Abstract
To characterize the populational diversity of Mourella caerulea, an endemic stingless bee from the Pampa biome, we collected workers of the stingless bee Mourella caerulea from 24 colonies of five localities in Southern Brazil and analyzed it using geometric morphometrics of forewings, mtDNA cytochrome oxidase I variability, and cuticular hydrocarbon (CHC) chemical analysis. The morphometric analysis discriminated the populations of M. caerulea from different physiographic regions. There was a positive correlation between morphometric and geographic distances. CHC profiles also differentiated the colonies from different localities. We found six particular haplotypes, nucleotide diversity (π) of 0.01631, and a haplotype diversity (Hd) of 0.74. In this sense, the comparison of the population belonging to different physiographic regions indicates that we need to give particular attention to M. caerulea at the moment of creating conservation strategies for South Brazilian Fauna, once it is the only species of this monospecific genus, and its populations are much differentiated from each other.
Similar content being viewed by others
1 Introduction
Mourella caerulea (Friese 1900) is the only species of the stingless bee genus Mourella Schwarz 1946 (Camargo and Wittmann 1989; Camargo and Pedro 2013). Its distribution is mainly associated to Pampa biome (Rio Grande do Sul State, in South Brazil, Argentine, and Paraguay), with a few records in areas of Campos de Cima da Serra from southern states of Brazil (Camargo and Wittmann 1989; Wittmann and Hoffman 1990; Silveira et al. 2002). This species nests on the ground (~ 50 cm from surface) and is an important pollinator of natural and agricultural ecosystems. It is known to visit flowers of onions, carrots, coriander (Witter and Blochtein 2003), canola (Halinski et al. 2015), and also native plants of the Pampa (Camargo and Wittmann 1989), and thus is an economically and ecologically essential species, necessary for native flora maintenance.
Due to its nesting habit, M. caerulea is a species vulnerable to inadequate soil management, standard practice in an environment where the main economic activities are agriculture and livestock.
The Pampa biome is divided into four physiographic regions: Campanha, with a predominance of grasslands, continental climate, and average altitude ranging from 60 to 120 m; Depressão Central, which is a transition area, close to Atlantic Rain Forest, with altitude usually inferior to 100 m; Serra do Sudeste, with an altitude ranging from 20 to 200 m with shrub vegetation, and height from 400 to 600 m with grasslands. It is also possible to find small forest vegetation; the last one is Encosta do Sudeste, which is near the great lakes, and its average altitude is less than 30 m, with a few hills up to 200 m and vegetation similar to the Serra do Sudeste region (Fortes 1979; Roesch et al. 2009). According to the Brazilian government, between 1970 and 2009, the Pampa area reduced more than 36% of its original size (MMA 2012).
Mourella caerulea was described in 1900, and the description of its nest was made only in 1989, from four colonies of Canguçu, Rio Grande do Sul (Camargo and Wittmann 1989). Up to now, nothing is known about the intraspecific diversity of this species and its relation with the Pampa biome.
In this sense, our objective was to characterize and map the population variability of the Pampa endemic stingless bee M. caerulea in the Rio Grande do Sul State, Brazil. Thus, we expected to provide valuable data into M. caerulea population structure and new information for its conservation. To achieve this objective, we used (1) geometric morphometrics of wings, to evaluate the morphological variation in population (Francoy and Imperatriz-Fonseca 2010); (2) cuticular hydrocarbon (CHC) profiles, which allow us to verify genetic (Falcón et al. 2014) and environmental effects (Howard and Blomquist 2005); and (3) the analysis of mitochondrial DNA, through nucleotide variation of the gene Cytochrome oxidase I (COI), commonly used to verify genetic variability inside Meliponini population (Arias et al. 2003).
2 Material and methods
2.1 Sampling
We collected workers of M. caerulea from 24 natural nests distributed in the following localities: 14 colonies from Alegrete (29° 47′ 5.29″ S 55° 46′ 32.37″ W), 3 from Candiota (31° 35′ 59.99″ S 53° 43′ 59.94″ W), 6 from Turuçu (31° 25′ 18.74″ S 52° 10′ 39.86″ W), and 1 from Viamão (30° 05′ 18.09″ S 51° 01′ 25.64″ W). Additionally, we collected bees visiting flowers in Bagé (31° 19′ 13.96″ S 54° 10′ 24.42″ W), from two places 8 km apart from each other (Fig. 1). As each analysis used a different number of individuals, these amounts will be described in the following sections.
2.2 Morphometric analysis
For this analysis, we used approximately 20 individuals per nest, except for the two different localities in Bagé, from which we used around 30 individuals per sampling locality (Table I). We removed the right forewing of each worker, placed it between microscope slides and photographed each wing. A .tps file was created using the software tpsUtil v. 1.40 (Rohlf 2009), and 13 landmarks were manually plotted in the intersections of wing veins (Fig. 2) using the software tpsDig v. 2.12 (Rohlf 2008). The landmark configurations were Procrustes aligned (Klingenberg and McIntyre 1998) using MorphoJ v. 1.03a (Klingenberg 2011), which also generates canonical variate analysis (CVA), Mahalanobis distances between the centroids of each group of samples, and pairwise discriminant analysis. MEGA v. 5.05 (Tamura et al. 2011) was used to build a neighbor-joining dendogram based on the Mahalanobis distances between the centroids of the distributions. To verify the correlation between the morphological and geographical distances of the localities, we performed a Mantel test using TFPGA (Miller 1997).
2.3 CHC profiles
CHC extraction, analysis, peak identification, normalization of relative peak area and derivatization reaction were made as described earlier (Falcón et al. 2014) with two adaptations: the volume for CHC extraction was 1 ml, and the re-suspended volume was 50 μl. We performed a PCA (R software v. 3.0.0) to check each CHC peak contribution for the differences among localities. The linear discriminant analysis was used to discriminate the localities based on CHC profiles using the software Past v. 3.04 (Hammer et al. 2001). We also used the R package pvclust v. 1.3-2 (Suzuki and Shimodaira 2006) to calculate the potential clustering of samples (localities) based on Euclidean distances and 10,000 bootstrap replications. This package returns the values of approximately unbiased p values for cluster support (AU) for each bootstrap support (BP). Groups with AU ≥ 95% were considered supported by these data. For this approach, we used five individuals per nest. In the case of Bagé, we used five bees per sampling site, since we collected them on the flowers. To estimate the correlation between the average Euclidean distances of CHC data and geographical distances, we used the Mantel test (R software v. 3.2.2, package ade4 v. 1.7-4).
2.4 Molecular analysis
We extracted total DNA from the middle leg of one individual per nest (sampling site in the case of Bagé) according to the methodology proposed by Walsh et al. (1991). We performed a PCR using conditions previously described (Françoso and Arias 2013; Simon et al. 1994). For the PCR, it was used 5 μl of DNA, 2 μl of each primer (10 pmol), 25 μl of 2× Promega PCRMaster Mix [Taq-DNA polymerase (0.05 units/μl), 2× reaction buffer, 400 μM of each dNTP, and 3 mM of MgCl2], and 16 μl of ultra-pure water. The amplified fragment was visualized in 1% agarose gel contrasted with Gel Red™, using UV light. Then, it was purified using Wizard® SV Gel and PCR Clean-Up System Kit (Promega) following the manufacturer’s instruction. The purified samples were sequenced using Sanger’s methodology. The sequences were visualized, edited, and aligned using the software Geneious 5.1.6 (Drummond et al. 2011). For the alignment, we used Muscle algorithm (Edgar 2004) with eight interactions. DnaSP v. 5.10 (Librado and Rozas 2009) was used to determine the number of polymorphic sites (S), haplotypes (h), haplotype diversity (Hd), nucleotide diversity (π), and the average of nucleotide differences (k). The haplotype network was built using NETWORK v. 4.6 (Polzin and Daneshmand 2003), through the algorithm median joining (Bandelt et al. 1999). MEGA v. 5.5 (Tamura et al. 2011) was used to calculate the average number of variable sites between and within populations. The distance matrix was calculated using the model of Kimura two-parameter (K2P) (Nei and Kumar 2000) and used to build a neighbor-joining dendrogram. For the dendrogram construction, we also used the COI sequences of Schwarziana quadripunctata (access number at National Center for Biotechnology Information (NCBI) data bank KC853379) and Scaura latitarsis (access number KC853332). We also sequenced, with the same pair of primers, the COI of the stingless bees Plebeia pugnax, Plebeia saiqui, and Plebeia droryana, for comparative purposes. For this approach, we used four samples per collection site of Bagé. The software Arlequin v. 3.5 (Excoffier and Lischer 2010) was used to estimate the pairwise Fst. We determined the correlation between the genetic and geographical distances through a Mantel test in the software TFPGA (Miller 1997).
3 Results
3.1 Geometric morphometrics
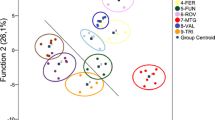
The two first canonical axes of the CVA explained approximately 90% of the variation among the sampled individuals. This result showed that the locations of Candiota, Turuçu and Viamão are morphologically similar. The CV1 discriminated better Alegrete and Bagé from the other localities. CV2 was also important to discriminate Bagé from the others (Online Resource 1). The cross-validation tests showed a high level of samples’ correct classification, with the smallest values being the comparison between Candiota and Turuçu with 78.57 and 84.75% of correct match, respectively. Mahalanobis distances and its dendrogram also support CVA results, grouping Candiota, Turuçu, and Viamão and separating Alegrete and Bagé (Table II, Fig. 3). The Mantel test indicated a significant positive correlation between morphological and geographical distances (r = 0.61, p = 0.05), which may indicate isolation by distance among populations.
3.2 CHC analysis
We identified 62 peaks of CHC, consisting of n-alkanes, unsaturated CHC (alkenes and alkadienes) and branched alkanes of chain length ranging from 21 (C21) to 35 (C35) carbon atoms. The PCA revealed that of the 20 peaks that contributed most to explaining the variation among localities, only 1 was an n-alkane (C32), 6 were branched alkanes, and the majority (13 peaks) were unsaturated CHC (Online resource 2). The discriminant analysis differentiated all analyzed groups, except Bagé from Candiota (Online Resource 3). The cluster analysis showed that samples from Alegrete form a separated group while the other localities clustered together. Although Viamão is inside of this larger group, its samples significantly clustered together (Fig. 4). The Mantel test indicated no statistical significance (r = 0.7763467, p = 0.1213), which corresponds to the absence of correlation between the average Euclidean distances of chemical data and geographical distances. Another interesting fact is that our results demonstrated that unsaturated and branched CHCs are the main responsible peaks for the differentiation of the groups and, based on the canonical analysis and cluster approach, Alegrete and Viamão are different from the other sampling places.
Dendrogram representing the Euclidean distances between samples based on CHC profiles. The first letter in each terminal represents the locality: Alegrete (A), Bagé (B), Candiota (C), Turuçu (T), Viamão (V). Cluster support (au—dark gray values) ≥ 95 are considered statistically significant. Bootstrap (bp—light gray values). Branches’ edges in gray. The localities of Alegrete and Viamão, which had all samples clustered together, as well as the major group that comprises Bagé, Candiota and Turuçu, are indicated by an arrow.
3.3 Molecular data
We obtained a 546-bp region from COI for population comparisons (accession numbers to NCBI data bank KY274850 to KY274882). The nucleotide composition was A = 32.1%, T = 45.3%, G = 10.2%, and C = 12.4%. We found 25 polymorphic sites and six different haplotypes (Table III). We found unique haplotypes for each sampling site (Online Resource 4), which is an indication of low gene flow among populations. Despite a large number of mutational steps between haplotypes from different localities, there was one single change in amino acid sequence, from methionine to leucine on haplotype 4 (in position 126). The neighbor-joining dendrogram showed that M. caerulea sequences grouped and that the samples from each site are discriminated through this method (Fig. 5). Along with these results, Fst values significantly discriminated populations of different localities (Table IV), except for Viamão. The K2P distance between sampling sites ranged from 0.6 to 3.1%, also in agreement with haplotype and Fst results (Online Resource 5). Additionally, Mantel test indicated a positive significant correlation between genetic and geographic distances (r = 0.51, p < 0.05).
4 Discussion
The three methodologies used in the present study indicated similar results regarding their effectiveness in the differentiation among samples from different localities. The high rates of correct classification using morphological and biochemical markers, as well as the existence of an elevated number of exclusive haplotypes, are in agreement to previous works involving bee populations (Francisco et al. 2008, 2013; Batalha-Filho et al. 2010; Brito and Arias 2010; Francisco and Arias 2010; Francoy et al. 2011; Brito et al. 2013; Bonatti et al. 2014). Otherwise, it is worth to mention that, as far our knowledge goes, studies involving the discrimination of Meliponini bee populations using CHC profiles are scarce and analyzed samples from few localities (Francisco et al. 2008; Ferreira-Caliman et al. 2012). It makes this the first study that analyzed a higher number of populations. As the results indicate, CHC can be considered a good marker to discriminate different bee populations and also be useful to track the geographic origin of samples. Another interesting fact here is that the majority of the compounds that influenced the discrimination of the groups are unsaturated CHC. This group is typically associated with derived functions, like nest mates, recognition, communication and others (Gibbs 2002; Châline et al. 2005).
The three markers are also in agreement in demonstrating the influence of the physiographic features of the sampling sites in the clustering of groups. The agreement between physiographic features and species differentiation has already been reported by other studies using mtDNA and geometric morphometrics of wings (Francisco et al. 2008; Francoy et al. 2011, 2016; Combey et al. 2013; Bonatti et al. 2014; Lima et al. 2014; Hurtado-Burillo et al. 2016). This influence may be due to some environmental effects on the heritability of the patterns of wing venation, which can vary between different parts of the wings (Monteiro et al. 2002). Eventual advantages of some haplotypes in similar physiographic regions may also be an explanation (Francoy et al. 2011, Bonatti et al. 2014). For the CHC, this influence is clearer, since environmental factors, such as temperature, diet and floral resources, have a direct impact over CHC (Gibbs 1998; Martin et al. 2012; Kather and Martin 2012). This significant influence may also be responsible for the lack of correlation between geographic and chemical distances among the studied populations.
On the other hand, the morphometric and molecular distances presented positive correlations with the geographic distance, indicating an isolation process caused by the geographic distances among the sampling sites (Telles and Diniz-Filho 2005). This fact may be due to specific characteristics of bees from Meliponini tribe. These bees are monandric, and this fact influences the genetic structure of the population (Peters et al. 1999). They also present strong queen philopatry, mainly due to the behavior of the colony during the swarming process (Nogueira-Neto 1997). In this process, the new queen leaves the original colony with some workers (Nogueira-Neto 1954). They nest in a location close enough to keep in touch with the mother colony, getting resources from it for a short period (Nogueira-Neto 1997). The small body size of M. caerulea workers works as a limiting factor in the flight range. For example, small bees such as workers of Tetragonisca angustula, Scaura latitarsis and Nannotrigona testaceicornis, which are approximately the same size of a M. caerulea worker, have their maximum flight distances between 621 and 951 m (Araújo et al. 2004). This short range of flight, together with the strong habitat fragmentation in South Brazil and the reduced capability of dispersion by the queens, is probably the main factor that determines the current population structuration.
M. caerulea is a stingless bee that nests exclusively underground, and its distribution is congruent to Pampa biome. Because of its nesting habits, M. caerulea populations do not suffer the effects of colony transportation since they are not reared for beekeeping as bees of the Melipona genus. Additionally, Pampa biome has been reduced due to urbanization and intense agriculture activity. Together with a low dispersion capability, our results suggest that the sampling sites are distinct and the structuration found here is directly influenced by geographical distance. The presence of exclusive haplotypes for each location and different morphometric and biochemical profiles indicate the need for particular attention for all occurrence area of M. caerulea, once the loss of any of these regions might correspond to a substantial negative impact on the diversity of this species.
As a final observation, all three markers seem to be adequate to evaluate population variability in bees and also to address specific questions regarding the relationships among these populations. Each one of them has its advantages and limitations, and they should be analyzed as such. However, the conjunction of the results may also indicate a broader picture, and it is worthy, in our opinion, to try to look at them all together.
References
Araújo, E. D., Costa, M., Chaud-Netto, J., Fowler, H. G. (2004) Body size and flight distance in stingless bees (Hymenoptera: Meliponini): inference of flight range and possible ecological implications. Braz. J. Biol. 64 (3B), 563–568
Arias, M. C., Francisco, F. D. O., Silvestre, D., Mello, G. A. R., Alves-dos-Santos, I. (2003) O DNA mitocondrial em estudos populacionais e evolutivos de meliponíneos, in: Mello, G.A.R. and Alves-dos-Santos, I. (Eds.),Apoidea Neotropica. Universidade do Extremo Sul Catarinense, Criciúma, pp. 305–309
Bandelt, H. J., Forster, P., Röhl, A. (1999) Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16 (1), 37–48
Batalha-Filho, H., Waldschmidt, A. M., Campos, L. A., Tavares, M. G., Fernandes-Salomão, T. M. (2010) Phylogeography and historical demography of the neotropical stingless bee Melipona quadrifasciata (Hymenoptera, Apidae): incongruence between morphology and mitochondrial DNA. Apidologie. 41 (5), 534–547
Bonatti, V., Simões, Z. L. P., Franco, F. F., Francoy, T. M. (2014) Evidence of at least two evolutionary lineages in Melipona subnitida (Apidae, Meliponini) suggested by mtDNA variability and geometric morphometrics of forewings. Naturwissenschaften. 101 (1), 17–24
Brito, R. M., Arias, M. C. (2010) Genetic structure of Partamona helleri (Apidae, Meliponini) from Neotropical Atlantic rainforest. Insect. Soc. 57 (4), 413–419.
Brito, R. M., Francisco, F. D. O., Françoso, E., Santiago, L. R., Arias, M. C. (2013) Very low mitochondrial variability in a stingless bee endemic to cerrado. Genet. Mol. Biol. 36 (1), 124–128
Camargo, J. M. F., Pedro, S. R. M (2013) Meliponini Lepeletier, 1836. In Moure, J. S., Urban, D., Melo, G. A. R. (Orgs). Catalogue of bees (Hymenoptera, Apoidea) in the Neotropical Region—online version. [online] http://www.moure.cria.org.br/catalogue (acessed on 22 March 16)
Camargo, J. M., Wittmann, D. (1989) Nest architecture and distribution of the primitive stingless bee, Mourella caerulea (Hymenoptera, Apidae, Meliponinae): Evidence for the origin of Plebeia (s. lat.) on the Gondwana continent. Stud. Neotrop. Fauna E. 24 (4), 213–229
Châline, N., Sandoz, J. C., Martin, S. J., Ratnieks, F. L., Jones, G. R. (2005) Learning and discrimination of individual cuticular hydrocarbons by honeybees (Apis mellifera). Chem. Senses. 30 (4), 327–335
Combey, R., Teixeira, J. S. G., Bonatti, V., Kwapong, P., Francoy, T. M. (2013) Geometric morphometrics reveals morphological differentiation within four African stingless bee species. Ann. Biol. Res. 4 (11), 93–103
Drummond, A. J., Ashton, B., Buxton, S., Cheung, M., Cooper, A., Duran, C., Moir, R. (2011) Geneious v5. 4.
Edgar, R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 (5), 1792–1797
Excoffier, L., Lischer, H. E. (2010). Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol Resour. 10 (3), 564–567.
Falcón, T., Ferreira-Caliman, M. J., Nunes, F. M. F., Tanaka, É. D., do Nascimento, F. S., Bitondi, M. M. G. (2014) Exoskeleton formation in Apis mellifera: cuticular hydrocarbons profiles and expression of desaturase and elongase genes during pupal and adult development. Insect Biochem. Molec. 50, 68–81
Ferreira-Caliman, M. J., Zucchi, R., & Nascimento, F. S. (2012). Cuticular hydrocarbons discriminate distinct colonies of Melipona marginata (Hymenoptera, Apinae, Meliponini). Sociobiology. 59 (3), 783
Fortes, A. B. (1979) Compêndio de Geografia do Rio Grande do Sul. Ed. Livraria Sulina, Porto Alegre.
Francisco, F. O., Arias, M. C. (2010) Inferences of evolutionary and ecological events that influenced the population structure of Plebeia remota, a stingless bee from Brazil. Apidologie. 41 (2), 216–224
Francisco, F. O., Nunes-Silva, P., Francoy, T. M., Wittmann, D., Imperatriz-Fonseca, V. L., Arias, M. C., Morgan, E. D. (2008) Morphometrical, biochemical and molecular tools for assessing biodiversity. An example in Plebeia remota (Holmberg, 1903)(Apidae, Meliponini). Insect. Soc. 55(3), 231–237
Francisco, F. O., Santiago, L. R., Arias, M. C. (2013) Molecular genetic diversity in populations of the stingless bee Plebeia remota: A case study. Genet. Mol. Biol. 36 (1), 118–123
Françoso, E., Arias, M. C. (2013) Cytochrome c oxidase I primers for corbiculate bees: DNA barcode and mini-barcode. Mol. Ecol. Resour. 13(5), 844–850
Francoy, T. M., Imperatriz-Fonseca, V. L. (2010) A morfometria geométrica de asas e a identificação automática de espécies de abelhas. Oecologia Australis. 14 (1), 317–321
Francoy, T. M., Grassi, M. L., Imperatriz-Fonseca, V. L., de Jesús May-Itzá, W., Quezada-Euán, J. J. G. (2011) Geometric morphometrics of the wing as a tool for assigning genetic lineages and geographic origin to Melipona beecheii (Hymenoptera: Meliponini). Apidologie. 42 (4), 499–507
Francoy, T. M., Bonatti, V., Viraktamath, S., Rajankar, B. R. (2016) Wing morphometrics indicates the existence of two distinct phenotypic clusters within population of Tetragonula iridipennis (Apidae: Meliponini) from India. Insec. Soc. 63 (1), 109–115
Gibbs, A. G. (1998) Water-proofing properties of cuticular lipids. Am. Zool. 38 (3), 471–482
Gibbs, A. G. (2002) Lipid melting and cuticular permeability: new insights into an old problem. J. Insect Physiol. 48 (4), 391–400
Halinski, R., Dorneles, A. L., Blochtein, B. (2015) Bee assemblage in habitats associated with Brassica napus L. Rev. Bras. Entomol. 59 (3), 222–228
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 4 (1), 1–9
Howard, R. W. Blomquist, G. J. (2005) Ecological, Behavioral, and Biochemical Aspects of Insect Hydrocarbons. Annu. Rev. Entomol. 50, 371–393
Hurtado-Burillo, M., Jara, L., May-Itzá, W. J., Quezada-Euán, J. J. G., Ruiz, C., De la Rúa, P. (2016). A geometric morphometric and microsatellite analyses of Scaptotrigona mexicana and S. pectoralis (Apidae: Meliponini) sheds light on the biodiversity of Mesoamerican stingless bees. J. Insect Conserv. 20(5), 753–763.
Kather, R., Martin, S. J. (2012) Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol. Entomol. 37(1), 25–32
Klingenberg, C. P. (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11(2), 353–357
Klingenberg, C. P., McIntyre, G. S. (1998) Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution. 52 (5), 1363–1375
Librado, P., Rozas, J. (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25 (11), 1451–1452
Lima, C. B., Nunes, L.A., Ribeiro, M.F., Carvalho, C. A. (2014) Population structure of Melipona subnitida Ducke (Hymenoptera: Apidae: Meliponini) at the southern limit of its distribution based on geometric morphometrics of forewings. Sociobiology. 61(4), 478–482
Martin, S. J., Shemilt, S., Drijfhout, F. P. (2012) Effect of time on colony odour stability in the ant Formica exsecta. Naturwissenschaften. 99 (4), 327–331
Miller, M.P. (1997) Tools for population genetic analyses (TFPGA): A windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author. http://www.marksgeneticsoftware.net/tfpga.htm. Accessed 30 Nov 2016
Ministério do Meio Ambiente (2012) Monitoramento dos Biomas Brasileiros: Bioma Pampa [online] http://www.mma.gov.br/estruturas/182/_arquivos/pampa2002_2009_182.pdf (acessed on 22 March 16)
Monteiro, L. R., Diniz-Filho, J.A.F., Reis, S.F., Araújo, E.D. (2002) Geometric estimates of heritability in biological shape. Evolution. 56 (3) 563–572
Nei, M., Kumar, S. (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford.
Nogueira-Neto, P. (1954) Notas bionômicas sobre meliponíneos: III–Sobre a enxameagem. Arq. Mus. Nac. 42, 419–451.
Nogueira-Neto, P. (1997) Vida e criação de abelhas indígenas sem ferrão. Editora Nogueirapis, São Paulo.
Peters, J. M., Queller, D. C., Imperatriz-Fonseca, V. L., Roubik, D. W., Strassmann, J. E. (1999) Mate number, kin selection and social conflicts in stingless bees and honeybees. P. Roy. Soc. Lond. B Bio. 266 (1417), 379–384
Polzin, T., Daneshmand, S. V. (2003) On Steiner trees and minimum spanning trees in hypergraphs. Oper. Res. Lett. 31(1), 12–20
Roesch, L. F. W., Vieira, F. C. B., Pereira, V. A., Schünemann, A. L., Teixeira, I. F., Senna, A. J. T., Stefenon, V. M. (2009) The Brazilian Pampa: a fragile biome. Diversity. 1 (2), 182–198.
Rohlf, F. J. (2008) TPSdig, v. 2.12. State University at Stony Brook, New York
Rohlf, F. J. (2009) tps Util v. 1.40. Ecology & Evolution. Stony Brook University, New York
Silveira, F. A., Melo, G. A., Almeida, E. A. (2002) Abelhas brasileiras: sistemática e identificação. Silveira, F.A., Belo Horizonte
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., Flook, P. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87 (6), 651–701
Suzuki, R., Shimodaira, H. (2006) Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 22(12), 1540–1542
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28 (10), 2731–2739
Telles, M. P.C., Diniz-Filho, J. A. F. (2005) Multiple Mantel tests and isolation-by-distance, taking into account long-term historical divergence. Genet. Mol. Res. 4 (4), 742–748
Walsh, P. S., Metzger, D. A., Higuchi, R. (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 10(4), 506–513.
Witter, S., Blochtein, B. (2003) Efeito da polinização por abelhas e outros insetos na produção de sementes de cebola. Pesq. Agropec. Bras. 38 (12), 1399–1407
Wittmann, D., Hoffmann, M. (1990) Bees of Rio Grande do Sul, Southern Brazil (Insecta, Hymenoptera, Apoidea). Iheringia Ser. Zool. 70, 17–43
Acknowledgments
Thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support (process number 2011/07857-9) to TMF and the fellowship provided to JSGT (process number 2011/02434-2). Thanks to Professor Fábio Nascimento who allowed us to use the GC/MS machine and Professor Zilá L. P. Simões for the support on molecular analysis.
Author information
Authors and Affiliations
Contributions
JSGT data acquisition, data analysis and wrote the paper. TMF supervisor and wrote the paper. SW data acquisition. MJFC and TF data analysis. All authors read and approved the final manuscript.
Corresponding author
Additional information
Manuscript editor: Yves Le Conte
Des analyses morphologiques, chimiques et moléculaires permettent la différenciation des populations à nidification souterraine sans aiguillon de l’abeille Mourella caerulea (Apidae: Meliponini)
abeilles sans aiguillon / Mourella caerulea / morphométrie géométrique / ADN mitochondrial / hydrocarbures cuticulaires
Morphologische, chemische und molekulare Analysen ermöglichen die Unterscheidung von Populationen der unterirdisch nistenden stachellosen Biene Mourella caerulea (Apidae: Meliponini)
Stachellose Bienen / Mourella caerulea / geometrische Morphometrie / mitochondriale DNA / kutikuläre Kohlenwasserstoffe
Rights and permissions
About this article
Cite this article
Galaschi-Teixeira, J.S., Falcon, T., Ferreira-Caliman, M.J. et al. Morphological, chemical, and molecular analyses differentiate populations of the subterranean nesting stingless bee Mourella caerulea (Apidae: Meliponini). Apidologie 49, 367–377 (2018). https://doi.org/10.1007/s13592-018-0563-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-018-0563-5