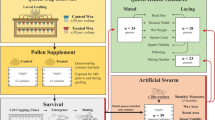
Abstract
Honey bees are incidentally exposed to pesticides such as the insect growth regulator methoxyfenozide (MEOF) during crop pollination, exposures that extend into the hive via contaminated stored food. We examined the sublethal effects of MEOF-contaminated pollen and queen cell wax on replacement queen development. MEOF-exposed colonies were largely able to produce replacement queens of similar physiological and reproductive quality as unexposed colonies. Newly established queens did not differ in their body mass, ovariole development, or protein and fatty acid contents in their ovaries and fat bodies. MEOF and control queens had similar glandular contents of queen mandibular pheromone (QMP) and queen retinue pheromone (QRP) compounds. However, MEOF queens stored less sperm in their spermathecae than control queens. Given that queen productivity is ultimately limited by sperm availability, MEOF contamination might shorten the functional lifespan of exposed queens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Honey bees pollinate crops throughout the world and are considered essential to global food security (National Research Council 2007). Recent declines among honey bees worldwide have been attributed to a variety of stressors including novel parasites and pathogens, reduced forage, poor nutrition, increased queen failures, and agrochemical exposures (Potts et al. 2010; Goulson et al. 2015; Lee et al. 2015; vanEngelsdorf et al. 2013). One of the most commonly encountered pesticides by bees is the insect growth regulator (IGR) methoxyfenozide (MEOF). This endocrine disruptor functions as an ecdysteroid receptor agonist and is particularly effective against immature lepidopterans due to high specificity for lepidopteran receptors (Tasei 2001; Carlson et al. 2001; Retnakaran et al. 2003). MEOF shows very low acute toxicity against honey bee larvae and adult workers (Wade et al. 2019; Zhu et al. 2015). Because of its low acute toxicity to non-target insects relative to other pesticide alternatives, MEOF is considered a reduced risk pesticide by the EPA (Mommaerts et al. 2006; Wade et al. 2019). As a result, MEOF applications against pests have increased, leading to more frequent pollinator exposures (Baker 2017; for non-bloom application, see Higbee-Siegel 2012; Wade et al. 2019). As a lipophillic compound, MEOF is detected in honey bee colonies in the USA especially in wax and pollen (Ostiguy et al. 2019; Johnson et al. 2010; Mullin et al. 2010). Most colony members are exposed to food-derived residues in honey, stored pollen, and wax inside the colony (Sanchez-Bayo and Goka 2014). Relatively few studies have examined sublethal effects of field-relevant MEOF exposures on honey bees. Notably, field-relevant MEOF exposures have few discernable sublethal effects on honey bee larvae and pupae (see Fine and Corby-Harris 2021 for review). By contrast, sublethal effects have been observed in adult workers exposed to MEOF, including enlarged hypopharyngeal gland size (Fine 2020, but see Meikle et al. 2019 at lower concentrations), decreased forager survival (Fisher et al. 2018), and decreased thermoregulation, reduced forager activity, and reduced forager populations in MEOF-exposed field colonies (Meikle et al. 2019). Other effects on individuals may be indirect. A recent study by Fine (2020) on queens in highly structured queen monitoring cages did not find an effect on oviposition, but found reduced egg eclosion in MEOF-exposed microcolonies.
Notably, the effects of MEOF exposure on colonies rearing developing queens has not been explored despite the vulnerability of these bees to disruption by stressors during queen replacement. Ecdysteroids likely play key roles in the development and reproduction of honey bees and queens, although specific mechanisms are not fully understood (Mello et al. 2014; Wegener et al. 2013). Developing queens are susceptible to physiological and behavioral disruption by pesticides of key functions both in the queens and the supporting workers, all of which must be achieved to avoid queen loss. Workers must detect the absence of the resident queen, rear new queens from appropriate-aged larvae, and support the developing queen (Pettis et al. 1995; Winston 1987). Young adult queens must kill rival queens, take nuptial flights during open mating, maintain stored sperm, and complete reproductive maturation to become established queens (Gilley and Tarpy 2005; Collins et al. 2004b; Winston 1987). Queens also risk greater exposure to dietary pesticide contaminants due to high food consumption rates. Queens consume considerably more food in the form of glandular secretions than workers both as larvae and as adults. Queens themselves consume considerably more food than workers as worker glandular secretions (royal jelly) both as immatures and adults (Crailsheim 1992). However, queens may be less exposed than workers to MEOF contaminants due to fewer task interactions (foraging, stored food and wax handling) with contaminated materials and lower direct consumption of foodborne residues (Purdy 2015; Böhme et al. 2018).
In this study, we examined whether MEOF impacts the ability of colonies to produce viable, well-mated replacement queens. We focused primarily on MEOF effects on the ability of colonies to rear, support, and produce replacement queens from larvae to established mated queens, as well as key developmental and reproductive metrics related to queen productivity (Delaney et al. 2011). We also assessed whether MEOF affects pheromone communications essential to maintenance of an established mated queen. Queens release the contact pheromone complexes QMP (queen mandibular pheromone) and QRP (queen retinue pheromone, which contains five QMP compounds) to workers to communicate their presence and quality to colony workers, to suppress supersedure, to promote queenright behavior, and solicit retinue care (QRP) (Slessor et al. 1988; Naumann et al. 1991; Pankiw et al. 1998; Pettis et al. 1995, 1997; Keeling et al. 2003). Both pathways rely on queen release of pheromones to workers and pheromone perception by workers, processes that are susceptible to interference by colony stressors.
2 Materials and methods
2.1 Experimental colonies
We monitored queen replacement during a simulated 8-week colony exposure to MEOF-contaminated pollen and wax in the late summer and early fall. Sixty nucleus colonies were established in early summer 2018 and 2019 at an apiary (University of Arizona Campus Agricultural Center, Tucson, AZ, USA; 32.27636, − 110.93845) in a riparian site dominated by desert plants rarely treated with pesticides. Each colony consisted of 4200 to 6500 workers headed by a commercial Italian queen. Forage was available from March to early June and late August to mid-November but limited during a pronounced mid-summer dearth.
2.2 Methoxyfenozide treatment
To maintain nutrition through the mid-summer dearth and early fall, colonies were supplemented weekly with 200 g pollen patty (1:1:1 dried corbicular pollen (Great Lakes Bee Supply, Galesburg, MI, USA): sucrose: Megabee (MegaBee Inc., San Diego, CA, USA)) for 8 consecutive weeks beginning at the end of July (7/30/18 and 7/29/19, 4 weeks before emergency queen rearing). At this time, colonies were randomly assigned to one of two treatment groups (methoxyfenozide-supplemented experimentals (MEOF) or unsupplemented controls (CON)). MEOF colonies were initially exposed to MEOF at field-relevant doses both in pollen patties (to simulate contaminated pollen) and in queen cup wax for 4 weeks before and 4 weeks after dequeening. Treatment concentrations were above (up to 2.7 ×) or within observed concentrations reported from field colonies in the literature (Johnson et al. 2010; Mullin et al. 2010; Meikle et al. 2019; Ostiguy et al. 2019). MEOF was added to pollen patty and cup wax at higher initial concentrations than concentrations observed in-hive upon deployment (346 ppb pollen patty and 23 ppb wax in formed cells; see results in Sect. 3.1) due to losses from chemical degradation, dilution with hive materials, and worker handling. MEOF colonies were given supplemental pollen patties initially containing 440 ppb MEOF (6.75 μg/μL stock solution in acetone; 65 µL stock solution/kg (wet mass) pollen patty mixed in water before addition to the patty; Sigma, Co, St. Louis, MO, USA). CON colonies were given patties supplemented with acetone only. MEOF was directly infused via carrier solvent into the wax coating of larval grafting queen cups (JZS BZS wide base cell cups, Mann Lake, Hackensack, MN, USA). Each queen cup was carefully coated with 100 μL melted comb wax. Each queen cup surface in MEOF colonies was infused with 50 μL of a 0.327 ng/μL stock solution in acetone to yield an initial concentration of 171 ppb MEOF in the wax coating (Sigma, Co, St. Louis, MO, USA). Queen cup wax used in control colonies (CON) was treated with acetone only. Queen cups were left out overnight to allow the acetone to evaporate.
2.3 Larval grafting, queen rearing, queen maturation, and queen establishment
Colonies were dequeened in late August (staggered from 8/27/18–8/28/18 and 8/25/19–8/27/19) and forced to rear emergency queens from grafted larvae and brood frame larvae to assess the effects of MEOF on queen rearing and development. At 4 days after dequeening, 30 1st instar larvae were grafted into queen cups (JZS BZS wide base cell cups, Mann Lake, Hackensack, MN, USA) on two cell bars in each colony. At 4 days after grafting (8 days after dequeening), queen cells were cut from the colony brood frames and the number of viable grafted cells noted. Colonies were then allowed to rear queens through adult emergence. At 19 days after dequeening, emerged adult queens were paint-marked with small unique colored dots. New adult queens were then allowed to openly mate, complete reproductive maturation, and engage in oviposition as established queen. Queens were terminally sampled on dry ice in October approximately 10 days after initial egg laying (43 days after dequeening; 10/9/18–10/10/18, 10/7/19–10/9/19). All tissues were frozen at − 80 °C until sample processing. Queens were sampled late enough to allow full establishment as an ovipositing queen yet early enough to avoid late fall oviposition declines associated with overwintering.
2.4 Queen development and reproductive quality
Sampled queens were assessed for physiological and reproductive quality and maturation. Each frozen queen was weighed, decapitated for later analysis of head queen pheromone contents, and dissected on wet ice to remove the ovaries, fat bodies (as the abdominal carcass attached to abdominal tergites and sternites), and spermatheca. One ovary was removed to obtain a count of ovarioles in PBS buffer under a dissecting microscope. The remaining ovary and abdominal carcass were analyzed separately for nutrient contents (total hydrolyzable protein and fatty acid) to provide estimates of nutrient provisioning in reproductive and non-reproductive tissues. Each tissue sample was brought up to 500 µL total volume with DI water, homogenized by Bead Beater, and subdivided among the nutrient assays.
Total hydrolyzed proteins were quantified by a bicinchonic acid (BCA) protein assay (Pierce Thomas Scientific, MA, USA) after acid hydrolysis. Dried tissue homogenates were subjected to a 16 h digest in 500 µL 12 N HCl at 70 °C under a nitrogen atmosphere, dried in a SpeedVac to remove acid residues, reconstituted in 1000 µL PBS buffer, and analyzed by the kit protocol. Total hydrolyzed protein contents were quantified by comparing the 562 nm absorbance of samples against bovine serum albumin (Sigma Inc., St. Louis, MO, USA) standard curves in a Gen-5 Plate Reader (Biotek, Inc., Winooski, UT, USA). Fatty acids were quantified by FAME (fatty acid methyl ester) analysis after conversion to their methyl ester equivalents (DeGrandi-Hoffman et al. 2018). Tissue homogenates were dried down and resolvated in 1000 µL Folch solution (2:1 chloroform: methanol) containing a pentadecanoic acid internal standard, then partitioned against 210 µL 0.25% KCl. One hundred sixty microliters of the chloroform:methanol layer was removed and analyzed for FAME contents by methods detailed in DeGrandi-Hoffman et al. (2018).
The amounts of nine QMP/QRP compounds present in queen mandibular glands were estimated from gland-containing heads by silylation derivatization techniques (after Slessor et al. 1990). Frozen queen heads were macerated in 2000 µL diethyl ether containing 60 µg of a cis-10-heptadecanoic acid internal standard, then extracted for 24 h at 27 °C in the dark (after Slessor et al. 1990; Keeling et al 2003). One thousand microliters of the extract supernatant was removed and analyzed by methods detailed in Carroll et al. (2018).
The number of sperm present in queen spermathecae was estimated using hemocytometer counts (Delaney et al. 2011). The spermatheca was dissected into 950 µL HEPES pH 7.4 buffer and 50 µL 10% bovine serum albumin (BSA; Sigma, St. Louis, MO, USA), vortexed to create a sperm suspension, and stained with 5 µL 2.4 mM propidium iodide (Live/Dead Sperm Viability kit, Thermo Fisher Scientific, Waltham, MA, USA). One microliter of stained sperm suspension was visualized and quantified on a hemocytometer by fluorescence microscopy (Nikon Eclipse 80i microscope with D-FL Epi Fluorescence attachment, Nikon Instruments, Melville, NY, USA) at a fluorescence emission of 617 nm.
2.5 Pesticide residues in workers and hive materials
Nest workers, stored pollen, honey, and royal jelly were sampled from colonies before and during treatments to assess both treatment exposures and background pesticide residues. Each sample type was collected and pooled equally by treatment group from all surviving colonies to provide approximately 3 g material for both MEOF-targeted and full pesticide panel analyses (USDA Agricultural Marketing Service National Science Laboratory in Gastonia, NC, USA).
2.6 Statistical analyses
For each dataset, comparisons were made across year and treatment. Datasets were checked for normality by PROC UNIVARIATE normality tests (SAS 9.2 2010) and examination of residuals. Datasets that lacked normality (fat body and ovary total protein and fatty acid contents, QMP/QRP pheromone contents) were compared by non-parametric Wilcoxon rank sum tests (NPAR1WAY SAS 9.2 2010). Datasets with normality (emergency cells, grafted cells, queen mass, ovarioles, sperm counts) were compared by 2-way ANOVA (PROC GLM, SAS 9.2 2010) with year and treatment as the main effects. There were no significant interactions between year and treatment.
3 Results
3.1 Colony pesticide residues
Honey bee exposure to MEOF was strictly limited to treated food and hive materials and the nest workers that handled such materials. MEOF residual levels were lower in the experimental hive materials than in the initial formulations. MEOF pollen patty contained 346 ppb MEOF and treated queen cup wax had 23 ppb MEOF after queen cup formation. MEOF nest workers contained 17 ppb MEOF with their gut contents removed. Notably, food secretions fed directly to queen larvae were not contaminated by MEOF in treated colonies, demonstrating that oral exposure may be low in developing queens. Royal jelly, honey, and frame wax from MEOF colonies had no detectable MEOF present, while stored pollen contained a trace (near the LOD of 2 ppb). MEOF was not detected in any bees, hive, or food materials from CON colonies. Colony hive and food materials from both treatments contained significant background residual compounds from miticide treatments (thymol 153–7220 ppb and mitraz (2,4-DMPF (decomposition product) 30–314 ppb)) applied months before.
3.2 Rearing and support of replacement queens
MEOF exposure had little effect on colonies’ abilities to rear queens and subsequent development and establishment of replacement queens. Most queens were lost during larval-pupal development or after adult emergence rather than during the initial phase of replacement queen rearing. Out of an initial 24 MEOF and 27 CON colonies, 100% and 100% made emergency queen cells while 97% and 97% reared grafted larvae on queen bars. Colonies differed by year but marginally not by treatment in the number of emergency queen cells constructed on brood frames (2-way ANOVA, F2,36 = 4.89, p = 0.033 (year); F2,36 = 3.69, p = 0.063 (treatment); see Online Resources 1)–there were slightly more emergency cells were constructed in 2019 than 2018. Colonies did not differ by treatment or year in the viable grafted larvae that took after grafting (2-way ANOVA, F2,36 = 2.75, p = 0.1062 (year); F2,36 = 0.00, p = 0.9441 (treatment); Figure 1). Eighty-eight percent of MEOF and 89% of CON colonies produced viable emerged virgin queens by 19 days after dequeening. Seventy-five percent of MEOF and 81% of CON colonies had established queens in place 43 days after dequeening. Of these queens, all but 2 of the MEOF colony queens and 1 of the CON colony queens contained stored sperm in their spermathecae. Unmarked queens of indeterminate origin were recovered in 3 MEOF and 3 CON colonies, with affected colonies excluded from statistical analysis of established queens.
Average number of viable grafted larvae (± S.E.) reared by methoxyfenozide-exposed (MEOF) and unexposed control (CON) colonies within 4 days after grafting. Thirty 1st instar larvae were grafted into each dequeened colony. The 2018 and 2019 colony totals are pooled by treatment (n = 23 (MEOF) or 26 (CON)).
3.3 Effects on queen development and reproductive maturation
Regardless of MEOF treatment, 2018 queens were of smaller and of lower reproductive quality than 2019 queens. The 2018 queens had smaller total body masses (2-way ANOVA, F2,35 = 6.45, p = 0.016 (year)), less total protein (Wilcoxon ranked sum test, Z = 2.4834, p = 0.017), oleic acid (Wilcoxon ranked sum test, Z = 2.1005, p = 0.042), and total fatty acids (Wilcoxon ranked sum test, Z = 2.1897, p = 0.035) in their ovaries than 2019 queens. The 2018 queens did not differ in ovariole number (2-way ANOVA, F2,34 = 2.18, p = 0.149 (year)), spermathecae sperm counts (2-way ANOVA, F2,35 = 0.07, p = 0.798 (year)), fat body total protein and total fatty acid contents (Wilcoxon ranked sum test, Z from − 1.9059 to 0.0866, p from 0.064 to 0.931), or other ovary fatty acid contents (hexadecanoic acid, linolenic acid, or octadecanoic acid) (Wilcoxon ranked sum test, Z from 1.0278 to 1.8024, p from 0.079 to 0.311) from 2019 queens.
MEOF treatment did not have significant effects on development and maturation of queen reproductive and non-reproductive tissues as estimated by nutrient contents. MEOF queens did not differ in body mass from CON queens (2-way ANOVA, F2,35 = 2.75, p = 0.106 (treatment); Online Resource 2). Ovaries and fat bodies of established queens did not differ by treatment in total protein contents (Wilcoxon ranked sum test, Z = 0.1767, p = 0.860 (treatment)(ovaries); Z = 1.2098, p = 0.234 (treatment)(fat bodies); see Online Resources 3), or total fatty acid contents (Wilcoxon ranked sum test, Z from − 0.2113 to 0.1767, p from 0.833 to 0.924 (fat bodies and ovaries); see Online Resources 4 and 5). Ovariole number (a proxy for ovary development) did not vary between MEOF and CON queens (2-way ANOVA, F2,35 = 0.04, p = 0.8522 (treatment); see Online Resources 6).
3.4 Effects on spermatheca sperm counts
Queens from MEOF colonies had marginally less sperm stored in their spermathecae than queens from CON colonies (2-way ANOVA, F2,35 = 4.57, p = 0.036; Figure 2). Queens with less stored sperm may produce fewer female workers over their functional lifespans as the colony’s sole producer of fertilized eggs and therefore might be replaced earlier on average.
Sperm contents (± S.E.) stored in spermatheca of newly established replacement queens reared by methoxyfenozide-exposed (MEOF) and unexposed control (CON) colonies. Treatment groups that do not share the same superscript significantly differ by Tukey’s HSD (p < 0.05). The 2018 and 2019 queen samples are pooled by treatment (n = 18 (MEOF) or 21 (CON)).
3.5 Effects on QMP/QRP queen pheromones
Queens did not differ by treatment in their glandular contents of pheromones critical to worker care (QRP), queen retention (QMP), and signals of queen quality (QMP) despite differences in spermatheca stored sperm contents. With one exception, head contents of 5 QMP/QRP and 4 QRP pheromone components did not vary significantly by year or between treatment groups (Wilcoxon ranked sum test, Z from − 0.9983 to 0.9431, p = 0.091 to 1.000). Queen heads contained less of the QRP component linolenic acid in 2018 than 2019 (Wilcoxon ranked sum test, Z = 2.3084, p = 0.027; see Online Resources 7, 8, and 9). Notably, three poorly mated queens lacking stored sperm had low levels of HVA, a QMP/QRP component associated with queen matedness.
4 Discussion
Our results indicate that MEOF does not reduce emergency rearing of young larvae into replacement queens either by inclusion in contaminated pollen or contact with contaminated wax. Queens reared in MEOF-exposed colonies did not experience any visible developmental abnormalities associated with methoxyfenozide (Carlson et al. 2001). The basis for sublethal effects in honey bees is poorly understood since MEOF likely does not bind as well to honey bee ecdysteroid receptors as well as lepidopteran receptors (Carlson et al. 2001). Curiously, we observed a non-significant trend toward fewer emergency queens cells on brood frames in MEOF colonies. This tendency may be related to reduced egg eclosion in MEOF colonies, which would lower the number of appropriately-aged worker larvae available for conversion to queen larvae (Fine 2020). Reduced fecundity and reduced egg eclosion have been observed in leafrollers exposed to MEOF (Sun et al. 2000). Fine and Corby-Harris (2021) noted that embryos in eggs may be particularly sensitive to endocrine disruption as they undergo ecdysteroid-mediated development. Similarly, queens from colonies treated with the IGR fenoxycarb experienced both reduced egg eclosion and increased queen losses (Milchreit et al. 2016). More serious queen losses occurred across both treatments during young adult queen development, a period when queens engage in risky rival queen elimination, mating flights, and reproductive maturation (Gilley and Tarpy 2005; Collins et al. 2004b; Winston 1987). Other pesticides have been shown to interfere both with queen development (Johnson et al. 2013 (diflubenzuron); Gajger et al. 2017 (thiamethoxam); Collins et al. 2004a (coumaphos)) and queen mating (Forfert et al. 2017 (thiamethoxam and clothianidin); Thompson et al. 2005 (fenoxycarb)).
One reason developing queens may be mostly unaffected by MEOF is that they largely avoid contact with MEOF-contaminated food. Our pesticide analysis indicates that workers, but not queens, encounter significant amounts of MEOF residues in their food. Adult workers may lower queen exposures by serving as intermediaries between contaminated stored food and hive materials and the queen herself (Purdy 2015; Böhme et al. 2018). Workers collect, handle, and store contaminated food materials, mold contaminated wax, and directly consume pesticide-contaminated semi-refined food materials as older larvae and young adults, both for their own nutrition and to redistribute nutrients to larvae, queens, drones, and other workers (Purdy 2015; Haydak 1970). In this process, workers may metabolize or be targeted by residues present in food. By contrast, queens feed throughout their lives exclusively on glandular secretions, a food material similar to the MEOF-free royal jelly sampled in our pesticide analysis (Crailsheim 1992), a feature that potentially protects queens from direct effects of dietary pesticides (Böhme et al 2018). Worker metabolism of MEOF residues may function as a form of social barrier protecting the queen from exogenous foodborne toxins present in raw forage materials.
One queen feature affected by MEOF exposure was spermatheca sperm contents. Low spermatheca sperm counts could arise from impacts on nuptial flight and mating behaviors or on post-mating features required to store and nutritionally support sperm in spermatheca. It is unlikely that differences in drone availability or quality were responsible for the difference in sperm content since unexposed queens shared the same apiary airspace as MEOF-exposed queens. While effects on queen flight were not examined, MEOF has been shown to reduce forager flight behavior at dawn in field colonies (Meikle et al. 2019). Pesticides with different modes of toxicity have shown variable effects on queen mating and sperm storage. Developmental exposure to the neonicotinoids thiamethoxam and clothianidin resulted in fewer queen matings with drones than unexposed queens (Forfert et al. 2017). By contrast, Walsh et al. (2021) found that developing queens exposed to amitraz, tau-fluvalinate and coumaphos, or chlorothalonil and chlorpyifos in wax either did not differ in their mating frequency or had higher mating frequencies (amitraz). Rangel and Tarpy (2015) found that queens reared on tau-fluvalinate and coumaphos-contaminated wax had higher mating frequencies than unexposed controls. In our study, we did not examine sperm viability or spermatheca development, both of which could limit sperm storage. Sperm storage in queen spermathecae, which requires significant physiological support (Collins et al. 2004b), has also been shown to be negatively impacted by certain agrochemicals but not others. Developmental exposures to the IGR fenoxycarb, the miticides tau-fluvalinate, coumaphos, and amitraz, the neonicotinoid thiamethoxam, the organophosphate chlopyrifos and chlorothalonil have all resulted in lower sperm viability or reduced queen viability in developing queens (Thompson et al. 2005; Gajger et al. 2017; Rangel and Tarpy 2015; but see Walsh et al. 2021). Effects of pesticide contaminants on developing queens appear to be complicated but observable in field colonies. Colonies with wax contaminated by multiple pesticides produced fewer viable queens with lower sperm counts, yet had similar mating frequencies, compared to untreated colonies (Milone and Tarpy 2021).
Lower sperm contents may shorten the reproductive lifetime productivity of the affected queen and induce earlier queen replacement via supersedure (Pettis et al. 1995, 1997). A queen’s ability to produce female workers from fertilized eggs is ultimately limited by the quantity of viable sperm stored within her spermatheca. Recently, a study by Walsh et al. (2020) detailed the effects of pesticide and miticide exposure on queen pheromone signaling and worker retinue support of the queen. Queens exposed to high levels of the miticides Amitraz, coumaphos, tau-fluvalinate, and the pesticides chlopyrifos and chlorothalonil not only experienced reduced oviposition rates but also recruited smaller retinue sizes and had lower contents of QMP pheromone component critical for queen retention. These pesticides did not affect worker rearing of new queens in a subsequent study, highlighting the complexity of pesticide effects on developing queens (Walsh et al. 2021). In this study, MEOF-treated queens did not have an altered QMP/QRP pheromone profile that reflected reduced sperm storage. However, effects on queen signaling may not occur in newly established queens at the beginning of their lifetime egg productivity when their spermathecae are maximally full. A more likely time for effects on pheromone signaling and queen-worker interactions would be later in the queen cycle when months of oviposition eventually result in spermatheca sperm depletion.
Few sublethal effects of MEOF on queens have been observed despite the centrality of ecdysteroids in regulating honey bee physiology and social structures. In particular, queens have high levels of ecdysteroids in their bodies (Robinson et al. 1991) and ecdysteroids mediate caste differentiation, reproductive development, and many behaviors in honey bees (Pandey and Bloch 2015). For honey bee queens, the combination of low ecdysteroid receptor binding and social shielding by attendant workers limits effects observed in target lepidopterans. The question remains whether developing queens are fully exposed to more potent agrochemicals or whether the social structure of the honey bee colony insulates them against external toxins (Purdy 2015).
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baker NT (2017) Estimated annual agricultural pesticide use by crop group for states of the conterminous United States, 1992–2014. U.S. Geological Survey Data Release. https://doi.org/10.5066/F7NP22KM
Böhme F, Bischoff G, Zebitz CPW, Rosenkranz P, Wallner K (2018) From field to food – will pesticide-contaminated pollen diet lead to a contamination of royal jelly? Apidologie 49:112–119
Carlson GR, Dhadialla TS, Hunter R, Jansson RK, Jany CS et al (2001) The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag Sci 57:115–119
Carroll MJ, Meikle WG, McFrederick QS, Rothman JA, Brown N et al (2018) Pre-almond supplemental forage improves colony survival and alters queen pheromone signaling in overwintering honey bee colonies. Apidologie 49:827–837
Collins AM, Pettis JS, Wilbanks R, Feldlaufer MF (2004a) Performance of honey bee (Apis mellifera) queens reared in beeswax cells impregnated with coumaphos. J Apic Res 43:128–134
Collins AM, Williams V, Evans JD (2004b) Sperm storage and antioxidant enzyme expression in the honey bee. Apis Mellifera Insect Mol Biol 13:141–146
Crailsheim K (1992) The flow of jelly within a honeybee colony. J Comp Physiol B 162:681–689
DeGrandi-Hoffman G, Gage S, Corby-Harris V, Carroll M et al (2018) Connecting the nutrient composition of seasonal pollens with changing nutritional needs of honey bee (Apis mellifera L.) colonies. J Insect Physiol 109:114–124
Delaney DA, Keller JJ, Caren JR, Tarpy DR (2011) The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 42:1–13
Fine JD (2020) Evaluation and comparison of the effects of three insect growth regulators on honey bee queen oviposition and egg eclosion. Ecotoxicol Environ Saf 205:111142
Fine JD, Corby-Harris V (2021) Beyond brood: the potential impacts of insect growth disruptors on the long-term health and performance of honey bee colonies. Apidologie 52:580–595
Fisher A, Colman C, Hoffmann C, Fritz B, Rangel J (2018) The effects of the insect growth regulators methoxyfenozide and pyriproxyfen and the acaricide bifenazate on the honey bee (Hymenoptera: Apidae) forager survival. J Econ Entomol 111:510–516
Forfert N, Troxler A, Retschnig G, Gauthier L, Straub L et al (2017) Neonicotinoid pesticides can reduce honeybee genetic diversity. PLoS ONE 12:e0186109
Gajger IT, Sakac M, Gregorc A (2017) Impact of thiamethoxam on honey bee queen (Apis mellifera carnica) reproductive morphology and physiology. Bull Environ Contam Toxicol 99:297–302
Gilley DC, Tarpy DR (2005) Three mechanisms of queen elimination in swarming honey bee colonies. Apidologie 36:461–474
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Haydak MH (1970) Honey bee nutrition. Annu Rev Entomol 15:143–156
Higbee BS, Siegel JP (2012) Field efficacy and application timing of methoxyfenozide, a reduced-risk treatment for control of navel orangeworm (Lepidoptera: Pyralidae) in almond. J Econ Entomol 105:1702–1711
Johnson R, Ellis M, Mullin C, Frazier M (2010) Pesticides and honey bee toxicity – USA. Apidologie 41:312–331
Johnson RM, Percel EG (2013) Effect of a fungicide and spray adjuvant on queen-rearing success in honey bees (Hymenoptera: Apidae). J Econ Entomol 106:1952–1957
Keeling C, Slessor K, Higo H, Winston M (2003) New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci USA 100:4486–4491
Lee KV, Steinhauer N, Rennich K, Wilson ME, Tarpy DR et al (2015) A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 46:292–305
Meikle WG, Corby-Harris V, Carroll MJ, Weiss M et al (2019) Exposure to sublethal concentrations of methoxyfenozide disrupts honey bee colony activity and thermoregulation. PLoS ONE 14:e0204635
Mello TRP, Aleixo AC, Pinheiro DG, Nunes FM et al (2014) Developmental regulation of ecdysone receptor (EcR) and EcR-controlled gene expression during pharate-adult development of honeybees (Apis mellifera). Front Genet 5:445
Milchreit K, Ruhnke H, Wegener J, Bienefeld K (2016) Effects of an insect growth regulator and a solvent on honeybee (Apis mellifera L.) brood development and queen viability. Ecotoxicol 25:530–537
Milone JP, Tarpy DR (2021) Effects of developmental exposure to pesticides in wax and pollen on honey bee (Apis mellifera) queen reproductive phenotypes. Sci Rep 11:1020
Mommaerts V, Sterk G, Smagghe G (2006) Bumblebees can be used in combination with juvenile hormone analogues and ecdysone agonists. Ecotoxicol 15:513–521
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds et al (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5:e9754
National Research Council (2007) Status of pollinators in North America. The National Academies of Science Press, Washington
Naumann K, Winston M, Slessor K, Prestwich G, Webster F (1991) Production and transmission of honey bee (Apis mellifera L.) mandibular gland pheromone. Beh Ecol Sociobiol 29:321–332
Ostiguy N, Drummond FA, Aronstein K, Eitzer B et al (2019) Honey bee exposure to pesticides: a four-year nationwide study. Insects 10:13
Pandey A, Bloch G (2015) Juvenile hormone and ecdysteroids as major regulators of brain and behavior in bees. Curr Opin Insect Sci 12:26–37
Pankiw T, Huang Z, Winston ML, Robinson GE (1998) Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J Insect Physiol 44:685–692
Pettis JS, Higo HA, Pankiw T, Winston ML (1997) Queen rearing suppression in the honey bee – evidence for a fecundity signal. Insectes Soc 44:311–322
Pettis JS, Winston ML, Collins AM (1995) Suppression of queen rearing in European and Africanized honey bees (Apis mellifera L.) by synthetic queen mandibular gland pheromone. Insectes Soc 42:113–121
Potts SG, Biesmeijer JC, Kremen C, Neumann P et al (2010) Global pollinator declines: trends, impacts, and drivers. Trends Ecol Evol 25:345–353
Purdy J (2015) Potential routes of exposure as a foundation for a risk assessment scheme: a conceptual model. Hazards of pesticides to bees. 12th Int Symp of the ICP-PR Bee Protection Group. Julius-Kühn-Arch 22–27. https://ojs.openagar.de/index.php/JKA/article/view/5312. Accessed 27 Feb 2022
Rangel J, Tarpy DR (2015) The combined effects of miticides on the mating health of honey bee (Apis mellifera L.) queens. J Apic Res 54:275–283
Retnakaran A, Krell P, Feng Q, Arif B (2003) Ecdysone agonists: mechanism and importance in controlling insect pests of agriculture and forestry. Arch Insect Biochem Physiol 54:187–199
Robinson GE, Strambi C, Strambi A, Feldlaufer MF (1991) Comparison of juvenile hormone and ecdysteroid hemolymph titers in adult worker and queen honey bees (Apis mellifera). J Insect Physiol 37:929–935
Sanchez-Bayo F, Goka K (2014) Pesticide residues and bees – a risk assessment. PLoS ONE 9:e94482
SAS 9.2 (2010) SAS Institute. Cary, North Carolina, USA
Slessor KN, Kaminski LA, King G, Winston ML (1990) Semiochemicals of the honeybee mandibular glands. J Chem Ecol 16:851–860
Slessor KN, Kaminski LA, King GG, Borden JH, Winston ML (1988) Semiochemical basis of the retinue response to queen honey bees. Nature 332:354–356
Sun X, Barrett BA, Biddinger DJ (2000) Fecundity and fertility reductions in adult leafrollers exposed to surfaces treated with theecdysteroid agonists tebufenozide and methoxyfenozide. Entomol Exp Appl 94:75–83
Tasei JN (2001) Effects of insect growth regulators on honey bees and non-Apis bees. A Review Apidologie 32:527–545
Thompson HM, Wilkins S, Battersby AH, Waite RJ, Wilkinson D (2005) The effects of four insect growth-regulating (IGR) insecticides on honeybee (Apis mellifera L.) colony development, queen rearing, and drone sperm production. Ecotoxicol 14:757–769
vanEngelsdorp D, Tarpy DR, Lengerich EJ, Pettis JS (2013) Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev Vet Med 108:225–233
Wade A, Lin C-H, Kurkul C, Regan ER (2019) Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects 10:20
Walsh EM, Khan O, Grunseich J, Helms AM et al (2021) Pesticide exposure during development does not affect the larval pheromones, feeding rates, or morphology of adult honey bee (Apis mellifera) queens. Frontiers Ecol Evol 9:681506
Walsh EM, Sweet S, Knap A, Ing N, Rangel J (2020) Queen honey bee (Apis mellifera) pheromone and reproductive behavior are affected by pesticide exposure during development. Behavior Ecol Sociobiol 74:33
Wegener J, Huang ZY, Lorenz MW, Lorenz JI, Bienefeld K (2013) New insights into the roles of juvenile hormone and ecdysteroids in honey bee reproduction. J Insect Physiol 59:655–661
Winston ML (1987) The biology of the honey bee. Harvard Press, Boston
Zhu YC, Adamczyk J, Rinderer T, Yao J, Danka R et al (2015) Spray toxicity and risk potential of 42 commonly used formulations of row crop pesticides to adult honey bees (Hymenoptera: Apidae). J Econ Entomol 6:2640–2647
Acknowledgements
We would like to thank Charlotte Meador, William Meikle, and Milagra Weiss for valuable input into this research project. We would also like to thank John Borden and Contech Enterprises for their gift of the synthetic QMP mixture. We would also like to thank an anonymous reviewer for critical insights that substantially improved this manuscript.
Funding
This research was funded by internal funds of the USDA-ARS (Project Plans 5342–21000-015-00D and 2022–21000-018-00D). The USDA-ARS is an equal opportunity employer and provider.
Author information
Authors and Affiliations
Contributions
MJC and VCH conceived this research and designed experiments. MJC, VCH, LS, and NB participated in the design and interpretation of the data. NB, LC, MJC, VCH, and DCR performed experiments and analysis. MJC, VCH, NB, LS, and DCR wrote the paper and participated in the revisions. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: David Tarpy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carroll, M.J., Corby-Harris, V., Brown, N. et al. Methoxyfenozide has minimal effects on replacement queens but may negatively affect sperm storage. Apidologie 53, 33 (2022). https://doi.org/10.1007/s13592-022-00940-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00940-7