Abstract
Soil emergence tents (e-tents) are a new tool for studying the nesting biology of ground-nesting bees. E-tents allow us to link nests with specific soil conditions; however, low success probabilities (≤ 20% of e-tents capture at least one bee) and long deployment times (> 72 h) limit their efficiency. We examined if adding scents—spearmint and lemongrass essential oils—increases how quickly e-tents capture bees actively nesting in the soil directly covered by the trap (“capture rate”), letting e-tents be moved more frequently to sample more area. Adding essential oils did not have a significant effect on the overall capture rate. However, in e-tents with spearmint essential oil, bees in the family Halictidae were captured 1.80× faster, and there was a trend for reduced capture rate for the family Andrenidae. The efficacy of adding scents to e-tents appears to be taxon-specific. Researchers interested in halictid nesting biology may increase the efficacy of e-tents with spearmint essential oil, but further work is needed to find a suitable attractant for other groups or whole communities.
Similar content being viewed by others
1 Introduction
Ground-nesting bees account for over 80% of bee species in the USA (Harmon-Threatt 2020) and play an intrinsic role in the pollination of crops and wild plant communities (Koh et al. 2016). However, relatively little is known about what influences nest site selection and success, limiting our ability to predict how bee communities will respond to anthropogenic changes (Harmon-Threatt 2020). One tool for studying ground-nesting bees’ nesting biology is soil emergence tents (e-tents) (Sardiñas and Kremen 2014; Anderson and Harmon-Threatt 2016; Pane and Harmon-Threatt 2017). Broadly, e-tents (Figure 1a) collect emerging insects from areas of covered soil or water by directing them to a container attached to the top and filled with a collecting fluid (e.g., soapy water). E-tents help researchers better understand the nesting patterns and species diversity of ground-nesting insects by linking captures with specific soil conditions (Sardiñas and Kremen 2014) or in response to environmental changes (Buckles and Harmon-Threatt 2019).
For ground-nesting bees, researchers have used e-tents in two ways: to collect the emerging offspring of successful nests from the previous growing season (e.g., Sardiñas and Kremen 2014) and to collect actively nesting female bees (e.g., Anderson and Harmon-Threatt 2016; Buckles and Harmon-Threatt 2019; Cope et al. 2019; Kim et al. 2006). In both cases, e-tents are placed randomly during periods when female bees are not actively foraging and presumed to be in their nests (Batra 1968)—early in the season or at dusk, respectively—and subsequently checked for captured individuals at predefined intervals. While capturing newly emerged bees provides information about successful nest sites, conditions that influenced nest site selection are unknown unless data were collected from the e-tent location during nesting the previous year. In many cases, these data are unavailable. Additionally, if e-tents must be deployed at the same spot all year, the amount of area sampled at a site is greatly diminished. Alternatively, short-term e-tent deployment for actively nesting bees can be done concurrently with measurements of environmental factors that may influence the selection of a nesting site, such as percent bare ground, topographical slope, and soil moisture (Potts et al. 2005; Pane and Harmon-Threatt 2017). This design also assumes that actively nesting female bees under an e-tent are captured or starve after some amount of time, allowing e-tents to be moved every few days to cover more area.
Despite their strengths, e-tents suffer from laborious set-up and low capture probabilities. Across multiple short-term deployment studies, 0–20% of e-tents at a site captured at least one bee (Anderson and Harmon-Threatt 2016; Pane and Harmon-Threatt 2017; Cope et al. 2019; Buckles and Harmon-Threatt 2019). One way to account for this limitation, at least in short-term deployment studies, is to move e-tents at regular intervals to sample more area at a site. While a previous study found that e-tents capture most bees within 72 h of deployment, how quickly bees trapped under e-tents were caught in the collecting fluid varied between sites (Pane and Harmon-Threatt 2017). Shortening the time it takes e-tents to capture bees would reduce the probability of a false zero and allow researchers to move e-tents more frequently, increasing the sampled area. Such an increase in the efficiency of this method can transform our understanding of ground-nesting bee biology and directly impact bee conservation strategies.
One way to increase the efficiency of e-tents may be to add attractants to the collecting fluid. Incorporating plant-based attractants into the soap-water mixture may induce positive chemotaxis in bees trapped within e-tents, coaxing them into the collection fluid more quickly than soapy water alone. Similar strategies have been employed to increase trap efficiency for other insects, including beetles (Mitchell et al. 2018), moths (Cunningham et al. 2004), and flies (Jang and Light 1996). A previous study suggested that bees may forgo foraging if their nest is covered and, thus, might only be captured after sufficient time has passed, potentially due to increased hunger or desire to continue nesting (Pane and Harmon-Threatt 2017). Bees can sense flower volatiles and use this information, in addition to visual stimuli, to discriminate food from nonfood objects and between different plant species (Kunze and Gumbert 2001; Klatt et al. 2013). Due to their role in mediating plant-pollinator interactions, adding plant volatiles—in the form of essential oils here—may attract bees trapped beneath the e-tent to the collecting fluid at the top of the trap, thus decreasing the amount of time necessary to capture actively nesting bees. In turn, an increase in capture efficiency should increase the information collected during a sampling period.
This study aimed to determine whether potentially attractive scents decrease the time it takes to capture actively nesting ground-nesting bees trapped beneath e-tents. If essential oils increase how quickly bees within e-tents are captured (herein referred to as “capture rate”), researchers will be able to collect more data within the same sampling period by moving e-tents more frequently and, as a result, sampling more area. We also tested if adding essential oils increased the total number of female bees captured or the probability that an e-tent captures at least one female bee. We predicted that these measures would be unaffected as the spatial distribution of bee nests likely plays a larger role, thus necessitating a methodology that allows more area to be sampled by reducing deployment duration and increasing the number of within-site locations.
2 Materials and methods
Study sites
This study was conducted at two restored prairie sites, 1.2 km apart, managed by the University of Illinois at Urbana-Champaign. The soils at both locations are classified as Catlin silt loam (Soil Survey Staff 2019). The average soil moisture across both sites was 12.2% (range 6.9 to 18.0%) and the average daytime temperature was 28.9 °C (range 25.5 to 32.8 °C). Florida-Orchard prairie (4047 m2; 40°05′49.2′′ N, 88°12′53.8′′ W) was converted from turfgrass to native prairie in 2013 and contains mowed paths but is otherwise allowed to grow naturally. The Pollinatarium prairie (5615 m2; 40°05′13.9′′ N, 88°12′56.2′′ W) was used by the University of Illinois Department of Crop Sciences until 2015 when it was planted with plugs. The Pollinatarium prairie was mowed in the early spring of 2019. Since nesting sites are challenging to locate and the nesting requirements of many bee species remain unknown (Sardiñas et al. 2016), we chose these locations because of previously detected ground-nesting bee activity (Pane and Harmon-Threatt 2017).
Scents
We chose lemongrass (Cymbopogon flexuosus) and spearmint (Mentha spicata) essential oils because they have been used in bee foraging assays (Thomas 2011; Balbuena et al. 2012), elicit electroantennogram responses in bees (Stopfer et al. 1997; Denker et al. 2010), and are readily available. Additionally, lemongrass essential oil contains the compound citrol, which is similar to the order-restoring pheromone produced by the Nasonov gland and is used as a honey bee swarm lure (Amrine et al. 2007). Spearmint belongs to the family Lamiaceae, a popular pollen and nectar source for bees (Westerhold et al. 2018). While the current literature concerning bee odor detection is heavily honey bee biased, the ability to detect these essential oils may be conserved in Hymenoptera beyond Anthophila (Arenas and Roces 2018).
Sampling
We placed 60 e-tents (BugDorm, Taichung, Taiwan; model BT2006; 60 × 60 × 60 cm; 108 × 32 mesh polyester netting) for 1 week periods in May, June, July, and August of 2019 resulting in 240 unique e-tent placements (1680 total trapping days). Bees that are nesting in the covered soil emerge in the morning and crawl or fly to the top of the e-tent where they are collected in a container of soapy water. Soap reduces the surface tension of the water, causing captured insects to drown. E-tents were staked in the ground, and flaps were covered with soil to prevent insects from escaping or entering. Thirty e-tents were used at each site each month, with 10 receiving only soapy water, 10 receiving soapy water with spearmint oil, and 10 receiving soapy water with lemongrass oil. The soapy water solutions consisted of 2 mL of Dawn Ultra dish soap (original scent) and 400 μL of essential oil (Spearmint: NOW Foods, Bloomingdale, IL, USA; Lemongrass: Aura Cacia, Norway, IA) per 3785 mL of water. This ratio of scent to water was slightly higher than the ratio previously shown to increase bee attraction (Slaa et al. 2003; Tutun et al. 2018). The collecting bottle of each e-tent was filled with ~ 100 mL of one of the three solutions. Tents were arranged in groups of three (Figure 1b), with one tent for each treatment, to minimize the chance that a nesting aggregation (Rosenheim 1990) only impacted one treatment.
E-tents were deployed at 19:00 and checked 12, 15, 18, 21, 24, 36, 39, 42, 45, 48, 72, 96, 120, 144, and 168 h after setup. At each time, water was strained into a separate container and replaced in the bottle. The essential oil-soap mixture was replaced at least every 2 days to maintain freshness. Most bees were expected to be captured within the first 48 h (Pane and Harmon-Threatt 2017), so e-tents were checked more frequently for the first 48 h to better detect changes in capture rate. Collected bees were placed in labeled Whirl-paks (Nasco, Fort Atkinson, Wisconsin, USA) in 70% ethanol until they could be pinned. Bees were identified to species level using a reference collection and identification keys (Gibbs 2011; Ascher and Pickering 2019). Bees in the genus Andrena were identified by Michael Arduser.
Statistical methods
We excluded all male bees and female bees from genera not thought to be ground-nesting from our analyses (Supplemental Table I). Male bees are not thought to spend their nights in active nests, opting instead to rest on vegetation and, therefore, may not be good indicators of nest-site preference at the scale of e-tents (Wcislo 2003; Miyanaga and Maeta 1998). Similarly, above-ground nesting bees like those collected from the genera Ceratina and Hylaeus nest in vegetation, and their captures do not provide a direct connection to the preferences of ground-nesting species (Kislow 1976; Rehan and Richards 2010; Vickruck et al. 2011; MacIvor and Packer 2015). Male bees and females from above-ground nesting species captured in our study were interpreted to have been on vegetation when e-tents were placed and inadvertently captured. We also removed one e-tent from July from the Pollinatarium prairie as it appeared to capture an emergence event of 28 bees and the focus of our study was on short-term e-tent methodology. We concluded that this was an emergence event as all bees were the same species, Lasioglossum hitchensi, and there was a pulse of male bees approximately 2 days before the female bees, a characteristic of bee emergence events (Szentgyörgyi and Woyciechowski 2013; Eickwort and Ginsberg 1980).
We used X2 tests to evaluate differences in the total number of female ground-nesting bees and females in the families Andrenidae and Halictidae. A generalized linear mixed-effects model (GLMM) with a binomial distribution was used to model the fixed effects of month and scent and the random effect of prairie on the probability that an e-tent captured at least one female ground-nesting bee using the ‘lme4’ package (Bates et al. 2015). We calculated post hoc Tukey contrasts using the ‘multcomp’ package (Hothorn et al. 2008) when significant main or interaction effects were detected. We modeled the rate of bees captured over the first 48 h using Cox proportional-hazards regression (Cox 1972) using the ‘rms’ package (Harrell 2017). Due to the study design, we cannot determine if there were any bees present within but not captured by the e-tents (e.g., deaths or, if surviving, not being funneled into the collecting jar). To partially account for this uncertainty, we right-censored bees captured after 48 h, allowing the Cox proportional-hazards regression to consider the probability that a bee is captured outside this time frame. Additionally, e-tents used to sample active ground-nesting bees are often deployed for 48 h or less (Cope et al. 2019; Pane and Harmon-Threatt 2017; Buckles and Harmon-Threatt 2019). Our regression model included the main effects of scent and bee family and their interaction. Contrasts were calculated using the ‘rms’ package when significant differences were detected. All model assumptions were met. All analyses were performed using R version 3.6.3 (R Core Team 2020).
3 Results
A total of 121 bees were collected over the 4 sampling periods and identified to species (Supplemental Table I). Eleven female above-ground nesting bees (no males collected), 17 male ground-nesting bees, and 28 (12 female and 16 male) ground-nesting bees from a suspected emergence event (see “Materials and methods” section) were removed from the analysis, leaving 65 ground-nesting female bees. Adding plant essential oils did not have a significant effect on the number of total (χ22 = 1.323, P = 0.516; Figure 2), andrenid (χ22 = 0.737, P = 0.692), or halictid (χ22 = 0.696, P = 0.706) bees. Similarly, there was no effect of scent on the probability that an e-tent successfully captured at least one female bee (χ22 = 0.206, P = 0.902; Figure 3). There was a significant effect of month on the probability that an e-tent captured bees (χ23 = 19.248, P < 0.001). There were more than twice as many successful e-tents in May compared to June (P = 0.005) and July (P = 0.005). All other pairwise comparisons were not significantly different (P > 0.157). There was no significant interaction between scent treatment and month (χ26 = 3.832, P = 0.699). Overall, 17.6% of e-tents captured ground-nesting female bees.
The estimated probability (± 95% confidence interval) of an e-tent capturing at least one female ground-nesting bee. Smaller, overlapping dots at 0 and 1 represent individual e-tents. Lower-case letters and horizontal bars denote significant differences such that points that do not share a common letter are significantly different (P < 0.05)
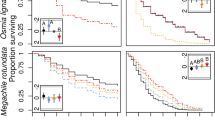
The overall Cox proportional-harzards regression model for ground-nesting bee capture rate over the first 48 h had a significant fit (Wald χ25 = 13.04, P = 0.023) and met the proportional-hazards assumption (χ23 = 7.71, P = 0.052). There was no significant main effect of e-tent scent on bee capture rate (Wald χ24 = 6.84, P = 0.145; Figure 4, Supplemental Fig. 1). However, there was a significant main effect of bee family (Wald χ23 = 12.55, P = 0.006) and a significant interaction between bee family and e-tent scent (Wald χ22 = 6.34, P = 0.042). For the family Andrenidae, there was a trend for slower capture rates in e-tents with spearmint essential oils when compared to control e-tents (Z = 1.63, P = 0.103; Figure 4, Supplemental Fig. 1). There were no differences in andrenid capture rates between lemongrass and control e-tents (Z = 0.35, P = 0.724) nor lemongrass and spearmint e-tents (Z = 1.14, P = 0.254). For the family Halictidae, e-tents with spearmint essential oils caught bees 1.80× faster than control e-tents (Z = 2.01, P = 0.045; Figure 4, Supplemental Fig. 1). There were no differences in halictid capture rates between lemongrass and control e-tents (Z = 0.51, P = 0.612) nor lemongrass and spearmint e-tents (Z = 1.41, P = 0.158). Andrenid bees were caught 2.44× faster in control e-tents than halictid bees (Z = 3.08, P = 0.002), and there was a similar trend in lemongrass e-tents (Z = 1.87, P = 0.061). However, there was not a significant difference between the capture rate of individuals belonging to these families in spearmint e-tents (Z = 0.52, P = 0.604).
4 Discussion
Adding essential oils had no effect on the number of female bees captured nor the probability that an e-tent captured at least one female bee. These metrics are presumably more related to the likelihood that an e-tent is placed over an active nest. The addition of essential oils to the collecting fluid of e-tents did not significantly affect the capture rate of actively nesting ground-nesting female bees when all taxonomic groups were taken together. Instead, the effect of essential oils differed for each observed family. For members of the family Halictidae—95.7% Lasioglossum species and 4.3% Halictus ligatus in our sample—the addition of spearmint essential oils markedly improved capture rate. However, for the family Andrenidae—84.2% Andrena species and 15.8% Calliopsis andreniformis in our sample—adding spearmint appeared to reduce the capture rate. Thus, benefits gained by adding essential oil for some taxa could be accompanied by adverse effects in other taxa. In other words, adding spearmint essential oil to e-tents in future studies only makes sense if the research question is specifically about the nesting biology of halictids or, more specifically, Lasioglossum. However, if the question pertains to andrenid species or the whole bee community, adding spearmint essential oils may decrease the e-tent method’s efficiency.
Diet breadth and the fact that essential oils are often extracted from portions of plants not used by bees, such as stem and leaves (Butnariu and Sarac 2018), may explain some of the variations in bee response to scent stimuli. For example, Lasioglossum—by far the most common genus of halictid bees in the current study—tend to be generalists, feeding on a wide array of floral resources, including plants in the family Lamiaceae (Adamson 2011), which includes spearmint. Alternatively, bees in the family Andrenidae have been shown to prefer pollen from flowers of genera Rudbeckia and Ratibida, part of the Asteraceae family (Neff and Simpson 1997) and pollen from fruit trees (Chambers 1946). While the results of this study suggest that adding essential oil to the collecting fluid could improve e-tent efficacy, selecting an appropriate scent for your group of interest will be important.
The current bee-essential oil literature may not be particularly helpful in selecting candidate essential oils to improve e-tent efficiency. Most previous studies have been limited to a few large-bodied, generalist, eusocial, non-ground-nesting species (da Silva et al. 2020; Thomas 2011; Balbuena et al. 2012; Stopfer et al. 1997; Denker et al. 2010), making it difficult to extend their results to predominately small-bodied, solitary, ground-nesting species. In fact, a recent study found that eusocial stingless bees and honey bees spent less time in areas with mint essential oil (da Silva et al. 2020), potentially suggesting that scents like spearmint may negatively affect capture rate in similar species. In addition to basing candidate scents on bee-plant association data, electroantennograms could be used to determine which odors non-model bees can detect (Park et al. 2002). This method is commonly used in chemical ecology to determine what pheromones elicit responses in insects (e.g., cerambycids; Wickham et al. 2016). Alternatively, candidate scents could be identified by adding them to white pan traps (the color of the collecting jar) and comparing bee capture rates. In either case, promising scents would need to be verified using similar methods to those presented in this paper. Additionally, these relatively quick assays could be used to test mixtures of scents for use in studies where broader portions of the ground-nesting bee community are of interest.
In addition to spiking collecting fluid with attractive scents, future studies could explore adding visual cues. Such cues could include changing the color of the collecting fluid or the color of the collection jar. Similar techniques have been effective for pan traps (e.g., bee bowls; Toler et al. 2005). However, one limitation of using visual attractants with e-tents is that the collection jar is obscured from the inside of the e-tent until insects enter the upper chamber. Adding color to the collection fluid or jar will likely only be effective if bees are currently entering the top of the collection jar but then returning to the e-tent’s body. Scent cues seem to be the more promising route as they can be detected in environments where visual cues are obstructed (Deisig et al. 2014).
The probability that an e-tent would successfully capture a female bee was more affected by the month of deployment than the scent treatment. May had the highest probability of catching at least one female bee—1.5-2 times greater than in June, July, or August—indicating that low e-tent capture probabilities reported in previous studies may be, in part, a function of the sampling period. A similar trend in the more widely-adopted bee sampling techniques of bee bowls (specialized pan traps) and aerial netting was noted in a previous study conducted in the Midwestern USA (Grundel et al. 2011). Although it is possible that the removal of individuals affected the probability that an e-tent would capture a female bee in subsequent months, particularly in long-lived or multivoltine species, we did not place e-tents in the exact same locations within sites between months. Therefore, we believe the removal of individuals had a negligible effect on subsequent sampling periods. While the aim of the current study was not to associate specific nesting conditions with female bee captures, a previous study at the same sites found that increasing soil moisture reduced the probability of an e-tent capturing a bee (Pane and Harmon-Threatt 2017), potentially explaining this temporal pattern. When using e-tents for future projects, it is important for researchers to choose their study months carefully and to sample across broader time points, if possible.
To our knowledge, this is the first study to attempt to increase e-tent efficacy in capturing bees present under the trap using attractive scents in the collecting fluid. While lemongrass and spearmint essential oils did not affect the number of female bees captured nor the probability that an e-tent would capture at least one female bee, spearmint had taxon-specific effects on capture rate. Further work is needed to identify additional attractants, but the utility of adding attractive scents in the study of some groups—here, spearmint and Halictidae—is promising. We hope that future incorporation of essential oils or other attractants will increase our ability to use e-tents to study ground-nesting bees more effectively.
Data Availability
All bee capture data including bee species identification are included in Supplemental Table 1. Interested parties may contact the corresponding author to obtain data files that include e-tents that did not capture at least one bee.
References
Adamson N. L. (2011) An assessment of non-Apis bees as fruit and vegetable crop pollinators in southwest Virginia. Virginia Polytechnic Institute and State University.
Amrine J. W., Noel R. C., Webb D. (2007) Results of 50% formic acid fumigation of honey bee hives [Apis mellifera ligustica (Hymenoptera: Apidae)] to control varroa mites (Acari: Varroidae) in brood combs in Florida, U.S.A. Int. J. Acarology 33 (2), 99–109.
Anderson N., Harmon-Threatt A. (2016) 07. The effects of seed mix diversity on soil conditions and nesting of bees in prairie restorations. North American Prairie Conference Proceedings 17, 104–112.
Arenas A., Roces F. (2018) Appetitive and aversive learning of plants odors inside different nest compartments by foraging leaf-cutting ants. J. Insect Physiol. 109, 85–92.
Ascher J. S., Pickering J.(2019) Discover Life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila) [online] https://www.discoverlife.org/mp/20q?search=Apoidea ().
Balbuena M. S., Molinas J., Farina W. M. (2012) Honeybee recruitment to scented food sources: correlations between in-hive social interactions and foraging decisions. Behav. Ecol. Sociobiol. 66, 445–452.
Bates D., Mächler M., Bolker B. M., Walker S. C. (2015) Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 (1), 1–48.
Batra S. W. T. (1968) Behavior of some social and solitary halictine bees within their nests: A comparative study (Hymenoptera: Halictidae). J. Kans. Entomol. Soc. 41 (1), 120–133.
Buckles B. J., Harmon-Threatt A. N. (2019) Bee diversity in tallgrass prairies affected by management and its effects on above- and below-ground resources. J. Appl. Ecol. 56 (11), 2443–2453.
Butnariu M., Sarac I. (2018) Essential oils from plants. J. Biotechnol. Biomed. Sci. 1 (4), 35–43.
Chambers V. H. (1946) An examination of the pollen loads of Andrena: The species that visit fruit trees. J. Anim. Ecol. 15 (1), 9–21.
Cope G. C., Campbell J. W., Grodsky S. M., Ellis J. D. (2019) Evaluation of nest-site selection of ground-nesting bees and wasps (Hymenoptera) using emergence traps. Can Entomol 151, 260–271.
Cox D. R. (1972) Regression models and life tables (with discussion). JR Statist. Soc. B 34, 187–220.
Cunningham J. P., Moore C. J., Zalucki M. P., West S. A. (2004) Learning, odour preference and flower foraging in moths. J. Exp. Biol. 207 (Pt 1), 87–94.
Deisig N., Dupuy F., Anton S., Renou M. (2014) Responses to pheromones in a complex odor world: Sensory processing and behavior. Insects 5 (2), 399–422.
Denker M., Finke R., Schaupp F., Grün S., Menzel R. (2010) Neural correlates of odor learning in the honeybee antennal lobe. Eur. J. Neurosci. 31 (1), 119–133.
Eickwort G. C., Ginsberg H. S. (1980) Foraging and mating behavior in Apoidea. Annu. Rev. Entomol. 25 (1), 421–446.
Gibbs J. (2011) Revision of the metallic Lasioglossum (Dialictus) of eastern North America (Hymenoptera: Halictidae: Halictini). Zootaxa 3073, 1–216.
Grundel R., Frohnapple K. J., Jean R. P., Pavlovic N. B. (2011) Effectiveness of bowl trapping and netting for inventory of a bee community. Environ. Entomol. 40 (2), 374–380.
Harmon-Threatt A. (2020) Influence of nesting characteristics on health of wild bee communities. Annu. Rev. Entomol. 65, 39–56.
Harrell F. E. Jr (2017) rms: Regression Modeling Strategies. R package version 5.1-1.
Hothorn T., Bretz F., Westfall P. (2008) Simultaneous Inference in General Parametric Models. Biom. J. 50 (3), 346–363.
Jang E. B., Light D. M. (1996) Olfactory semiochemicals of tephritids, in: McPheron B. A. and Steck G. J. (Eds.), Fruit Fly Pests: A World Assessment of Their Biology and Management. St. Lucie Press Delray Beach, FL, USA. pp. 73–90.
Kim J., Williams N., Kremen C. (2006) Effects of cultivation and proximity to natural habitat on ground-nesting native bees in California sunflower fields. J. Kans. Entomol. Soc. 79 (4), 309–320.
Kislow J. C (1976) The comparative biology of two species of small carpenter bees, Ceratina strenua T. Smith and C. calcarata Robertson (Hymenoptera, Xylocopinae). University of Georgia.
Klatt B. K., Burmeister C., Westphal C., Tscharntke T., Fragstein M. von (2013) Flower volatiles, crop varieties and bee responses. PLoS One 8 (8), e72724.
Koh I., Lonsdorf E. V., Williams N. M., Brittain C., Isaacs R., et al. (2016) Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. U. S. A. 113 (1), 140–145.
Kunze J., Gumbert A. (2001) The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 12 (4), 447–456.
MacIvor J. S., Packer L. (2015) "Bee hotels" as tools for native pollinator conservation: A premature verdict? PLoS One 10 (3), e0122126.
Mitchell R. F., Ray A. M., Hanks L. M., Millar J. G. (2018) The common natural products (S)-α-Terpineol and (E)-2-Hexenol are important pheromone components of Megacyllene antennata (Coleoptera: Cerambycidae). Environ. Entomol. 47 (6), 1547–1552.
Miyanaga R., Maeta Y. (1998) Notes on a male sleeping aggregation of Lasioglossum (Ctenonomia) kumejimense (Hymenoptera: Halictidae). Entomol. Sci. 1 (3), 357–358.
Neff J. L., Simpson B. B. (1997) Nesting and foraging behavior of Andrena (Callandrena) rudbeckiae Robertson (Hymenoptera: Apoidea: Andrenidae) in Texas. J. Kans. Entomol. Soc. 70 (2), 100–113.
Pane A. M., Harmon-Threatt A. N. (2017) An assessment of the efficacy and peak catch rates of emergence tents for measuring bee nesting. Appl. Plant Sci. 5 (6), 1–6.
Park K. C., Ochieng S. A., Zhu J., Baker T. C. (2002) Odor discrimination using insect electroantennogram responses from an insect antennal array. Chem. Senses 27 (4), 343–352.
Potts S. G., Vulliamy B., Roberts S., O'Toole C., Dafni A., et al. (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 30 (1), 78–85.
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Rehan S. M., Richards M. H. (2010) Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Can. Entomol. 142 (1), 65–74.
Rosenheim J. A. (1990) Density-dependent parasitism and the evolution of aggregated nesting in the solitary Hymenoptera. Ann. Entomol. Soc. Am. 83 (3), 277–286.
Sardiñas H. S., Kremen C. (2014) Evaluating nesting microhabitat for ground-nesting bees using emergence traps. Basic Appl. Ecol. 15 (2), 161–168.
Sardiñas H. S., Ponisio L. C., Kremen C. (2016) Hedgerow presence does not enhance indicators of nest-site habitat quality or nesting rates of ground-nesting bees. Restor. Ecol. 24 (4), 499–505.
Silva I. M. da, Zanuncio J. C., Brügger B. P., Soares M. A., Zanuncio A. J. V., et al. (2020) Selectivity of the botanical compounds to the pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 10 (4820).
Slaa E. J., Wassenberg J., Biesmeijer J. C. (2003) The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 28 (3), 369–379.
Soil Survey Staff (2019) Gridded Soil Survey Geographic (gSSURGO) Database for Illinois. https://gdg.sc.egov.usda.gov/.
Stopfer M., Bhagavan S., Smith B. H., Laurent G. (1997) Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390 (6655), 70–74.
Szentgyörgyi H., Woyciechowski M. (2013) Cocoon orientation in the nests of red mason bees (Osmia bicornis) is affected by cocoon size and available space. Apidologie 44 (3), 334–341.
Thomas V. (2011) Learned associations and preferential foraging in honey bees (Apis mellifera), (Dawson S. (Ed.)). Franklin and Marshall College.
Toler T. R., Evans E. W., Tepedino V. J. (2005) Pan-trapping for bees (Hymenoptera: Apiformes) in Utah's West Desert: The importance of color diversity. Pan-Pac. entomol. 81 (3–4), 103–113.
Tutun H., Koç N., Kart A. (2018) Plant essential oils used against some bee diseases. TURJAF 6 (1), 34–45.
Vickruck J. L., Rehan S. M., Sheffield C. S., Richards M. H. (2011) Nesting biology and DNA barcode analysis of Ceratina dupla and C. mikmaqi, and comparisons with C. calcarata (Hymenoptera: Apidae: Xylocopinae). Can. Entomol. 143 (3), 254–262.
Wcislo W. T. (2003) A Male Sleeping Roost of a Sweat Bee, Augochlorella neglectula (Ckll.) (Hymenoptera: Halictidae), in Panamá. J. Kans. Entomol. Soc. 76 (1), 55–59.
Westerhold C. M., Wortman S., Todd K., Golick D. (2018) Knowledge of pollinator conservation and associated plant recommendations in the horticultural retail industry. Horttechnology 28 (4), 529–535.
Wickham J. D., Millar J. G., Hanks L. M., Zou Y., Wong J. C. H., et al. (2016) (2 R, 3 S)-2, 3-Octanediol, a female-produced sex pheromone of Megopis costipennis (Coleoptera: Cerambycidae: Prioninae). Environ. Entomol. 45 (1), 223–228.
Acknowledgements
We would like to thank John Marlin for allowing access to research sites and Mike Arduser for help with bee identification. We would also like to thank Ryan Leonard, Katherine Barie, Benjamin Yeager, Elizabeth Moscoso Anderson, Morgan Mackert, Zoe Trujillo, and Benjamin Chiavini for help with e-tent assembly and deployment.
Funding
This research was made possible, in part, through funding to ACG by the University of Illinois at Urbana-Champaign Office of Undergraduate Research; to ACG and NLA by the Sharon Gray Memorial Award, which was made possible by a generous gift from her family, friends, and colleagues to the School of Integrative Biology at the University of Illinois at Urbana-Champaign; to ANHT by USDA-NIFA award number 2018-67013-27537; and to NLA by the U.S. Department of Education Graduates Assistance in Areas of National Need Fellowship through the University of Illinois School of Integrative Biology.
Author information
Authors and Affiliations
Contributions
ACG, ANHT, and NLA designed the experiment and revised the paper; ACG and NLA collected the data; ACG wrote the first draft; NLA analyzed the data; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the publishing of this study.
Code availability
Analyses were conducted in accordance with standard usages of freely available software and packages (see the Materials and methods section above). Interested parties may contact the corresponding author to obtain the specific files.
Additional information
Manuscript Editor: Cedric Alaux
L'ajout d'huiles essentielles aux cages d’éclosion a des effets spécifiques aux taxons sur l'efficacité du piégeage des abeilles sauvages nichant au sol.
Halictidae / cage d’éclosion / nidification au sol / huiles essentielles / substance attractive.
Der Zusatz von Duftölen in Schlupfzelten hat taxon-spezifische Effekte auf die Wirksamkeit der Fallen bei bodennistenden Wildbienen.
Halictidae / Schlupfzelte / Bodennister / Duftöle / Lockstoffe.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributions: ACG, ANHT, and NLA designed the experiment and revised the paper; ACG and NLA collected the data; ACG wrote the first draft; NLA analyzed the data; all authors read and approved the final manuscript.
Supplementary Information
ESM 1.
(DOCX 293 kb)
Rights and permissions
About this article
Cite this article
Grommes, A.C., Harmon-Threatt, A.N. & Anderson, N.L. Adding essential oils to emergence tents has taxon-specific effects on trapping efficiency of ground-nesting bees. Apidologie 52, 378–387 (2021). https://doi.org/10.1007/s13592-020-00827-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00827-5