Abstract
Stingless bees collect plant latex and resin to produce cerumen and propolis. Cerumen is primarily used for nest construction, such as brood cells, storage pots, and involucrum. Propolis is mainly used as a sealing material and for predator and pathogen defense. Knowledge about the botanical origin of these materials is vital for sustainable bee management. We performed (i) direct observation method by field surveys and (ii) indirect assumption method via pollen analysis of corbicular and in-hive stored latex/resin, cerumen, and propolis of Tetragonula iridipennis. By the direct observation method, we identified 25 plant species as latex/resin sources of the stingless bees; frequently encountered were Artocarpus heterophyllus, Calotropis gigantea, Ficus benghalensis, Ficus religiosa, Mangifera indica, Tabernaemontana divaricata, and Vachellia nilotica. From pollen analyses, we found diverse pollen types, including pollens of polleniferous plants. Comparatively higher pollen content was found in cerumen and propolis samples than in the in-hive stored latex/resin and corbicular latex/resin loads. But all the pollen types do not indicate actual latex/resin sources for the bee species. These pollen types came from the foraging environment and additionally during the transport of latex/resin within the hive and the processing of latex/resin into cerumen and propolis. Therefore, we can conclude that the pollen content of corbicular and in-hive stored latex/resin, cerumen, and propolis is not truly inferring its botanical origin; it requires alternative techniques like the direct observation method or chemical profiling.
Similar content being viewed by others
1 Introduction
Stingless bees are closely related to honeybees and are members of the Meliponini tribe of the Apidae family. They are referred to as “stingless” because they do not have functional stinging apparatus. They can be found across Australasian, Indo-Malayan, and Neotropical tropical, subtropical, and Afrotropical regions (Camargo 1988; Michener 2000; Kajobe 2007). In India, several species of stingless bees were recognized (Rasmussen 2013; Rahman et al. 2015; Viraktamath and Thangjam 2021) with the dominance of Tetragonula iridipennis (Smith, 1854) (Layek and Karmakar 2018a; Rahman et al. 2015). Stingless bees are eusocial and have one queen, a few drones, and thousands of workers in their perennial colonies (Wille 1983). Most species are cavity-nesting mainly in building walls and tree trunks (Roubik 2006; Danaraddi et al. 2009; Layek and Karmakar 2018a), and sometimes also found in termite mounds (Roubik 2006) and ridges of agricultural fields (Layek and Karmakar 2018a).
The nest components (including food pots, brood cells, involucrum, and other supporting strands) of stingless bees are made up of cerumen. In contrast, propolis utilizes as a sealing material and for protective purposes, and is found in the joining point of the top cover sheet of a managed hive. Additionally, when soil is mixed with propolis, the product is called geopropolis. To make these nesting materials (i.e., cerumen and propolis), they collect plant latex and resin (Layek et al. 2021a) and mix them with bee wax and other substances. Cerumen and propolis have a wide spectrum of pharmacological activities such as antimicrobial (Park et al. 1998), antioxidant (Kumazawa et al. 2004), antihypertensive (Maruyama et al. 2009), and anticancer properties (Kimoto et al. 1998). For these, cerumen and propolis have long been utilized in traditional medicine to treat diseases and improve health (Choudhari et al. 2012; Flores et al. 2018; Yam-Puk et al. 2019).
Stingless bees are generalist, polylectic foragers with significant floral constancy (Layek and Karmakar 2018a). These characteristics make them effective pollinators of several flowering plants, including wild and cultivated crops (Heard 1999; Slaa et al. 2006; Sanches et al. 2017; Layek et al. 2022c). In many regions of the world, bees and other pollinators are declining (Kremen et al. 2007; Kennedy et al. 2013), and their elimination has adverse impacts on plant-pollinator interactions and the functional composition of ecosystems. To overcome these negative impacts, the management of honeybees is traditional. Recently, researchers and beekeepers started using stingless bees for agricultural pollination and hive products (Nunes-Silva et al. 2013; Layek et al. 2021b, 2022a).
The successful management of stingless bees largely depends on the vegetation in their immediate surroundings. The availability of latex and resin sources is vital for colony growth and leads to sustainable meliponiculture. In this regard, determining the botanical origin of cerumen and propolis is essential. However, there is no available data about the latex/resin source of the Tetragonula iridipennis. Therefore, the present study was conducted to determine the botanical origin of the cerumen and propolis of Indian stingless bees (Tetragonula iridipennis).
2 Materials and methods
2.1 Sampling sites
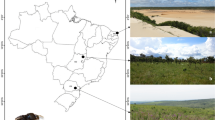
We collected samples from three managed colonies kept in three regions—(i) the Vidyasagar University (VU) campus of Paschim Medinipur district, (ii) Jenadihi village in Bankura district, and (iii) Bolpur in Birbhum district of West Bengal, India (Figure 1 which is generated using QGIS). In Jenadihi, hive surrounding (~ 1 km) vegetation comprises wild and cultivated trees, weeds, and crops. In the VU campus and Bolpur, hive surrounding vegetation consists mainly of trees, with a few weeds and ornamental plants.
2.2 Collection of samples
We collected corbicular latex and resin loads, in-hive stored latex/resin loads, cerumen, and propolis samples of Indian stingless bees (Tetragonula iridipennis) from November 2021 to October 2022. For collecting corbicular latex/resin loads, we blocked the nest entrance for a short time (5–10 min) by plugging a paper cap. Then, returning latex and resin foragers (Figure 2) were captured at the nest entrance. The two loads of a latex/resin forager were collected (here, we took on a clean glass slide). Some foragers return with sticky cream-colored corbicular loads (maybe latex or resin); we cannot differentiate them as latex or resin foragers. Therefore, we excluded them from our sampling. We caught 4–5 latex/resin foragers on a sampling day during the morning (8.00–11.00 h). For collecting corbicular latex/resin loads, we performed two sampling days per month per site. This way, we collected 348 pairs of corbicular loads (166 pairs of latex loads and 182 pairs of resin loads) covering all the months. For collecting in-hive stored latex/resin, cerumen, and propolis samples, we uncovered the top sheet of the managed hives. Then, we collected stored latex/resin (lighter than cerumen and propolis; Figure 3A) found on the inner wall of the nest. We collected propolis samples from the joining point of the top cover sheet (Figure 3B). As cerumen samples, we collected honey pots (Figure 3C) and involucrum (Figure 3D). In the case of honey pots used as cerumen samples, after rupturing, the pots were washed with distilled water, blotted, and weighed. On a sampling day, we collected 50–300 mg of cerumen (comprising one sample), 50–300 mg of propolis, and 50–200 mg of stored latex/resin (if available and constituting one sample) from a hive. To collect stored latex/resin, cerumen, and propolis samples, we performed one sampling day per month per site. A total of 31 stored latex/resin samples, 25 cerumen, and 11 propolis samples were collected.
2.3 Palynological analyses of the collected samples
For the corbicular latex/resin loads (in pairs), which were taken on a glass slide, we added glycerine water (one drop) and mixed the loads well with a needle. After using a coverslip, we studied the pollen content with the help of a compound microscope. Leica DM1000 was used for microscopic analysis, while Leica DFC295 was used to capture microphotographs of some pollen grains at the appropriate magnifications. We considered the number of pollen grains per pair loads for quantitative analysis. Using the mean values, we also estimated the number of pollen grains per gram of latex and resin by considering the average weight of a pair of latex and resin loads (i.e., 2.26 mg for latex loads and 1.20 mg for resin loads; Layek et al. 2021a).
For in-hive stored latex/resin samples, we added 5 mL ethanol (70%) and incubated them for 5 days at room temperature (20–27 °C). The samples were stirred with a glass rod to mix the latex/resin well. Then, we added 2 mL KOH (50–70%) solution and incubated for 2 days. After homogenization, we took a 10 µL sample solution on a glass side. Then, we counted the number of pollen grains in the 10 µL sample. The method was repeated three times and obtained mean values. Then, we estimated the number of pollen grains per gram of latex/resin sample as follows: N = (n × 1000 × St) / (10 × Swt), where N is the number of pollen grains per gram of sample, n is the average number of pollen per 10 µL sample solution, St is the volume of the stock sample solution in mL, and Swt is the weight of sample which was recorded during sampling. After the quantitative study, the remaining sample solution was centrifuged at 2500 rpm for 5 min. The sediment containing pollens was processed by the acetolysis method of Erdtman (1960) to increase the visibility of diagnostic characteristics of the pollen types (Layek et al. 2022b). Then, we studied the pollen types’ composition of the samples.
To find out an appropriate processing method for pollen analysis of cerumen and propolis samples, initially, we used three methods—(i) ethanol-based method (described previously for the analysis of stored latex/resin), (ii) KOH-based method, and (iii) H2SO4-based method. In the KOH-based method, we added 3 mL KOH (50–70%) solution to the sample and incubated it for 4 days at room temperature. Then, we stirred with a glass rod to homogenize the sample. Then, we added 2 mL ethanol (70%) and incubated it for another 3 days. Again, we stirred the solution to homogenize the sample. In the H2SO4-based method, we added 3 mL of concentrated H2SO4 to the sample and incubated it for 4 days at room temperature. After that, we stirred the sample. Then, we added 1 mL of distilled water drop by drop and shook. Then, we added 1 mL ethanol (70%) and incubated it for 3 days. After that, we shook the solution to homogenize the sample. Among these three methods, the H2SO4-based method was most effective in dissolving the cerumen and propolis samples. Therefore, we followed this method to analyze the rest cerumen and propolis samples. After homogenizing a sample, we add 2 mL of 20% KOH to slightly reduce the acidity of the solution of the cerumen or propolis (here, we do not use a higher concentration of KOH solution to avoid salt crystal formation). Then, we took a 10 µL sample solution and followed the method described earlier for in-hive stored latex/resin samples for quantitative and later qualitative analysis.
Pollen types were identified using reference slides available in the palynology and plant reproductive biology lab under the Department of Botany & Forestry of Vidyasagar University. Additionally, the published articles (Pal and Karmakar 2013; Layek and Karmakar 2016, 2018b; Layek et al. 2020) having images of pollen grains from these regions are also considered. When species-level identification was not possible, we employed the pollen type system (Joosten and Klerk 2002).
Based on the occurrence of a pollen type within the corbicular latex, corbicular resin, in-hive stored latex/resin, cerumen, and propolis samples, we classified the pollen types into four groups: (i) very frequent (found in > 30% of the samples), (ii) frequent (found in 10–30% of the samples), (iii) less frequent (found in 3– < 10% of the samples), and (iv) rare (found in < 3% of the analyzed samples).
We counted 100–300 pollen grains per sample (for in-hive stored latex/resin, cerumen, and propolis samples). Regarding the pollen content of individual samples, we also segregated the pollen types into one of the four groups (as proposed by Louveaux et al. (1978) for quantitative pollen analysis of honey sample): predominant (> 45%), secondary pollen type (16–45%), important minor (3– < 16%), and minor (< 3%).
2.4 Field surveys
On a sunny day, we surveyed the nest surrounding vegetation, focusing on the injured plant parts’ exudate latex or resin. Field observations were performed during the peak activity time of the Tetragonula iridipennis, i.e., 9.00–11.00 h (Layek and Karmakar 2018a). We recorded the plant sources and number of bees collecting latex/resin. The plant species indicated by frequent pollen types in latex/resin, cerumen, and propolis samples were also observed, whether the stingless bees (Tetragonula iridipennis) collect latex/resin.
2.5 Statistical analyses
We calculated the mean and standard deviation using descriptive data analysis. To evaluate if the data (i.e., quantitative pollen contents of in-hive stored latex/resin, cerumen, and propolis samples) were normally distributed, we employed the Shapiro–Wilk tests. On normally distributed data, we applied a parametric test, one-way ANOVA, and p ≤ 0.05 was considered statistically significant. The above-mentioned statistical studies were carried out using SPSS (v. 16.0, Chicago, IL, USA) and Microsoft Excel software.
3 Results
3.1 Palynological analyses
Among the analyzed 348 pairs of corbicular latex/resin loads, 83.33% of loads had pollen grains (in latex loads: 80.72%; in resin loads: 85.71%). Each pair (two loads) may have zero, one, two, or more pollen types (Table I). Most analyzed loads have a single pollen type (in latex loads: 66.26%; in resin loads: 71.98%). Twenty-three pollen types were recognized from corbicular latex loads and 27 from resin loads (Table II). Within the latex loads, very frequent pollen types were Borassus flabellifer, Eucalyptus type, and Peltophorum pterocarpum, and frequent pollen types were Brassica type and Phoenix sylvestris. The pollen spectrum of resin loads showed a similar type of very frequent and frequent pollen types, with the addition of Lannea coromandelica as very frequent pollen types. Some of the latex loads contained pollen types of resinous plants (Lannea coromandelica and Spondias pinnata), while some resin loads contained pollen types of plants having latex (e.g., Artocarpus heterophyllus).
From pollen analysis of in-hive stored latex/resin, we recognized 39 pollen types. The number of pollen types per sample ranges from 3 to 7 (Supplementary Table I) with an average value of 4.39 ± 0.95 (mean ± SD, n = 31). Predominant pollen types were Borassus flabellifer, Brassica type, Eucalyptus type, Peltophorum pterocarpum, and Trema orientalis. Regarding the occurrence of the pollen types within the analyzed samples, very frequent pollen types (i.e., the occurrence was > 30% among the studied samples) were Borassus flabellifer, Brassica type, Eucalyptus type, and Peltophorum pterocarpum (Table II).
From pollen analysis of 25 cerumen samples, we identified 43 pollen types. The number of pollen types per sample was 5.68 ± 1.31 (mean ± SD, n = 25), ranges 4–9 (Supplementary Table II). Predominant pollen types were Borassus flabellifer, Brassica type, Eucalyptus type, Lannea coromandelica, Peltophorum pterocarpum, and Trema orientalis. The very frequent pollen types were Borassus flabellifer, Cocos nucifera, Eucalyptus type, Peltophorum pterocarpum, and Phoenix sylvestris.
From pollen analysis of 11 propolis samples, we identified 29 pollen types. The number of pollen types per sample ranges from 4 to 9 (Supplementary Table III) with an average of 5.64 ± 1.50 (mean ± SD, n = 11). Predominant pollen types were Borassus flabellifer, Brassica type, Eucalyptus type, and Peltophorum pterocarpum. In the studied propolis samples, the very frequently occurred pollen types were Borassus flabellifer, Brassica type, Eucalyptus type, Helianthus annuus, Mikania scandens, Phoenix sylvestris, and Trema orientalis.
When we considered all types of samples (i.e., corbicular latex, corbicular resin, in-hive stored latex/resin, cerumen, and propolis), a total of 45 pollen types belonging to 27 plant families were identified. A few pollen grains (found in the analyzed samples) cannot identify up to species, genus, or family level. Figure 4 shows microphotographs of a few different pollen types. The obtained pollen spectra remain quite ambiguous regarding the botanical origin of these samples (latex, resin, cerumen, or propolis). Several frequently occurring pollen types (~ plant species) like Borassus flabellifer and Phoenix sylvestris are not to be vital latex/resin sources for the bee species. On the other hand, some plant species (Ficus benghalensis and Ficus religiosa) that provide a significant amount of latex/resin (determined through field observations) are missing in the obtained pollen spectra.
Microphotographs of some pollen types. A Acacia auriculiformis. B Borassus flabellifer. C Brassica type. D Citrus type. E Coriandrum sativum. F Eucalyptus type. G Holoptelea integrifolia. H Lannea coromandelica. I Peltophorum pterocarpum. J Phoenix sylvestris. K Solanum melongena. L Trema orientalis. Scale bars: 10 µm.
The number of pollen grains per pair of corbicular loads does not differ between the latex and resin loads (F1, 346 = 1.95, p = 0.16). Each pair of corbicular latex loads contained 0–13 (3.11 ± 2.84, mean ± SD, n = 166) pollen grains, and resin loads had 0–18 (3.63 ± 3.58, mean ± SD, n = 182) pollen grains. However, considering the number of pollen grains per unit weight (here, 1 g), resin loads have greater pollen (2983.33 pollen grains/g) than latex loads (1371.68 pollen grains/g) (Table III). Pollen content of the in-hive stored latex/resin, cerumen, and propolis samples significantly differed (F2, 64 = 73.25, p < 0.001). However, pollen content does not differ between cerumen and propolis samples (F1, 34 = 1.54, p = 0.22). Cerumen and propolis samples have greater pollen content (cerumen: 176,099.81 ± 38,139.59, mean ± SD, n = 25; propolis: 159,627.98 ± 33,009.35, mean ± SD, n = 11) than the in-hive stored latex/resin (85,486.43 ± 16,726.09, mean ± SD, n = 31).
3.2 Field surveys
From the field surveys, we recorded 25 plant species as latex/resin sources for the stingless bee Tetragonula iridipennis (Table IV). Important (i.e., frequently observed) resin/latex sources were Artocarpus heterophyllus, Calotropis gigantea, Ficus benghalensis, Ficus religiosa, Mangifera indica, Tabernaemontana divaricata, and Vachellia nilotica. Highly represented plant families were Anacardiaceae, Apocynaceae, Fabaceae, and Moraceae. The bee species collected latex/resin from different plant parts, including injured bark of stem, young leaves, and immature fruits.
4 Discussion
We studied the pollen content of corbicular latex and resin loads simply by adding glycerine water. To dissolve and homogenize the in-hive stored latex/resin samples, we used ethanol and KOH, while for pollen analysis of cerumen and propolis, initially, we used three methods (i.e., ethanolic extract, KOH treatment, and H2SO4 treatment) to select a suitable protocol for further analysis. Barth (1998) proposed ethanol treatment followed by KOH treatment. But we found that the cerumen and propolis samples of Tetragonula iridipennis did not homogenize well with ethanol and KOH. The third method (i.e., H2SO4 treatment) remained more effective.
There are few studies on the palynological analysis of propolis, geopropolis, and cerumen. The majority of pollen analyses performed worldwide used honeybee Apis mellifera propolis (Ricciardelli D’Albore 1979; Barth et al. 1999; Luz et al. 2009; Freitas et al. 2011). Only a few studies came from stingless bees (Barth 2006; Freitas et al. 2012). The present studies on Indian stingless bees (Tetragonula iridipennis) will be the first and assume significant value in sustainable meliponiculture.
The pollen spectra obtained from corbicular latex/resin loads, in-hive stored latex/resin, cerumen, and propolis contained diverse pollen types. The obtained pollen spectra highly matched the polleniferous flora for the stingless bees (Trigona iridipennis = Tetragonula iridipennis) reported by Layek and Karmakar (2018a) and Bisui et al. (2019). Therefore, pollen analysis of these samples (latex/resin, cerumen, and propolis) may be a valuable tool in determining the regional flora (Barth and Luz 2003). In our study, frequent pollen types were Acacia auriculiformis, Borassus flabellifer, Cocos nucifera, Eucalyptus type, Peltophorum pterocarpum, and Trema orientalis. The predominance of Eucalyptus type was also reported from geopropolis analysis (Barth and Luz 2003; Barth 2006). The dominant pollen types within the pollen spectra of cerumen, propolis, and geopropolis depend on the surrounding vegetation of the sampling colonies. Barth et al. (2009) reported the dominance of some hygrophilous plants (Cedrela sp., Chrysophyllus sp., Cuphea sp., and Ludwigia sp.) in geopropolis samples collected from the Brazilian state. Freitas et al. (2012) documented the plant families Arecaceae, Cecropiaceae, Fabaceae, Myrtaceae, Rubiaceae, and Solanaceae in South American countries. Babaeva et al. (2021) found the predominance of some entomophilous plants (e.g., Centaurea sp., Cyanus sp., and Helianthus sp.) and anemophilous plants (e.g., Ambrosia sp.) of Asteraceae family in propolis samples collected from Caucasus and Volga.
Most corbicular latex/resin loads have pollen grains. In corbicular latex/resin loads, pollen may come from airborne pollen deposited on latex/resin collected by the foragers. Sometimes nectar and pollen foragers may change their allocated task and become latex/resin foragers (Layek et al. 2021a). In that case, remnant pollen within the corbicula may also be a leading pollen source in corbicular latex/resin loads. The quantitative pollen analysis revealed that in-hive stored latex/resin, cerumen, and propolis have more pollen grains than corbicular latex/resin loads. The greater pollen content of in-hive stored latex/resin, cerumen, and propolis can be explained by (i) pollen adhering to the internal tunnel surface from returning nectar and pollen foragers. When a latex/resin forager enters into the nest, corbicular latex/resin loads acquire pollens by contacting the tunnel surface, and (ii) the addition of pollen also takes place during the processing of latex/resin into cerumen and propolis. For that, pollen spectra specify the polleniferous flora for the bee species rather than its latex/resin sources, as pollen spectra do not correctly match the actual latex/resin-providing plants (determined by direct field observation). Several plant species were recorded as resin/latex sources for the stingless bees, including dominant Artocarpus heterophyllus, Ficus benghalensis, Mangifera indica, and Moringa oleifera. Some of the listed latex/resin-providing plants’ (e.g., Ficus benghalensis, Ficus religiosa) pollen types are not represented within the obtained pollen spectra from cerumen, propolis, or other samples. Furthermore, some pollen types of vital latex/resin-providing plants (e.g., Artocarpus heterophyllus and Moringa oleifera) occurred in the pollen spectra of latex/resin, cerumen, and propolis as rare and less frequent pollen types. Therefore, pollen spectra obtained from the latex/resin, cerumen, and propolis samples cannot be treated as the botanical origin of latex/resin sources. Through direct field observations, we can accurately depict the plant sources. However, the field survey method in determining latex/resin sources is rare, as it is more laborious and time-consuming than palynological analyses.
5 Conclusion
The corbicular latex/resin loads, in-hive stored latex/resin, cerumen, and propolis contained diverse pollen types. Pollen grains came to the corbicular latex/resin loads via airborne pollens from the surrounding environment or previous exposure of the foragers to the floral pollen sources. The addition of pollen grains also takes place in the in-hive stored latex/resin during the transport of corbicular latex/resin loads through the internal tunnel of the nest and during the processing of stored latex/resin into the cerumen and propolis. Through direct field observations, several plant species (here, 25) were recognized as latex/resin sources of the stingless bee species Tetragonula iridipennis. The most frequent were Artocarpus heterophyllus, Calotropis gigantea, Ficus benghalensis, Ficus religiosa, Mangifera indica, Tabernaemontana divaricata, and Vachellia nilotica. The field survey–based listed plants (i.e., accurate latex/resin sources for the bee species) do not match appropriately with the obtained pollen types from palynological analyses of the samples. Pollen spectra obtained from these samples (corbicular latex/resin loads, in-hive stored latex/resin, cerumen, and propolis) may be indicators of pollen sources of the stingless bee species. Therefore, the pollen content of corbicular and in-hive stored latex/resin, cerumen, and propolis does not truly infer its botanical origin; it requires alternative techniques like direct observation or chemical profiling.
Data availability
All relevant data are within the manuscript and its supplementary files.
Code availability
Not applicable.
References
Babaeva EY, Polevova SV, Zugkiev BG (2021) Identification of propolis and honey sources pollen analysis of the south of North Caucasus and the lower Volga. Scien Stud Res Chem Chemic Engin Biotec Food Indus 22(4):437–451
Barth OM (1998) Pollen analysis of Brazilian propolis. Grana 37(2):97–101. https://doi.org/10.1080/00173139809362650
Barth OM (2006) Palynological analysis of geopropolis samples obtained from six species of Meliponinae in the Campus of the Universidade de Ribeirão Preto, USP, Brazil. Apiacta 41(2):71–85
Barth OM, Barros MA, Freitas FO (2009) Análise palinológica em amostras arqueológicas de geoprópolis do vale do Rio Peruaçu, Januária, Minas Gerais, Brasil. AMHNJB 19(1):277–290
Barth OM, Dutra VML, Justo RL (1999) Análise polínica de algumas amostras de própolis do Brasil Meridional. Ciênc Rural 29:663–667. https://doi.org/10.1590/S0103-84781999000400016
Barth OM, Luz CFPD (2003) Palynological analysis of Brazilian geopropolis sediments. Grana 42(2):121–127. https://doi.org/10.1080/00173130310012512
Bisui S, Layek U, Karmakar P (2019) Comparing the pollen forage pattern of stingless bee (Trigona iridipennis Smith) between rural and semi-urban areas of West Bengal, India. J Asia-Pac Entomol 22(3):714–722. https://doi.org/10.1016/j.aspen.2019.05.008
Camargo JMF (1988) Meliponinae (Hymenoptera, Apidae) da coleção do Instituto di entomologia agraria, Portici, Itália. Rev Bras Entomol 32:351–374
Choudhari MK, Punekar SA, Ranade RV, Paknikar KM (2012) Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J Ethnopharmacol 141:363–367. https://doi.org/10.1016/j.jep.2012.02.047
Danaraddi CS, Viraktamath S, Basavanagoud K, Bhat ARS (2009) Nesting habits and nest structure of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. Karnataka J Agric Sci 22(2):310–313
Erdtman G (1960) The acetolysis method. A revised description. Sven Bot Tidskr 54:561–564
Flores FF, Hilgert NI, Lupo LC (2018) Melliferous insects and the uses assigned to their products in the northern Yungas of Salta, Argentina. J Ethnobiol Ethnomed 14:1–5. https://doi.org/10.1186/s13002-018-0222-y
Freitas ADS, Barth OM, de Oliveira SÉ, Matsuda AH, de Almeida-Muradian LB (2011) A palynological analysis of Brazilian propolis samples. J ApiProd ApiMed Sci 3(2):67–74. https://doi.org/10.3896/IBRA.4.03.2.01
Freitas AS, Vit P, Barth OM (2012) Pollen profile of geopropolis samples collected by native bees (Meliponini) in some South American countries. Sociobiology 59:1465–1482
Heard TA (1999) The role of stingless bees in crop pollination. Ann Rev Entomol 44(1):183–206. https://doi.org/10.1146/annurev.ento.44.1.183
Joosten H, De Klerk P (2002) What’s in a name? Some thoughts on pollen classification, identification, and nomenclature in Quaternary palynology. Rev Palaeobot Palynol 122:29–45. https://doi.org/10.1016/S0034-6667(02)00090-8
Kajobe R (2007) Nesting biology of equatorial Afrotropical stingless bees (Apidae; Meliponini) in Bwindi Impenetrable National Park. Uganda J Apic Res 46(4):245–255. https://doi.org/10.1080/00218839.2007.11101403
Kennedy CM, Lonsdorf E, Neel MC et al (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16:584–599. https://doi.org/10.1111/ele.12082
Kimoto T, Arai S, Kohguchi M, Aga M, Nomura Y, Micallef MJ, Kurimoto M, Mito K (1998) Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian propolis. Cancer Detec Prevent 22:506–515. https://doi.org/10.1046/j.1525-1500.1998.00020.x
Kremen C, Williams NM, Aizen MA et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314. https://doi.org/10.1111/j.1461-0248.2007.01018.x
Kumazawa S, Hamasaka T, Nakayama T (2004) Antioxidant activity of propolis of various geographic origins. Food Chem 84:329–339. https://doi.org/10.1016/S0308-8146(03)00216-4
Layek U, Bisui S, Karmakar P (2021a) Flight range and resource loading-unloading behavior of stingless bee Tetragonula iridipennis (Smith). J Apic Res. https://doi.org/10.1080/00218839.2021.1994259
Layek U, Das A, Karmakar P (2022a) Supplemental stingless bee pollination in fennel (Foeniculum vulgare Mill.): an assessment of impacts on native pollinators and crop yield. Front Sustain Food Syst 6:820264. https://doi.org/10.3389/fsufs.2022.820264
Layek U, Das N, Kundu A, Karmakar P (2022b) Methods employed in the determining nectar and pollen sources for bees: a review of the global scenario. Ann Entomol Soc Am 115(6):417–426. https://doi.org/10.1093/aesa/saac013
Layek U, Das U, Karmakar P (2022c) The pollination efficiency of a pollinator depends on its foraging strategy, flowering phenology, and the flower characteristics of a plant species. J Asia-Pac Entomol 25(2):101882. https://doi.org/10.1016/j.aspen.2022.101882
Layek U, Karmakar P (2016) Bee plants used as nectar sources by Apis florea Fabricius in Bankura and Paschim Medinipur districts. West Bengal. Geophytology 46(1):1–14
Layek U, Karmakar P (2018a) Nesting characteristics, floral resources, and foraging activity of Trigona iridipennis Smith in Bankura district of West Bengal. India Insect Soc 65(1):117–132. https://doi.org/10.1007/s00040-017-0593-4
Layek U, Karmakar P (2018b) Pollen analysis of Apis dorsata Fabricius honeys in Bankura and Paschim Medinipur districts, West Bengal. India Grana 57(4):298–310. https://doi.org/10.1080/00173134.2017.1390604
Layek U, Kundu A, Bisui S, Karmakar P (2021b) Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: a comparative study. Ann Agric Sci 66:38–45. https://doi.org/10.1016/j.aoas.2021.02.004
Layek U, Manna SS, Karmakar P (2020) Pollen foraging behaviour of honey bee (Apis mellifera L.) in southern West Bengal, India. Palynology 44(1):114–126. https://doi.org/10.1080/01916122.2018.1533898
Louveaux J, Maurizio A, Vorwohl G (1978) Methods of Melissopalynology. Bee World 59:139–157
Luz CD, Barth OM, Bacha Junior GL (2009) Análise palinológica de própolis vermelha do Brasil: subsídios para a certificação de sua origem botânica e geográfica. Mensagem Doce 102:10–15
Maruyama H, Sumitou Y, Sakamoto T, Araki Y, Hara H (2009) Antihypertensive effects of flavonoids isolated from Brazilian green propolis in spontaneously hypertensive rats. Biol Pharm Bull 32:1244–1250. https://doi.org/10.1248/bpb.32.1244
Michener CD (2000) The bees of the world. Johns Hopkins University Press, Baltimore
Nunes-Silva P, Hrncir M, Silva CI, Roldao YS, Imperatriz-Fonseca VL (2013) Stingless bees, Melipona fasciculata, as efficient pollinators of eggplant (Solanum melongena) in greenhouses. Apidologie 44:537–546. https://doi.org/10.1007/s13592-013-0204-y
Pal PK, Karmakar P (2013) Pollen analysis in understanding the foraging behaviour of Apis mellifera in Gangetic West Bengal. Geophytology 42:93–114
Park YM, Koo MH, Abreu JAS, Ikegaki M, Cury JA, Rosalen PL (1998) Antimicrobial activity of propolis on oral microorganisms. Curr Microbiol 36:24–38. https://doi.org/10.1007/s002849900274
Rahman A, Das PK, Rajkumari P, Saikia J, Sharmah D (2015) Stingless bees (Hymenoptera: Apidae: Meliponini): diversity and distribution in India. Int J Sci Res 4:77–81
Rasmussen C (2013) Stingless bees (Hymenoptera: Apidae: Meliponini) of the Indian subcontinent: Diversity, taxonomy and current status of knowledge. Zootaxa 3647(3):401–428. https://doi.org/10.11646/zootaxa.3647.3.1
Ricciardelli D’Albore G (1979) L’origine géographique de la propolis. Apidologie 10(3):241–267
Roubik DW (2006) Stingless bee nesting biology. Apidologie 37:124–143. https://doi.org/10.1051/apido:2006026
Sanches MA, Pereira AM, Serrão JE (2017) Pharmacological actions of extracts of propolis of stingless bees (Meliponini). J Apic Res 56:50–57. https://doi.org/10.1080/00218839.2016.1260856
Slaa EJ, Chaves LAS, Malagodi-Braga KS, Hofstede FE (2006) Stingless bees in applied pollination: practice and perspectives. Apidologie 37(2):293–315. https://doi.org/10.1051/apido:2006022
Viraktamath S, Thangjam R (2021) Two new species of Tetragonula (Hymenoptera: Apidae: Meliponini) from North-East India with notes on their nest structure. Biologia 76(6):1691–1704. https://doi.org/10.2478/s11756-020-00662-0
Wille A (1983) Biology of stingless bees. Ann Rev Entomol 28:41–64
Yam-Puc A, Santana-Hernández AA, Yah-Nahuat PN, Ramón-Sierra JM, Cáceres-Farfán MR, Borges-Argáez RL, Ortiz-Vázquez E (2019) Pentacyclic triterpenes and other constituents in propolis extract from Melipona beecheii collected in Yucatan, México. Rev Bras Farmacogn 29:358–363. https://doi.org/10.1016/j.bjp.2019.01.006
Acknowledgements
We are thankful to the authority of Vidyasagar University for providing us with the necessary laboratory facilities. We also thank the anonymous reviewers for their valuable comments to improve the manuscript.
Author information
Authors and Affiliations
Contributions
UL and PK conceived the ideas. UL, ND, and SKD collected samples. UL and PK designed the methodology. UL and ND analyzed the samples. UL wrote the manuscript (draft copy). PK and ND revised the manuscript. All authors approved the manuscript and agreed to publish.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: Klaus Hartfelder
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Layek, U., Das, N., Kumar De, S. et al. The botanical origin of cerumen and propolis of Indian stingless bees (Tetragonula iridipennis Smith): pollen spectrum does not accurately indicate latex and resin sources. Apidologie 54, 18 (2023). https://doi.org/10.1007/s13592-023-00994-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-00994-1