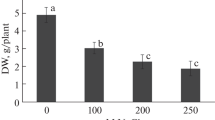
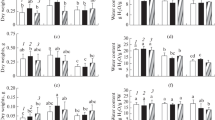
Abstract
This study aimed to better understand the limited natural distribution of the endangered ornamental plant Amsonia orientalis Decne. by focusing on salt stress, a common limiting factor of plant growth. Plants were subjected to in vitro salt stress at concentrations between 25 and 150 mM. In general, shoot and root lengths, root number, and total protein, chlorophyll a and carotenoid content were negatively influenced at NaCl concentrations above 25 mM. Hydrogen peroxide, malondialdehyde and proline content all gradually increased with increasing salt concentration. Activity levels of the antioxidant enzymes catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) were all at their maximum in plants cultured in medium containing 50 mM NaCl. Compared to control cultures, an overall upward trend in POD activity was observed with increasing salt concentration, while the activity levels of SOD and CAT increased at lower concentrations but were limited at elevated concentrations of NaCl. These results suggest that A. orientalis prefers soils with no or very low salt but can tolerate NaCl up to a concentration of 50 mM.
Similar content being viewed by others
Literature Cited
Abogadallah GM (2010) Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Acemi A, Özen F, Kiran R (2012) Development of an efficient callus production protocol for Amsonia orientalis: A critically endangered medicinal plant. Eurasia J Biosci 6:105–112
Acemi A, Özen F, Kıran R (2013) In vitro propagation of Amsonia orientalis Decne. from nodal segments of adult plants. Propag Ornam Plants 13:25–32
Aebi H (1974) Methods of enzymatic analysis. In Bergmeyer HU, Ed, Catalase. Academic Press, New York, pp 673–675
Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48:555–560
Amira MS, Qados A (2011) Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J Saudi Soc Agri Sci 10:7–15
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bern Convention (1979) Convention on the conservation of European wildlife and natural habitats. Available via https://rm.coe.int/CoERMPublicCommonSearchServices/DisplayDCTMCo ntent?documentId=0900001680304354 Accessed 03 January 2016
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Çelik Ö, Atak Ç (2012) The effect of salt stress on antioxidative enzymes and proline content of two Turkish tobacco varieties. Turk J Biol 36:339–356
Chen C, Tao C, Peng H, Ding Y (2007) Genetic analysis of salt stress responses in asparagus bean (Vigna unguiculata L. ssp. sesquipedalis Verdc.). J Hered 98:655–665
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Doupis G, Chartzoulakis K, Beis A, Patakas A (2011) Allometric and biochemical responses of grapevines subjected to drought and enhanced ultraviolet-B radiation. Aust J Grape Wine Res 17:36–42
Ellouzi H, Ben HK, Cela J, Munné-Bosch S, Abdelly C (2011) Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol Plant 142:128–143
Esfandiari E, Shekari F, Shekari F, Esfandiari M (2007) The effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not Bot Horti Agrobot 35:48–56
Galvan-Ampudia CS, Testerink C (2011) Salt stress signals shape the plant root. Curr Opin Plant Biol 14:296–302
Gechev TS, Van Brausegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Gürkanlı CT, Özkoç İ, Aydın EB, Acemi A, Özen F (2014) Genetic diversity of Amsonia orientalis. Biologia 69:742–749
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179
Halperin JH, Kochian LV, Lynch JP (1997) Salinity stress inhibits calcium loading into the xylem of excised barley (Hordium vulgare) roots. New Phytol 135:419–427
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Huang Z, Zhao L, Chen D, Liang M, Liu Z, Shao H, Long X (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 8(4):e62085
Husen A, Iqbal M, Aref IM (2016) IAA-induced alteration in growth and photosynthesis of pea (Pisum sativum L.) plants grown under salt stress. J Environ Biol 37:42–429
Kar M, Mishra D (1976) Catalase, peroxidase, polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Koca H, Bor M, Özdemir F, Türkkan İ (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Lichtenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mattioni C, Lacerenza NG, Troccoli A, De Leonardis AM, Di Fonzo N (1997) Water and salt stress-induced alterations in proline metabolism of Triticum durum seedlings. Physiol Plant 101:787–792
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 52:17–26
Moghaieb REA, Saneoka H, Fujita K (2004) Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophyte plants, Salicornia europaea and Suaeda maritima. Plant Sci 166:1345–1349
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Neto ADA, Prisco JT, Enéas-Filho J, Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Niknam V, Meratan AA, Ghaffari SM (2011) The salt stress on lipid peroxidation and antioxidative enzymes in callus of two Acanthophyllum species. In Vitro Cell Dev Biol Plant 47:297–308
Noreen Z, Ashraf M (2009a) Changes in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environ Exp Bot 67:395–302
Noreen Z, Ashraf M (2009b). Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol 166:1764–1774
Özen F (2006) Autoecology of a species being endangered in Turkey: Amsonia orientalis Decne. (Apocynaceae). Journal of Balikesir University Institute of Science and Technology 8:4–9
Sibole JV, Cabot C, Poschenrieder C, Barceló J (2003) Efficient leaf ion partitioning, an overriding condition for abscisic acid-controlled stomatal and leaf growth responses to NaCl salinization in two legumes. J Exp Bot 54:2111–2119
Shu S, Yuan LY, Guo SR, Sun J, Yuan YH (2013) Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem 63:209–216
Stone JR, Yang S (2006) Hydrogen peroxide: A signaling messenger. Antioxid Redox Signal 8:243–270
Tort N, Türkyılmaz B (2004) A physiological investigation on the mechanisms of salinity tolerance in some barley culture forms. EGE UNIVERSITY JOURNAL OF THE FACULTY OF SCIENCE 27:1–16
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Zhu JK, Hasegawa PM, Bressan RA (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16:253–277
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acemi, A., Duman, Y., Karakuş, Y.Y. et al. Analysis of plant growth and biochemical parameters in Amsonia orientalis after in vitro salt stress. Hortic. Environ. Biotechnol. 58, 231–239 (2017). https://doi.org/10.1007/s13580-017-0215-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-017-0215-0