Abstract
Honey stores of Apis mellifera colonies are replaced with sugar water by beekeepers, which may result in malnutrition. Nutritional supplements have been developed, but the importance of bacterial probiotics and vitamins is poorly understood. Given that supplementary feeding with vitamins and probiotics may enhance worker weight and longevity, this might suggest a feasible approach to mitigate winter colony losses. Here, we conducted a laboratory hoarding cage study with freshly emerged winter bees, which were treated with the antibiotic tetracycline to reduce gut bacteria obtained post-emergence and subsequently assigned to feeding regimes: sucrose only, sucrose + pollen, probiotics (low and high dosage), probiotics + pollen (low and high dosage), or b-vitamins (low and high dosage) (N = 8 treatments, 29 workers/cage × 8 replicates). In parallel, another age cohort of bees remained on their frame (= Frame) to establish their gut microbiota and were subsequently fed with sucrose only or sucrose + pollen (N = 2 treatments, 29 workers/cage × 4 replicates). The most beneficial effects on body weights were found in workers given ad libitum access to pollen, notably in the Frame Sucrose + Pollen group, confirming the inherent importance of post-emergent gut flora inoculation and the role of gut bacteria in protein digestion. Furthermore, both Frame groups and the antibiotic-treated workers fed with probiotic low + pollen survived longer than all other groups, highlighting a fundamental host-microbial relationship. On the other hand, our current treatments alone, post-tetracycline, did not yield any positive results. In contrast, high dosages of both probiotic and b-vitamins significantly reduced lifespan compared to their low concentration counterparts, probably due to dysbiosis and toxicity, suggesting that the outcome was dose-dependent. These results highlight that bacterial and b-vitamin supplementation can alter longevity with advisable caution since harmful concentrations appear to exist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Adequate nutrition is paramount for honey bee, Apis mellifera, colony survival (reviewed in Brodschneider & Crailsheim, 2010). Recent winter colony losses have brought immense awareness to apiculture (Neumann & Carreck, 2010) and inadequate nutrition has been identified as one of several key indicators for hive failure (reviewed in Brodschneider et al., 2010; van Engelsdorp et al., 2010). Various reasons explain why a colony might lack proper nutrition, such as reduced availability to forage due to altered landscapes from agriculture (Naug, 2009) or over intense honey harvesting from beekeepers (reviewed in Brodschneider & Crailsheim, 2010). In order to sustain colony development, honey bees forage pollen and nectar from spring to autumn flower efflorescence (Klein et al., 2019) and store the food in the forms of bee bread and honey for the times of reduced foraging, i.e., winter (Winston, 1991). Traditionally, beekeepers remove honey stores and replace it with simple sucrose solution, yet often times, micronutrients such as vitamins are wholly neglected in the supplementing process of adult workers. If an over-intense honey harvest occurs, replaced solely with sucrose solution, this may result in low micronutrient dense energy stores for the critical over-wintering time.
Substantial A. mellifera nutritional research has demonstrated the importance of pollen (Frias et al., 2016), showing its vital ties between nurse and emergent bees (Haydak, 1935), as well as causal associations with honey bee health proxies, namely, longevity (Maurizio, 1954) and body weight (Retschnig et al., 2021). Fortunately, decade’s long in-depth reflection has been dedicated to the nutritional requirements of honey bees, with a particular emphasis on macronutrients (reviewed in Brodschneider & Crailsheim, 2010; reviewed in Pudasaini et al., 2020). In line with such research, evidence-based macronutrient supplementation practices are readily available for beekeepers to consult (Standifer et al., 1977), and commercially made products are available (Somerville, 2000). On the other hand, gaps in the present understanding of the micronutritional needs (i.e., vitamins) of adult honey bees persist.
To date, little work has been done that focuses on a branch of micronutrients, b-vitamins, and their requirements for A. mellifera workers (reviewed in Brodschneider & Crailsheim, 2010). Insects are predominantly unable to synthesize eight essential b-vitamins themselves (Douglas, 2017), but have been reported to require them for metabolic processes like fatty acid synthesis (biotin, vitamin B7) or nucleic acid biosynthesis (folic acid, vitamin B9) (Dadd, 1973). In A. mellifera, pyridoxine (vitamin B6) is necessary for brood rearing (Haydak & Dietz, 1972) and measurable doses of required pyridoxine needed for brood rearing have already been published (Anderson & Dietz, 1976). Furthermore, recent data suggest that b-vitamins positively contribute to colony performance (Jovanovic et al., 2021). Insects need a steady supply of b-vitamins, given their limited capacity of being able to store them (Douglas, 2017), and typically, they obtain them from a combination of their external environment as well as host-microbial symbionts, otherwise referred to as their gut microbiome (Salem et al., 2014).
Nutrition-based interactions with a host’s microbiota are well established in animals (Douglas, 1998; Duncan et al., 2008), and animal health is considerably intertwined with the bacterial community within the digestive tract (Martinson et al., 2012). This mutualistic host-microorganism relationship in A. mellifera workers includes vitamin production (Kwong et al., 2014), as well as plant secondary metabolite detoxification (Kešnerová et al., 2017), immune function support (Tejerina et al., 2021; Vásquez et al., 2012), and integral links to longevity (Raymann et al., 2017). Emergent bees are born sterile and are fully inoculated with their core microbiota shortly after emergence, primarily from physical contact with the nest down to social interactions with nestmates (Martinson et al., 2012), forming a core microbiota composed primarily of lactobacilli that, if left undisturbed, are highly conserved throughout a worker bee’s lifespan (Kwong & Moran, 2016). Given their apparent beneficial effects, Lactobacillus supplementation (i.e., probiotics) to honey bees may prolong lifespan thereby providing a feasible gateway to improving winter colony survival.
At present, b-vitamin and probiotic supplementation lack concrete quantitative and qualitative data for A. mellifera. B-vitamins have been labeled as generally safe for insects even if administered at high doses (Douglas, 2017); however, habitual exposure to elevated levels of b-vitamins has shown to be harmful in other taxa (Phillips et al., 1978). Furthermore, even if certain strains of probiotics are beneficial, a possible reversal of benefits from probiotics can occur in an overgrowth scenario (Hayashi et al., 2017). Both of these aspects highlight the relevant importance of proper dosing. Furthermore, in order to better isolate b-vitamin dosing, decoupling innate b-vitamin synthesis from A. mellifera microbiomes is also necessary a step to consider. Therefore, the present study aims to undertake hoarding cage experiments to explore if b-vitamins and probiotics administered at different dosages influence antibiotic-treated winter worker bee longevity and body weights, with the goal of undercovering a starting point for micronutrient and probiotic supplementation. We would expect to see an improvement on the measured parameters as a result from supplementation.
2 Material and methods
2.1 Experimental setup
The experiment was conducted from October 22nd to December 31st 2019 (local autumn and winter seasons) at the Institute of Bee Health in Bern, Switzerland. To obtain defined age cohorts of newly emerged workers, in addition to isolating late autumn brood to ensure long-lived winter workers, queens from local A. mellifera colonies (N = 5) were caged onto open drawn comb, and after 20 days, capped worker brood frames were incubated until adult emergence (48 h, 34.5 °C, > 60% RH) (Williams et al., 2013). After 48 h, newly emerged workers from all colonies were mixed, standardizing the impact of genetics between colonies, and randomly placed in hoarding cages (N = 64 cages, 29 bees/cage, N = total workers 1856) (Williams et al., 2013). After being completely cleared of emergent bees, the same frames were incubated again, and 24 h later, a second group of freshly emerged workers were marked individually (UniPosca color tip pens), put back on their original frame, and incubated at 30 °C and > 60% RH (Williams et al., 2013) for 48 additional hours, resulting in 72-h old worker bees. Afterwards, the workers were removed and also mixed in a single container and assigned to cages (N = 8, 29 bees/cage, total workers N = 232). The first group of workers were treated with tetracycline for 72 h to reduce gut microbiota and then assigned to one out of eight treatments (Table I, groups 3–10), while in parallel to the antibiotic treatment, workers from the 2nd incubation remained on their frames, retaining access to nutrients and bacteria, and were designated to one out of two feeding regiments (Table I, groups 1–2, hereafter referred to as “Frame” bees). All workers were maintained at 30 °C and > 60% RH (Williams et al., 2013) until the last worker died.
2.2 Treatments
2.2.1 Antibiotic
Tetracycline hydrochloride (©Sigma-Aldrich) was assessed in two hoarding cage pre-trials, first with freshly emerged winter bees and later with freshly emerged summer bees, and was found as the best suitable substance to inactivate the gut microbiota without significantly influencing longevity (Figs. 3 and 4). Following those results for this trial, a tetracycline-sucrose solution (50% (w/v), 500 µg/mL tetracycline hydrochloride) was prepared and supplied ad libitum to the antibiotic-treated groups for 72 h.
2.2.2 Dietary treatments
Sucrose solution (50% w/v) made with sterilized tap water was mixed with either vitamins, probiotics, or left blank (control), and fed to all workers ad libitum (Table I). All treatment solutions were prepared freshly on a weekly basis and stored at 4 °C. Four treatment groups (2, 4, 9, and 10) were additionally fed ad libitum non-sterilized corbicula pollen from honey bees (Swiss Pollen, Bienen Roth). The pollen was stored at −24 °C, and prior to feeding, was thawed, ground, and packed into 1.5-mL microcentrifuge tubes with a clipped tip and supplied to workers. Syringes and pollen tubes were changed with fresh sterilized ones on a bi-weekly basis.
2.2.3 B-vitamins
Two b-vitamin dosages were used (1) Vitamin Low (300 mg/L) and (2) Vitamin High (9000 mg/L). The Vitamin Low dosage was chosen based on b-vitamin estimates in the natural diet (as reviewed in Bogdanov et al., 2008) and daily food consumption (Winston 1991), and the high dosage was chosen to explore possible toxic effects (Douglas, 2017). Pure vitamin isolates were purchased from Hänseler AG (B1, B2, B3, B5, B6, B8), PureBulk (B9), and Sanofi Chimie BP (B12). Stock concentrations were made by solving each vitamin in deionized water, then adding those to a 50% (w/v) sucrose solution resulting in two mixes (Table II).
2.2.4 Probiotics
The pharmaceutical product Lactibiane Voyage, which contains a mix of Lactobacillus acidophilus (strain LA 201), Lactobacillus plantarum (strain LA 301), and Lactobacillus casei (strain LA 205), was used to make two probiotic dosages: (1) Probiotic Low: 4.1 × 106 CFU/L and (2) Probiotic High: 4.1 × 107 CFU/L, similar to total viable CFUs in A. mellifera honey crops (Vásquez et al., 2012). The probiotics were dissolved in deionized water and directly mixed with 50% (w/v) sucrose solution to obtain the final dosages.
To confirm that the bacteria were alive throughout the treatment process, the presence of Lactobacillus bacteria was confirmed by culture on Lactobacillus specific MRS agar plates and identification was made using matrix-assisted-laser-desorption/ionization-time-of-flight-mass-spectrometry (MALDI-TOF MS) (Microflex LT, Bruker, Daltonics) using the Biotyper software with Bruker MBT 7854 MSP Library. Species detection was performed using default parameters and identification was confirmed when the identification score was ≥ 2.0. Samples were taken from live workers on days 10 and 20 from the treatments: (1) Sucrose + Pollen, (2) Probiotic High, and (3) Probiotic High + Pollen (N = 5 workers/treatment/day). Samples were dissected following steps from Carreck et al. (2013), and cultured on Lactobacillus specific MRS agar (© Sigma-Aldrich) plates and incubated for 48 h at 37 °C under anaerobic conditions.
2.3 Mortality, sucrose consumption, and body weight
Mortality and sucrose-solution consumption were recorded daily. Dead workers were removed and stored at −80 °C. To estimate consumption of sucrose solution per worker per day, the syringes were weighed daily and the differences in weight were divided by the number of live workers present in the cage at that time. To account for evaporation from the incubator, ten control syringes were filled with sucrose solution and put in cages without workers, incubated, and measured daily, and the average evaporation rates were used to adjust sucrose-solution consumption across all treatments (OECD, 2017). Due to treatment-induced mortality, individual body weights of up to three live workers per cage were measured when possible on days 7, 14, and 21 (Supplement Table S1.1) using a Mettler AT 400 scale with a precision range of 10−4 g. From these workers, up to two were subsampled when possible from each treatment group and each day (Supplement Table S2.1) and dried to assess dry body weight (Brodschneider et al., 2009). To do so, samples were stored in liquid nitrogen, transferred into a Zirbus Sublimator VaCO 2 (Bad Grund, Germany), lyophilized, re-weighed using the same Mettler AT 400 scale, and repeated until constant dry weights (Table S2.1).
2.4 Statistical analyses
Statistical analyses were performed using the program, R, version 3.6.2 (R Core Team, 2008). For survival analysis, the packages “survival” (Therneau, 2021; Therneau & Grambsch, 2000) and “surminer” (Kosinski et al., 2020) were used to calculate and plot Kaplan–Meier survival curves. The Surfdiff function was used to calculate survival curves and log rank testing (rho = 0) as well as to perform a chi-squared test. The pairwise_survdiff function was used for multiple comparisons from the survival analysis between all treatment groups, and the resulting p values were adjusted for multiple comparisons using a Bonferroni method (Bonferroni, 1936). All data for the analysis of the body weight and sucrose consumption were log-transformed and used in linear mixed effect models (lmer), using the R package “lme4” (Bates et al., 2015) and fitted using “REML [‘lmerMod’],” while defining “cage” and “day” as random factors. Histograms and qqPlots of the model residuals were used to verify model assumptions. Post hoc lmer pairwise testing was done on the sucrose consumption and body weight data using the “multcomp” package (Hothorn et al., 2008), selecting “Tukey” comparison of means, with “Holm” correction (Holm, 1979) from the extracted fitted values of the model. To obtain the compact letter display (Piepho, 2004) used in Figs. 1 and 2, the function cld() was used from the “multcomp” package (Hothorn et al., 2008), and the function multcompLetters() from the multcompView package (Graves et al., 2019) for Figs. 3, 4, and 5. Finally, additional linear mixed effect models were carried out for the body weight and sucrose consumption data, with the explanatory variables (i.e., treatments) expressed as indicator variables (excluding “frame”), with “cage” and “day” defined as random factors, to estimate the effects of the explanatory variables as well as to test for any significant interactions.
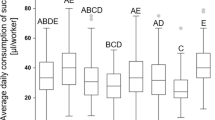
Boxplots of the predicted dry body weight values of workers, Apis mellifera, from 10 treatment groups, extracted from a linear mixed effect model (lmer) which was calculated using the log-transformed dry body weight of the workers, dependent on diet (treatment), and “cage” and “day” as random variables (N = 10 treatments, N = 1–16 workers per treatment, total N = 356 total workers). Post hoc testing from the lmer, comparing group means (Tukey), with Holm correction was used to determine statistical significance. Compact letter display indicates statistical difference between treatments (ps < 0.05).
Boxplots of the fitted daily sucrose solution consumption values of workers, Apis mellifera, per worker/per cage from 10 treatment groups, extracted from a linear mixed effect model (lmer) calculated with the log-transformed consumption rates of the workers, dependent on diet (treatment), and “cage” and “day” as random variables (N = 10 treatments, N = 4–8 cages per treatment, N = 2232 total measurements). Post hoc testing from the lmer, comparing group means (Tukey), with Holm correction was used to determine statistical significance. Compact letter display indicates statistical difference between treatments (ps < 0.05).
Kaplan–Meier survival curves of three groups of experimental winter workers, two of which were treated with tetracycline at dosages of 400 μg/mL or 700 μg/mL (Tetracycline-400 and Tetracycline-700) supplied in sucrose solution (50%, w/v) and a control supplied with sucrose solution only (50%, w/v). N = 90 workers per treatment group (3 cages/group with 30 bees each). N = 270 total workers. Significant differences between the survival of the experimental workers are indicated by compact letter display based on log rank tests and Bonferroni p adjusted values (ps < 0.05).
Kaplan–Meier survival curves of two groups of experimental summer workers, one of which was treated with tetracycline at dosages of 500 μg/mL (Tetracycline-500) supplied in sucrose solution (50%, w/v) and a control supplied with sucrose solution only (50%, w/v). N = 180 workers per treatment group (6 cages/group with 30 bees each). N = 360 total workers. Significant differences between the survival of the experimental workers based on chi-squared testing (rho = 0) are indicated by compact letter display (p < 0.05).
Kaplan–Meier survival curves of experimental workers, Apis mellifera, from 10 treatment groups, of which eight received a 72-h tetracycline treatment: Sucrose, Sucrose + Pollen, Probiotic Low, Probiotic High, Probiotic Low + Pollen, Probiotic High + Pollen, Vitamin Low, and Vitamin High (N = 232 workers per group). The two groups: Frame Sucrose and Frame Sucrose + Pollen, remained 72 h on their frame (no antibiotic treatment, N = 116 workers per group). Significant differences between the survival of the experimental workers are indicated by compact letter display based on log rank tests and Bonferroni p adjusted values (ps < 0.05).
3 Results
3.1 Probiotics treatment confirmation
The Lactobacillus bacteria fed were found in the mid- and hindguts of workers, confirming a successful probiotic feeding method. Samples from the group “Sucrose + Pollen” yielded negative results from both days 10 and 20 of sampling, not growing any Lactobacillus containing colonies on the agar plates. However, on both sample days from the groups “Probiotic High” and “Probiotic High + Pollen,” live bacteria from the supplemented species Lactobacillus acidophilus (strain LA 201), Lactobacillus plantarum (strain LA 301), and Lactobacillus casei (strain LA 205) were successfully detected.
3.2 Body weight
The dry body weight of all experimental workers ranged from 0.0152 to 0.0565 g (N = 1–16 workers per treatment, total N = 356, Table S2.1). Exploratory analysis showed significant variation caused by our treatments (analysis of variance (ANOVA) of linear mixed effect model (lmer) (F(9,346) = 13.4, p < 0.001)). Post hoc testing on the fitted (i.e., predicted) values extracted from the lmer (Fig. 1) revealed three distinct groups (lmer, Tukey multiple mean comparison, Holm adjusted p values, all ps < 0.05). Highest body weights were detected in the group “Frame Sucrose + Pollen” (Fig. 1, letter C), followed by the “Frame Sucrose,” “Sucrose + Pollen,” “Probiotic High,” and “Probiotic Low + Pollen” groups (Fig. 1, letter B). The lowest body weights were recorded in the groups “Sucrose,” “Vitamin Low,” “Vitamin High,” “Probiotic Low,” and “Probiotic High + Pollen” (Fig. 1, letter A). Furthermore, an additional multiple regression model ran with indicator variables, excluding “Frame,” revealed that pollen had a positive influence on body weight (lmer, coefficient = 0.0056, p < 0.001) as well as high levels of probiotics (lmer, coefficient = 0.0035, p = 0.03), low levels of probiotics negatively influenced body weight (lmer, coefficient = − 0.0033, p = 0.04) and lastly, that there was a significant negative interaction between the two categories “Probiotic High” and “Pollen” resulting in significant reduction in body weight (lmer, coefficient = 0.0105, p < 0.001).
3.3 Sucrose consumption
Sucrose solution consumption data were used to calculate an average consumption rate per worker/per cage/per day (N = 4–8 cages per treatment, N = 2232 total measurements). Consumption rate values ranged from 0.00 to 0.137 g. Control cages without workers showed < 0.01% evaporation due to the incubators, and all consumption values were adjusted accordingly to account for evaporation. Exploratory analysis showed significant variation caused by the treatments (analysis of variance (ANOVA) of linear mixed effect model (lmer) (F(9,2222) = 18.39, p < 0.000)). Post hoc testing on the fitted values from the lmer (lmer, Tukey multiple mean comparison, Holm adjusted p values, all ps < 0.05) showed “Frame Sucrose + Pollen” group as consuming consistently the most across all other treatments (Fig. 2, letter C), while all other groups consumed statistical similar amounts (Fig. 2, letters A, B, and AB). A multiple regression model ran with indicator variables, excluding “Frame,” was preformed and the model summary reveals that high levels of b-vitamins negatively influenced sucrose consumption (lmer, coefficient − 0.0042, p = 0.05) as well as low levels of probiotics (lmer, coefficient − 0.0039, p < 0.05), and finally that there were no significant interactions.
3.4 Tetracycline assessment
The winter bees from the first hoarding cage trial assessment of tetracycline had a lifespan of 1 to 41 days (N = 3 treatment groups, N = 3 replicates/treatment, N = 30 workers/treatment, N = 90 total workers/group, N = 270 total workers). No significant differences were found between the non-antibiotic-treated control “Sucrose” and the two tetracycline dosages, Tetracycline-400 (400 μg/mL) and Tetracycline-700 (700 μg/mL) (Kaplan–Meier, log rank test, all ps > 0.05, letter A, Fig. 3).
The summer bees from the second hoarding trial assessment of tetracycline had a lifespan of 1 to 55 days (N = 2 treatment groups, N = 6 replicates/treatment, N = 30 workers/treatment, N = 180 total workers/group, N = 360 total workers). No significant difference was found between the non-antibiotic-treated control “Sucrose” and the tetracycline dosage, Tetracycline-500 (500 μg/mL) (Kaplan–Meier, log rank test, all ps > 0.05, letter A, Fig. 4).
3.5 Longevity
The experimental workers from the ten treatment groups lived between 1 and 70 days (N = 1856 workers in tetracycline-treated groups and N = 232 workers in “Frame” treatment groups, total N = 2088, Fig. 5). Log rank testing resulted in significant differences in survival probability through time between groups (Table III, p value < 0.001). Best survival was observed in the three treatment groups “Frame Sucrose” and “Frame Sucrose + Pollen,” and “Probiotic Low + Pollen,” which had statistically similar outcomes (Kaplan–Meier, log rank test, all ps > 0.05, letter A, Fig. 5). No significant differences were found between the negative and positive controls “Sucrose” and “Sucrose + Pollen,” as well as the treatment groups “Probiotic High,” “Probiotic Low,” and “Vitamin Low” (Kaplan–Meier, log rank test, all ps > 0.05, letter B, Fig. 5). Compared to these groups, “Probiotic High + Pollen” showed a significantly higher mortality (Kaplan–Meier, log rank test, all ps < 0.05, letter C, Fig. 5). Workers that received “Vitamin High” as a treatment showed the highest mortality, significantly higher than all other treatment groups (Kaplan–Meier, log rank test, all ps > 0.05, letter D, Fig. 5). The survival data showed that the treatment outcomes were dosage-dependent. The two vitamin concentrations either had no effect on longevity (“Vitamin Low”) or negatively affected longevity of the workers (“Vitamin High”) when compared to the control groups: “Sucrose” and “Sucrose + Pollen” (Kaplan–Meier, log rank test, all ps > 0.05, letters “D” and “B,” Fig. 5). In the absence of pollen, the probiotic treatments had statistically similar survival outcomes as the control groups; however, when the probiotics were supplied together with pollen, the effects did significantly change (Kaplan–Meier, log rank test, all ps > 0.05, letters “C” and “A,” Fig. 5). The observed effect was positive for the combination “Probiotic Low + Pollen” and negative for “Probiotic High + Pollen.”
4 Discussion
The data obtained here provide no evidence for a beneficial effect of the applied dosages of b-vitamin or probiotic supplementation on longevity and body weight of antibiotic-treated honey bee workers. The data further demonstrate that supplementing sucrose solution fortified with b-vitamins or probiotics (Lactobacillus spp.) can significantly influence workers’ longevity, and above all, that ad libitum access to pollen as well as natural post-emergent gut flora inoculation (i.e., Frame) are key to increasing longevity, underscored by the two “Frame” groups as well as “Probiotic Low + Pollen.” Interestingly, this favorable outcome was distinctly dosage-dependent, drawing a striking contrast between “Probiotic High + Pollen” vs. “Probiotic Low + Pollen,” and similar consequences were observed in the two vitamin dosages “Vitamin High” vs. “Vitamin Low,” where in both instances, the high dosages proved to be detrimental. Finally, the data provide supporting evidence for the importance of pollen as well as an intact gut microbiota on worker body weights, where the “Frame Sucrose + Pollen” weighed the most of any group.
In A. mellifera studies, both fresh and dry weights have been recommended as measurable health parameters (EFSA, 2016), and here, dry weight was used for statistical analyses because it excludes water weight values and therefore, yields closer-to-true biomass data. The body weight results of this study suggest a significant positive effect from ad libitum access to pollen, while the Frame Sucrose + Pollen bees with an intact gut-microbiota weighed the most. Considering that pollen is both a substantial part of honey bee diets and has a high nutritive value of both macro- and micronutrients necessary for development, coupled with the gut microbiota’s role in aiding digestion (Kwong & Moran, 2016), the likelihood of such an outcome is expected and coheres with previously published literature (Haydak, 1970; Roulston & Cane, 2000). Interestingly, pollen had differing inter-treatment effects on worker weights, where in most cases, it was beneficial. However, in one particular case, the experimental workers that were part of the treatment “Probiotic High + Pollen” scored statistically worse than their counterpart “Probiotic Low + Pollen,” and ultimately finished in the lowest weight category. The linear model results also suggest a highly significant antagonistic interaction between pollen and probiotics in high dosages. It is important to outline that these workers were continually fed artificially elevated numbers of Lactobacillus bacteria while retaining ad libitum access to pollen, a known energy source for Lactobacillus bacteria (Vásquez & Olofsson, 2009), and it is possible this created an ideal scenario for Lactobacillus overgrowth. Such instances of gut dysbiosis have been shown to be harmful in other taxa, such as impeding digestion in mice (Hayashi et al., 2017), and has also been correlated with increased mortality in honey bees (Maes et al., 2016). Additionally, Lactobacillus bacteria produce lactic acid as a byproduct of carbohydrate digestion, creating a low pH environment favorable to Lactobacillus bacteria, giving them an additional advantage for out competing other flora (Sanhueza et al., 2015), but harmful for the bees.
The results of the body weight data are complimented by the sucrose-consumption data, where the “Frame Sucrose + Pollen” group both consumed and weighed the most. Honey bees strive to maintain a stable protein to carbohydrate (P/C) ratio, where an unbalanced P/C ratio, such as too high levels of protein, can have detrimental consequences (Altaye et al., 2010). Although pollen was not a significant predictor for sucrose consumption, pollen consumption data would have allowed the present study to properly perform a correlation analysis in order to confirm if a stable P/C ratio was indeed present here.
The longevity results demonstrate that the cage conditions seem to drastically alter the average lifespan of winter bees. Under natural conditions, winter bees normally live 150 days or longer (Fukuda & Sekiguchi, 1996), yet here, the oldest workers reached 70 days, accentuating an inherent flaw with the cage design for studying winter bees. Collectively, this draws attention to the difficulty of translating effects observed in laboratory trials to predicted outcomes in natural hive conditions, and published efforts to make hoarding cage improvements have been done (Huang et al., 2014). Despite here applied measures such as randomization of workers to control for genetic variation between colonies, as well as the imitation of in-hive conditions (i.e., temperature, light, etc.), many aspects still diverge from a natural hive scenario.
In line with this, the workers that were exposed to the closest to real-life scenario, the “Frame” groups, had the best survival outcomes, concurrent with the “Probiotic Low + Pollen” group. The underlying commonalities between the longest-lived workers in this study were microbiota (i.e., “Frame” and probiotic groups) and, most importantly, pollen. Nutrition-based interactions with a host’s microbiota are well established in animals (Bäckhed et al., 2005; Douglas, 2018), and in general, it is recognized that an insect’s microbiota facilitates many beneficial interactions, such as digestion facilitation (Brune, 2014), vitamin production (Salem et al., 2014), and detoxification (Kešnerová et al., 2017). Vásquez et al. (2012) found that nine different Apis species have estimated total ranges of 106–107 Lactobacillus bacteria in their honey crop, with similar spectrums of lactobacilli found in honey, pollen, and bee bread. The “Probiotic Low + Pollen” group received concentrations in these ranges in their diet following a tetracycline treatment, and it is arguable that this was the proper dosage for restoring vital microbial-gut symbiotic functions. In contrast to the latter group, all other workers that underwent the antibiotic treatment showed higher mortality, even though no negative effects on survival were observed in pre-trials of this treatment method. Additionally, it is noteworthy to state that the workers in the “Probiotic High + Pollen” group had a severe decrease in lifespan, likely due to the previous mentioned overabundance of lactobacilli from being fed excessive high doses of bacteria. Finally, microbial endosymbionts in insects can be highly host-specific (Xu et al., 2020), and Lactibiane Voyage contains probiotic species targeted for human digestion. Alternative solutions with probiotics containing bee-specific bacterial strains would be an interesting area of investigation for honey bee health.
Although tetracycline should not be harmful to A. mellifera because it inhibits protein synthesis in prokaryotic organisms (Chopra & Roberts, 2001) in addition to our longevity pre-trial data results being analogous with the aforementioned statement, such instances can nonetheless not be ruled out. Long-term consequences associated to antibiotic exposure in bees has been documented in other studies, notably increased mortality (Marceau et al., 2021; Raymann et al., 2017), disruption in protein digestion (du Rand et al., 2020; Retschnig et al., 2021), and negative influence on sucrose consumption (Retschnig et al., 2021). The pairwise comparison results from the sucrose consumption data (Fig. 2) demonstrate that no differences were found between the “Frame Sucrose” and the micronutrient or probiotic supplemented bees, but the lack of a non-tetracycline without “frame” treatment limits formal statistical conclusions if there were true negative effects on sucrose consumption or body weight from antibiotic exposure.
The “Vitamin High” group was the worst performing treatment, with the lowest likelihood of survival. Shortly after starting the experiment, there was an appearance of trembling, falling, and behavioral agitation in their cages compared to their counterpart “Vitamin Low” and all other treatment groups, hinting at possible signs of toxicity, as seen in honey bees (Suchail et al., 2004; Ludicke & Nieh, 2020) (Fig. 6). This was strictly an anecdotal observation, and it would have been beneficial to this study to have had followed formal laboratory protocols to measure such abnormal activity. Interestingly, A. mellifera lifespans have been correlated with energy expenditure (Neukirch, 1982), and it is possible the “Vitamin High” workers over exhausted themselves resulting in premature death. Intriguingly, it may not just be the high dosages of b-vitamins themselves that were pernicious, but a combined habitual long-term exposure to them over extended periods of time which ended up being harmful. Such instances have been seen in other taxa, such as ataxia, muscle weakness and loss of balance in beagles (Phillips et al., 1978), osteo-related complications in humans (Meyer et al., 2019) as well as many other harmful ailments in humans due to heighten long-term consumption of b-vitamins (Titcomb & Tanumihardjo, 2019). Large taxonomic differences between mammals and insects exist; however, inferring ideas from mammalian studies offer important reasons for speculation and thus, emphasizes the need for future studies around the idea of b-vitamin dosages in A. mellifera studies to avoid toxicity issues. Lastly, honey bees, when kept in cages, do not defecate (Köhler et al., 2013), blocking the pathway of expelling amassed waste in their rectums, a studied trigger of early death in honey bees (Altaye et al., 2010). This aspect may have led to accumulated high dosages of b-vitamins in the experimental workers of the present study and has possibly resulted in the observed harmful effects and contributed to the decreased lifespan.
Video of experimental honey bee workers, Apis mellifera, in hoarding cages. (A) B-vitamin Low treatment (300 mg/L), (B) Sucrose (control), and (C) B-Vitamin High treatment (9 g/L). The treatment workers (C) display clinical symptoms of B-vitamin poisoning in honey bees (hyperactivity; URL: https://youtu.be/450-eQjvBUE).
The present study provides no direct evidence of a beneficial effect solely from the concentrations of the supplements used on the investigated health parameters in antibiotic-treated winter bees. The most beneficial effects were observed in the workers subjected to the closest real-life scenario (i.e., access to pollen and natural post-emergent gut flora inoculation). Conversely, strong detrimental effects were observed for high amounts of b-vitamins and probiotics, highlighting caution before considering any field application of micronutrients at our studied concentrations. Combined efforts of improving hoarding cage designs in parallel with range-finding tests to determine micronutrient dosage safety are of utmost importance in future studies.
Data availability
The raw data of the study are available at the Dryad repository: https://doi.org/10.5061/dryad.b5mkkwhdq.
Code availability
All statistical code used for the present study is found in the supplementary material (see Supplementary Material, R script).
References
Altaye, S. Z., Pirk, C. W. W., Crewe, R. M., & Nicolson, S. W. (2010). Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. Journal of Experimental Biology, 213(19). https://doi.org/10.1242/jeb.046953
Anderson, L. M., & Dietz, A. (1976). Pyridoxine requirement of the honey bee (Apis mellifera) for brood rearing. Apidologie, 7(1). https://doi.org/10.1051/apido:19760105
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., & Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science, 307(5717). https://doi.org/10.1126/science.1104816
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1-48. https://doi.org/10.18637/jss.v067.i01
Bogdanov, S., Jurendic, T., Sieber, R., & Gallmann, P. (2008). Honey for nutrition and health: A review. Journal of the American College of Nutrition, 27(6). https://doi.org/10.1080/07315724.2008.10719745
Bonferroni, C. E. (1936). Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni Del R Istituto Superiore Di Scienze Economiche e Commerciali Di Firenze, 8.
Brodschneider, R., & Crailsheim, K. (2010). Nutrition and health in honey bees. Apidologie, 41(3). https://doi.org/10.1051/apido/2010012
Brodschneider, R., Moosbeckhofer, R., & Crailsheim, K. (2010). Surveys as a tool to record winter losses of honey bee colonies: A two year case study in Austria and South Tyrol. Journal of Apicultural Research, 49(1). https://doi.org/10.3896/IBRA.1.49.1.04
Brodschneider, R., Riessberger-Gallé, U., & Crailsheim, K. (2009). Flight performance of artificially reared honeybees (Apis mellifera). Apidologie, 40(4). https://doi.org/10.1051/apido/2009006
Brune, A. (2014). Symbiotic digestion of lignocellulose in termite guts. Nature Reviews Microbiology, 12(3). https://doi.org/10.1038/nrmicro3182
Carreck, N. L., Andree, M., Brent, C. S., Cox-Foster, D., Dade, H. A., Ellis, J. D., Hatjina, F., & van Englesdorp, D. (2013). Standard methods for Apis mellifera anatomy and dissection. Journal of Apicultural Research, 52(4). https://doi.org/10.3896/IBRA.1.52.4.03
Chopra, I., & Roberts, M. (2001). Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews, 65(2). https://doi.org/10.1128/mmbr.65.2.232-260.2001
Dadd, R. H. (1973). Insect nutrition: current developments and metabolic implications. Annual review of entomology, 18(381). https://doi.org/10.1146/annurev.en.18.010173.002121
Douglas, A. E. (1998). Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annual Review of Entomology, 43(17). https://doi.org/10.1146/annurev.ento.43.1.17
Douglas, A. E. (2017). The B vitamin nutrition of insects: the contributions of diet, microbiome and horizontally acquired genes. Current Opinion in Insect Science, 23(65). https://doi.org/10.1016/j.cois.2017.07.012
Douglas, A. E. (2018). Gut microbes alter the walking activity of fruit flies. Nature, 563(7731). https://doi.org/10.1038/d41586-018-07080-y
du Rand, E. E., Stutzer, C., Human, H., Pirk, C. W. W., & Nicolson, S. W. (2020). Antibiotic treatment impairs protein digestion in the honeybee, Apis mellifera. Apidologie, 51(1). https://doi.org/10.1007/s13592-019-00718-4
Duncan, S. H., Lobley, G. E., Holtrop, G., Ince, J., Johnstone, A. M., Louis, P., & Flint, H. J. (2008). Human colonic microbiota associated with diet, obesity and weight loss. International Journal of Obesity, 32(11). https://doi.org/10.1038/ijo.2008.155
EFSA. (2016). Panel Scientific opinion on assessing the health status of managed honeybee colonies (HEALTHY-B): a toolbox to facilitate harmonised data collection. EFSA Journal, 14(10). https://doi.org/10.2903/j.efsa.2016.4578
Frias, B. E. D., Barbosa, C. D., & Lourenço, A. P. (2016). Pollen nutrition in honey bees (Apis mellifera): impact on adult health. Apidologie, 47(1). https://doi.org/10.1007/s13592-015-0373-y
Fukuda, H., & Sekiguchi, K. (1996). Seasonal change of the honeybee worker longevity in Sapporo, North Japan, with notes on some factors affecting the life-span, Japanese Journal of Ecology, 16(5). https://doi.org/10.18960/seitai.16.5_206
Graves, S., Piepho, HP., Selzer, L., Dorai-Raj, S. (2019). multcompView: Visualizations of Paired Comparisons. R package version 0.1–8. https://CRAN.R-project.org/package=multcompView
Hayashi, A., Mikami, Y., Miyamoto, K., Kamada, N., Sato, T., Mizuno, S., Naganuma, M., Teratani, T., Aoki, R., Fukuda, S., Suda, W., Hattori, M., Amagai, M., Ohyama, M., & Kanai, T. (2017). Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Reports, 20(7). https://doi.org/10.1016/j.celrep.2017.07.057
Haydak, M. H. (1970). Honey Bee Nutrition 1. Annual Reviews of Entomology, 15(1). https://doi.org/10.1146/annurev.en.15.010170.001043
Haydak, M. H. (1935). Brood Rearing by Honeybees Confined to a Pure Carbohydrate Diet. Journal of Economic Entomology, 28(4). https://doi.org/10.1093/jee/28.4.657
Haydak, M. H., & Dietz, A. (1972). Cholesterol, pantothenic acid, pyridoxine and thiamine requirements of honeybees for brood rearing. Journal of Apicultural Research, 11(2). https://doi.org/10.1080/00218839.1972.11099707
Holm, S. (1979). A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics, 6(2). http://www.jstor.org/stable/4615733
Huang, S.K., Csaki, T., Doublet, V., Dussaubat, C., Evans, J.D., Gajda, A.M., Gregorc, A., Hamilton, M.C., Kamler, M., Lecocq, A., Muz, M.N., Neumann, P., Özkirim, A., Schiesser, A., Sohr, A. R., Tanner,G., Tozkar, C, Ö., Williams, G. R., Wu, L., Zheng, H., & Chen, Y. P. (2014). Evaluation of Cage Designs and Feeding Regimes for Honey Bee (Hymenoptera: Apidae) Laboratory Experiments, Journal of Economic Entomology, 107(1). https://doi.org/10.1603/EC13213
Hothorn,T., Frank Bretz, F., & Westfall, P. (2008). Simultaneous Inference in General Parametric Models. Biometrical Journal, 50(3). 346-363
Jovanovic, N. M., Glavinic, U., Delic, B., Vejnovic, B., Aleksic, N., Mladjan, V., & Stanimirovic, Z. (2021). Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Preventive Veterinary Medicine, 190. https://doi.org/10.1016/j.prevetmed.2021.105322
Kešnerová, L., Mars, R. A. T., Ellegaard, K. M., Troilo, M., Sauer, U., & Engel, P. (2017). Disentangling metabolic functions of bacteria in the honey bee gut. PloS Biology, 15(12). https://doi.org/10.1371/journal.pbio.2003467
Klein, S., Pasquaretta, C., He, X. J., Perry, C., Søvik, E., Devaud, J. M., Barron, A. B., & Lihoreau, M. (2019). Honey bees increase their foraging performance and frequency of pollen trips through experience. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-42677-x
Köhler, A., Nicolson, S. W., & Pirk, C. W. W. (2013). A new design for honey bee hoarding cages for laboratory experiments. In Journal of Apicultural Research, 52(2). https://doi.org/10.3896/IBRA.1.52.2.03
Kosinski, M., Kassambara, A., & Biecek, P. (2020). Survminer: Drawing Survival Curves using “ggplot2.” In R package version 0.4.7.
Kwong, W. K., Engel, P., Koch, H., & Moran, N. A. (2014). Genomics and host specialization of honey bee and bumble bee gut symbionts. Proceedings of the National Academy of Sciences of the United States of America, 111(31). https://doi.org/10.1073/pnas.1405838111
Kwong, W. K., & Moran, N. A. (2016). Gut microbial communities of social bees. Nature Reviews Microbiology, 14(6). https://doi.org/10.1038/nrmicro.2016.43
Ludicke, J. C., & Nieh, J. C. (2020). Thiamethoxam impairs honey bee visual learning, alters decision times, and increases abnormal behaviors. Ecotoxicology and Environmental Safety, 193. https://doi.org/10.1016/j.ecoenv.2020.110367
Maes, P. W., Rodrigues, P. A. P., Oliver, R., Mott, B. M., & Anderson, K. E. (2016). Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Molecular Ecology, 25(21). https://doi.org/10.1111/mec.13862
Marceau, T., Archer, C. R., Bulson, L., & Wilfert, L. (2021). Dose-dependent effects of antibiotic intake on Bombus terrestris (Linnaeus, 1758) dietary intake, survival and parasite infection prevalence. Journal of Invertebrate Pathology, 182. https://doi.org/10.1016/j.jip.2021.107580
Martinson, V. G., Moy, J., & Moran, N. A. (2012). Establishment of characteristic gut bacteria during development of the honeybee worker. Applied and Environmental Microbiology, 78(8). https://doi.org/10.1128/AEM.07810-11
Maurizo, A. (1954). Pollenernährung und Lebensvorgänge bei der Honigbiene (Apis mellifera L.). Landwirtsch. Jarh. Schweiz, 245.
Meyer, H. E., Willett, W. C., Fung, T. T., Holvik, K., & Feskanich, D. (2019). Association of high intakes of vitamins B6 and B12 from food and supplements with risk of hip fracture among Postmenopausal Women in the Nurses’ Health Study. JAMA Network Open, 2(5). https://doi.org/10.1001/jamanetworkopen.2019.3591
Naug, D. (2009). Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biological Conservation, 142(10). https://doi.org/10.1016/j.biocon.2009.04.007
Neukirch, A. (1982). Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. Journal of Comparative Physiology, 146(1). https://doi.org/10.1007/BF00688714
Neumann, P., & Carreck, N. L. (2010). Honey bee colony losses. Journal of Apicultural Research, 49(1). https://doi.org/10.3896/IBRA.1.49.1.01
OECD (2017), Test No. 245: Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding), OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, 9. https://doi.org/10.1787/9789264284081-en.
Phillips, W. E. J., Mills, J. H. L., Charbonneau, S. M., Tryphonas, L., Hatina, G. v., Zawidzka, Z., Bryce, F. R., & Munro, I. C. (1978). Subacute toxicity of pyridoxine hydrochloride in the beagle dog. Toxicology and Applied Pharmacology, 44(2). https://doi.org/10.1016/0041-008X(78)90194-1
Piepho, HP. (2004), An Algorithm for a Letter-Based Representation of All-Pairwise Comparisons, Journal of Computational and Graphical Statistics, 13(2). 456-466.
Pudasaini, R., Dhital, B., & Chaudhary, S. (2020). Nutritional requirement and its role on honeybee: a review. Journal of Agriculture and Natural Resources, 3(2). https://doi.org/10.3126/janr.v3i2.32544
Raymann, K., Shaffer, Z., & Moran, N. A. (2017). Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PloS Biology, 15(3). https://doi.org/10.1371/journal.pbio.2001861
R Core Team. (2008). R: a Language and Environment for Statistical Computing. In http://www.R-project.org/ (Vol. 2).
Retschnig, G., Rich, J., Crailsheim, K., Pfister, J., Perreten, V., & Neumann, P. (2021). You are what you eat: relative importance of diet, gut microbiota and nestmates for honey bee, Apis mellifera, worker health. Apidologie, 52(3). https://doi.org/10.1007/s13592-021-00851-z
Roulston, T. H., & Cane, J. H. (2000). Pollen nutritional content and digestibility for animals. Plant Systematics and Evolution, 222(1). https://doi.org/10.1007/BF00984102
Salem, H., Bauer, E., Strauss, A. S., Vogel, H., Marz, M., & Kaltenpoth, M. (2014). Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proceedings of the Royal Society B: Biological Sciences, 281(1796). https://doi.org/10.1098/rspb.2014.1838
Sanhueza, E., Paredes-Osses, E., González, C. L., & García, A. (2015). Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the pH acid acclimated variant. Electronic Journal of Biotechnology, 18(5). https://doi.org/10.1016/j.ejbt.2015.06.005
Somerville, D. (2000). Honey bee nutrition and supplementary feeding, Agnote DAI/178, NSW Agriculture. Online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0008/117494/honey-bee-nutrition-supplementary-feeding.pdf, (accessed 03.04.2021)
Standifer, L. N., Moeller, F. E., Kauffeld, N. M., Herbert, E. W., Jr., & Shimanuki, H. (1977). Supplemental Feeding of Honey Bee Colonies. United States Department of Agriculture, 413.
Suchail, S., Debrauwer, L., & Belzunces, L. P. (2004). Metabolism of imidacloprid in Apis mellifera. Pest Management Science, 60(3). https://doi.org/10.1002/ps.772
Tejerina, M. R., Cabana, M. J., & Benitez-Ahrendts, M. R. (2021). Strains of Lactobacillus spp. reduce chalkbrood in Apis mellifera. Journal of Invertebrate Pathology, 178. https://doi.org/10.1016/j.jip.2020.107521
Therneau, T. M. (2021). Survival: A Package for Survival Analysis in R. R Package Version 2.38.
Therneau, T. M., & Grambsch, P. M. (2000). The Cox Model BT – Modeling Survival Data: Extending the Cox Model. In Statistics for Biology and Health.
Titcomb, T. J., & Tanumihardjo, S. A. (2019). Global Concerns with B Vitamin Statuses: Biofortification, Fortification, Hidden Hunger, Interactions, and Toxicity. Comprehensive Reviews in Food Science and Food Safety, 18(6). https://doi.org/10.1111/1541-4337.12491
van Engelsdorp, D., Speybroeck, N., Evans, J. D., Nguyen, B. K., Mullin, C., Frazier, M., Frazier, J., Cox-Foster, D., Chen, Y., Tarpy, D. R., Haubruge, E., Pettis, J. S., & Saegerman, C. (2010). Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. Journal of Economic Entomology, 103(5). https://doi.org/10.1603/EC09429
Vásquez, A., Forsgren, E., Fries, I., Paxton, R. J., Flaberg, E., Szekely, L., & Olofsson, T. C. (2012). Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PloS ONE, 7(3). https://doi.org/10.1371/journal.pone.0033188
Vásquez, A., & Olofsson, T. C. (2009). The lactic acid bacteria involved in the production of bee pollen and bee bread. Journal of Apicultural Research, 48(3). https://doi.org/10.3896/IBRA.1.48.3.07
Williams, G. R., Alaux, C., Costa, C., Csáki, T., Doublet, V., Eisenhardt, D., Fries, I., Kuhn, R., McMahon, D. P., Medrzycki, P., Murray, T. E., Natsopoulou, M. E., Neumann, P., Oliver, R., Paxton, R. J., Pernal, S. F., Shutler, D., Tanner, G., van der Steen, J. J. M., & Brodschneider, R. (2013). Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. Journal of Apicultural Research, 52(1). https://doi.org/10.3896/IBRA.1.52.1.04
Winston, Mark L. (1991). The biology of the honey bee. Cambridge, Mass: Harvard University Press, Cambridge, Massachusetts
Xu, S., Jiang, L., Qiao, G., & Chen, J. (2020). The Bacterial Flora Associated with the Polyphagous Aphid Aphis gossypii Glover (Hemiptera: Aphididae) Is Strongly Affected by Host Plants. Microbial Ecology, 79(4). https://doi.org/10.1007/s00248-019-01435-2
Acknowledgements
We thank Alexandra Collaud for technical assistance.
Funding
Open access funding provided by University of Bern. Financial support was granted by Ricola Foundation Nature and Culture (GR, PN) and the Vinetum Foundation (PN).
Author information
Authors and Affiliations
Contributions
AB, GR, and PN designed and AB, VR, and JP conducted the experiment; AB and GR analyzed the data; AB, GR, and PN wrote the manuscript; and VR, JP, and VP revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Consent to participate
Not applicable.
Consent for publication
All authors have read and approved the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Mathieu Lihoreau
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brown, A., Rodriguez, V., Pfister, J. et al. The dose makes the poison: feeding of antibiotic-treated winter honey bees, Apis mellifera, with probiotics and b-vitamins. Apidologie 53, 19 (2022). https://doi.org/10.1007/s13592-022-00927-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00927-4