Abstract
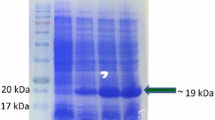
Enormous economic losses caused by infection of peppers with Cucumber mosaic virus (CMV) have spurred the development of CMV-resistant peppers using genetic techniques. However, growing concern from the general public over genetically modified (GM) crops has focused attention on safety and labeling issues. In this study, we developed and optimized a qualitative and quantitative enzyme-linked immunosorbent assay (ELISA) to detect CMV-resistant GM peppers. Two types of antibodies, virion-derived polyclonal antibody (VPAb) prepared from a CMV-Fny virion-immunized rabbit and peptide-based polyclonal antibody (PPAb) prepared from rabbits immunized with a peptide fragment of CMVcoat protein (CMV-CP) (LPDSVTEYDKKLVSR) predicted from the nucleotide sequence were prepared, and their affinities were compared. Optimized ELISAs using VPAb and PPAb as the primary antibodies, respectively, were carried out to measure the level of CMV-CP in GM peppers cultivated at an officially approved facility in Korea. Color development (reflecting CMV-CP levels) was 2.5-fold higher when PPAb was used as the primary antibody compared to VPAb as the primary antibody. No statistical differences were observed among GM pepper cultivars. These results imply that PPAb has higher specificity for CMV-CP than VPAb does, and that the analytical system presented in this study can be used to evaluate the level of CMV-CP in genetically modified peppers.
Similar content being viewed by others
Literature Cited
Agindotan, B.O., G. Thottappilly, A. Uwaifo, and S. Winter. 2003. Production of monoclonal and polyclonal antibodies against a Nigerian isolate of banana streak virus. Afr. J. Biotechnol. 2:171–178.
Bang, S.N., Y.S. Jung, S.J. Eom, G.B. Kim, K.H. Chung, G.P. Lee, D.Y. Son, K.W. Park, J.S. Hong, K.H. Ryu, and C. Lee. 2011. Assessment of the cucumber mosaic virus coat protein by expression evaluation in a genetically modified pepper and Esherichia coli BL21. J. Food. Biochem. 36:432–440.
Blystad, D.R. 2014. Diagnosis of plant viruses with emphasis on the ELISA-method. http://apfmo.org/dokument/Ana/Mostar_sept%202009.pdf. Accessed 10 April 2014.
Bradford, M.M. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of proteindye-binding. Anal. Biochem. 72:248–254.
Brent, P., D. Bittisnich, S. Brooke-Taylor, N. Galway, L. Graf, M. Healy, and L. Kelly. 2003. Regulation of genetically modified foods in Australia and New Zealand. Food Control 14:409–416.
Cai, W.Q., R.X. Fang, H.S. Shang, X. Wang, F.L. Zhang, Y.R. Li, J.C. Zhang, X.Y. Cheng, G.L. Wang, and K.Q. Mang. 2003. Development of CMV- and TMV-resistant transgenic chili pepper: field performance and biosafety assessment. Mol. Breed. 11:25–35.
Castro, S.M., J.A. Saraiva, F.M.J. Domingues, and I. Delgadillo. 2011. Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsium annum L.). LWT-Food Sci. Technol. 44:363–369.
Chen, Z.L., H. Gu, Y. Li, Y. Su, P. Wu, Z. Jiang, X. Ming, J. Tian, N. Pan, and L.J. Qu. 2003. Safety assessment for genetically modified sweet pepper and tomato. Toxicology 188:297–307.
Clark, M.F. and A.N. Adams. 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 34:475–483.
Cunha, M.G., T.S. Medina, S.G. Oliveira, A.N. Marinho, M.M. Povoa, and A.K.C. Ribeiro-dos-Santos. 2009. Development of a polymerase chain reaction (PCR) method based on amplification of mitochondrial DNA to detect Plasmodium falciparum and Plasmodium vivax. Acta Trop. 111:35–38.
Hidayat, S.H., D. Haryadi, and E. Nurhayati. 2009. Use of serologicalbased assay for the detection of pepper yellow leaf curl Indonesia virus. Plant Pathol. J. 25:328–332.
Holdenrieder, S., P. Stieber, L.Y.S. Chan, S. Geiger, A. Kremer, D. Nagel, and Y.M. Dennis. 2005. Cell-free DNA in serum and plasma: Comparison of ELISA and quantitative PCR. Clin. Chem. 51:1544–1546.
Holst-Jensen, A., Y. Bertheau, Md. Loose, L. Grohmann, S. Hamels, L. Hougs, D. Morisset, S. Pecoraro, M. Pla, M.Vd. Bulcke, and D. Wulff. 2012. Detecting un-authorized genetically modified organisms (GMOs) and derived materials. Biotechnol. Adv. 30:1318–1335.
Janknecht, R., G. Martynoff, J. Lou, R.A. Hipskind, A. Nordheim, and H.G. Stunneberg. 1991. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc. Natl. Acad. Sci. USA. 88:8972–8976.
Jensen, T.H., G. Ajjouri, K.J. Handberg, M.J. Slomka, V.J. Coward, V.J. Cherbonnel, L. Peter, and P.H. Jorgensen. 2013. An enzyme-linked immunosorbent assay for detection of avian influenza virus subtypes H5 and H7 antibodies. Acta Vet. Scand. 55:84.
Kazutoyo, M., C.O. Andrew, O.V. Muratova, L.H. Miller, A. Saul, and C.A. Long. 2008. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria caccines. Vaccine 26:193–200.
Lanfermeijer, F.C., J. Dijkhuis, M.G.J. Sturre, Pd. Haan, and J. Hille. 2003. Cloning and characterization of the durable tomato mosaix virus resistance gen Tm-22 from Lycopersicon esculentum. Plant Mol. Biol. 52:1037–1049.
Lee, H.I., J.H. Kim, and M.C. Yea. 2010. Immunocapture RT-PCR for detection of seed-borne viruses on Cucurbitaceae crops. Res. Plant Dis. 16:121–124.
Lee, Y.H., M. Jung, S.H. Shin, J.H. Lee, S.H. Choi, N.M. Her, J.H. Lee, K.H. Ryu, K.Y. Paek, and C.H. Harn. 2009. Transgenic peppers that are highly tolerant to a new CMV pathotype. Plant Cell Rep. 28:223–232.
Lee, Y.H., H.S. Kim, J.Y. Kim, M. Jung, Y.S. Park, J.S. Lee, S.H. Choi, N.H. Her, J.H. Lee, N.I. Hyung, C.H. Lee, S.G. Yang, and C.H. Harn. 2004. A new selection method for pepper transformation: callus-mediated shoot formation. Plant Cell Rep. 23:50–58.
Loughran, S.T. and D. Walls. 2011. Purification of poly-histisine-tagged proteins. Methods Mol. Biol. 681:311–335.
Manns, J.M. 2011. SDS-Polyacrylmide gel electrophoresis (SDS-PAGE) of proteins. Curr. Protoc. Microbiol. 22:A.3M.1-A.3M.13. DOI: 10.1002/9780471729259.mca03ms22
Mekuria, G., S.A. Ramesh, E. Alberts, T. Bertozzi, M. Wirthensohn, G. Collins, and M. Sedgley. 2003. Comparison of ELISA and RT-PCR for the detection of prunus necrotic ring spot virus and prune dwarf virus in almond (Prumusdulcis). J. Virol. Methods 114:65–69.
Meredith, G.D., H.Y. Wu, and N.L. Allbritton. 2004. Targeted protein functionalization using His-tags. Bioconjugate Chem. 15:969–982.
Plagemann, P.G.W. 2004. The primary GP5 neutralization epitope of North American isolates of porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunop. 102:265–278.
Quemada, H.D., D. Gonsalves, and J.L. Slightom. 1991 Expression of coat protein gene from cucumber mosaic virus strain C in tobacco: Protection against infections by CMV strains transmitted mechanically or by aphids. Phytopathology 81:794–802.
Ryu, H.J., J.S. Kim, K.Y. Kim, B.R. Nam, M.J. Nam, W.B. Shim, N.S. Kim, Y,J. Cho, and D.H. Chung. 2010. Production of monoclonal antibody against Escherichia coli O157:H7 and development of enzyme linked immunosorbent assay. Kor. J. Food Sci. Technol. 42:329–334.
Seo, P.S., S.Y. Heo, E.J. Han, J.W. Seo, S.H. Ghim, and C.H. Kim. 2009. Bacterial expression and purification of human papillomavirus type 18 L1. Biotechnol. Bioproc. Eng. 14:168–174.
Sun, W., K. Jiao, S. Zhang, C. Zhang, and Z. Zhang. 2001. Electrochemical detection for horseradish peroxidase-based enzyme immunoassay using p-amino phenol as substrate and its application in detection of plant virus. Anal. Chim. Acta 434:43–50.
Talukder, Y., R. Gopal, N. Andrews, M. Glenn, J. Breurer, and D. Brown. 2005. Development and evaluation of varicella zoster virus ELISA for oral fluid suitable for epidemiological studies. J. Virol. Methods 128:162–167.
Xie, Y., X. Jiao, X. Zhou, H. Liu, Y. Ni, and J. Wu. 2013. Highly sensitive serological methods for detecting tomato yellow leaf curl virus in tomato plants and whiteflies. Virol. J. 10:142.
Yu, C., J. Wu, and X. Zhou. 2005. Detection and subgrouping of cucumber mosaic virus isolates by TAS-ELISA and immunocapture RT-PCR. J. Virol. Methods 123:155–161.
Zein, H.S., J.A. Teixeira da Silve, and K. Miyatake. 2009a. Antigenic properties of the coat of cucumber mosaic virus using monoclonal antibodies. J. Virol. Methods 162:223–230.
Zein, H.S., J.A. Teixeira da Silve, and K. Miyatake. 2009b. Monoclonal antibodies specific to cucumber mosaic virus coat protein possess DNA-hydrolyzing activity. Mol. Immunol. 46:1527–1533.
Author information
Authors and Affiliations
Corresponding author
Additional information
These author contributed equally to this work.
Rights and permissions
About this article
Cite this article
Choi, J., Phat, C., Kim, E. et al. Improved detection of Cucumber mosaic virus coat protein (CMV-CP) in genetically modified pepper (Capsicum annuum) using a polyclonal antibody to a synthetic CP peptide. Hortic. Environ. Biotechnol. 56, 316–323 (2015). https://doi.org/10.1007/s13580-015-0139-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-015-0139-5