkranz P (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41:332–352
Grindrod I, Martin SJ (2021) Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents? Proc R Soc B 288:20211375. https://doi.org/10.1098/rspb.2021.1375
Harbo JR, Harris JW (2005) Suppressed mite reproduction explained by the behaviour of adult bees. J. Apicult. Res. 44:21–23
Hatjina F, Costa C, Büchler R, Uzunov A, Drazic M, Filipi J, Charistos L, Ruottinen L, Andonov S, Meixner MD, Bienkowska M, Dariusz G, Panasiuk B, Le Conte Y, Wilde J, Berg S, Bouga M, Dyrba W, Kiprijanovska H, Korpela S, Kryger P, Lodesani M, Pechhacker H, Petrov P, Kezic N (2014) Population dynamics of European honey bee genotypes under different environmental conditions. J Apic Res 53:233–247
Imdorf A, Buehlmann G, Gerig L, Kilchenmann V, Wille H (2019) Examination of the method for estimating the brood area and number of worker bees in free-flying bee colonies. Apidologie 18:137–146
Imdorf A, Bühlmann G, Gerig L, Kilchenmann V, Wille H (1987) Überprüfung der Schätzmethode zur Ermittlung der Brutfläche und der Anzahl Arbeiterinnen in freifliegenden Bienenvölkern. Apidologie 18:137–146
Janczyk A, Meixner MD, Tofilski A (2020) Morphometric identification of the endemic Maltese honey bee (Apis mellifera ruttneri). J. Apic. Res. 60:157–164. https://doi.org/10.1080/00218839.2020.1827705
Meixner MD, Francis RM, Gajda A, Kryger P, Andonov S, Uzunov A, Topolska G, Costa C, Amiri E, Berg S, Bienkowska M, Bouga M, Büchler R, Dyrba W, Gurgulova K, Hatjina F, Ivanova E, Janes M, Kezic N, Korpela S, Le Conte Y, Panasiuk B, Pechhacker H, Tsoktouridis G, Vaccari G, Wilde J (2014) Occurrence of parasites and pathogens in honey bee colonies used in a European genotype-environment interactions experiment. J Apic Res 53:215–229
Momeni J, Parejo M, Nielsen RO, Langa J, Montes I, Papoutsis L, Farajzadeh L, Bendixen C, SMARTBEES WP3 DIVERSITY COLLABORATORS, Vingborg R, Bouga M, Kryger P, Meixner MD, Estonba A (2021) Authoritative subspecies diagnosis tool for European honey bees based on ancestry informative SNPs. BMC Genomics 22:101. https://doi.org/10.1186/s12864-021-07379-7
Mondet F, Parejo M, Meixner MD, Costa C, Kryger P, Andonov S, Servin B, Basso B, Bienkowska M, Cauia E, Dahle B, Hatjina F, Kovacic M, Kretavicius J, Lima AS, Panasiuk B, Pinto MA, Uzunov A, Wilde J, Büchler R (2020) Evaluation of suppressed mite reproduction (SMR) reveals potential for varroa resistance in European honey bees (Apis mellifera L.). Insects, https://doi.org/10.3390/insects11090595
NAP (2019) National Agricultural Policy for the Maltese Islands 2018 – 2028. Parliamentary secretary for agriculture, fisheries and animal rights. Atriga Consult
National Livestock Database (Animal Register Section), www.agrikoltura.gov.mt, 2022.
Oddie M, Büchler R, Dahle B, Kovacic M, Le Conte Y, Locke B, de Miranda JR, Mondet F, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8.
OIE (2018) Nosemosis of honey bees. OIE Terrestrial Manual OIE 744–749.
Oleksa A, Wilde J, Tofilski A, Chybicki IJ (2013) Partial reproductive isolation between European subspecies of honey bees. Apidologie 44:611–619
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96–119
Ruttner H (1972) Technical recommendations for methods of evaluating performance of bee colonies. In F. Ruttner, Controlled mating and selection of the honey bee Bucharest, Apimondia 87–92.
Ruttner F (1988) Biogeography and Taxonomy of Honeybees. Springer Verlag, Berlin, Heidelberg and New York
Sheppard WS, Arias MC, Grech A, Meixner MD (1997) Apis mellifera ruttneri, a new honey bee subspecies from Malta. Apidologie 28:287–293
Tiemann K, Brückner D (1993) Zum Schwarmverhalten der Sizilianischen Honigbiene Apis mellifera sicula (Montagano 1911). Apidologie 24:365–374
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36:592–606
Uzunov A, Brascamp P, Du M, Büchler R (2022) Initiation and implementation of honey bee breeding programs. Bee World 99:50–55
Uzunov A, Costa C, Panasiuk B, Meixner M, Kryger P, Hatjina F, Bouga M, Andonov S, Bienkowska M, Le Conte Y, Wilde J, Gerula D, Kiprijanovska H, Filipi J, Petrov P, Ruottinen L, Pechhacker H, Berg S, Dyrba W, Ivanova E, Büchler R (2014) Swarming, defensive and hygienic behaviour in honey bee colonies of different genetic origin in a pan-European experiment. J. Apicult. Res. 53:248–260. https://doi.org/10.3896/IBRA.1.53.2.06
Uzunov A, Meixner M, Büchler R, Galea T (2018) Conservation of the endemic Maltese honey bee (Apis mellifera ruttneri). SmartBees Newsletter 5.
Winston ML (1987) The biology of the Honeybee. Harvard University Press, Cambridge
Zammit-Mangion M, Meixner M, Mifsud D, Sammut S, Camilleri L (2017) Thorough morphological and genetic evidence confirm the existence of the endemic honey bee of the Maltese Islands Apis mellifera ruttneri: recommendations for conservation. J Apic Res 56(5):514–522. https://doi.org/10.1080/00218839.2017.1371522
Acknowledgements
This work would not have been possible without the assistance of so many people who in some way or other helped us during the entire experiment. We are grateful to the University of Malta for financial assistance and for providing the space for one of the experimental setups. We also thank Mr. Renie Scicluna who provided us with his fields in Siggiewi for the second experimental station. We are also grateful to Mr. Christopher Busutill of Evolve Ltd., for party funding the honey bee boxes. We thank the Engineering Department of the University of Malta for producing metal frames under boxes to better manage ant attacks. We thank Dr. Raffaele Dall’Olio who made the necessary arrangements to get the Apis mellifera ligustica from a registered beekeeper in Bologna and to Mr. Tonio Mallia, who accompanied one of the authors (DM) to get these queens to Malta. We acknowledge the help of Ms. Martina Deskoska, a student from Ss Cyril and Methodius University in Skopje (Faculty for Agricultural Sciences and Food) from Macedonia for assistance with laboratory work. Several laboratory technicians in Kirchhain assisted us with laboratory analysis, and breeders within Breeds of Origin Conservancy, in particular Jorge Spiteri and Victor Busuttil, assisted with the collection of data. We also thank Dr. James Ciarlo for his assistance in gathering data related to weather conditions in Malta as well as Dr. Pantaleo Gemma and Mr. Francesco Luca Alexander for providing data on the number of colonies in Malta. We acknowledge the support from the University of Malta Academic Work Resources Fund.
Funding
The research was supported by the research fund of Prof. David Mifsud (RFSRA 01–01), University of Malta, the Academic Work Resource Fund of Prof. Marion Zammit Mangion, University of Malta and by Landesbetrieb Landwirtschaft Hessen.
Author information
Authors and Affiliations
Contributions
AU developed the study idea, collected data and samples, statistically analysed the data, and wrote the manuscript. DM developed the study idea, managed the honey bee colonies, collected data and samples, and wrote the manuscript. TG managed the honey bee colonies, collected data and samples, and wrote the manuscript. SC managed the honey bee colonies, collected data and samples, and wrote the manuscript. MZM collected data and samples and wrote the manuscript. MDM developed the study idea, analysed the samples in the laboratory, statistically analysed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is an observational study, and no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: Cedric Alaux
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uzunov, A., Mifsud, D., Galea, T. et al. Development, behaviour, productivity, and health status of the native honey bee Apis mellifera ruttneri vs. the introduced A. m. ligustica in Malta. Apidologie 54, 34 (2023). https://doi.org/10.1007/s13592-023-01008-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01008-w