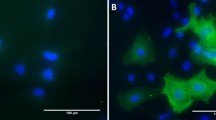
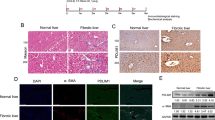
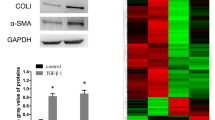
Abstract
Liver fibrosis is a pathological response driven by the activation of hepatic stellate cell (HSC). However, the mechanisms of liver fibrosis and HSC activation are complicated and far from being fully understood. We aimed to explore the candidate genes involved in HSC activation during liver fibrogenesis. Five genes (LBH, LGALS3, LOXL1, S100A6 and SPP1) were recurrent in the DEGs derived from the seven datasets. The expression of these genes gradually increased as liver fibrosis staging advanced, suggesting they might be candidate genes involved in HSC activation during hepatic fibrosis. These candidate genes were predicted to be coregulated by miRNAs such as hsa-miR-125a-5p and has-miR-125b, or by transcription factors including JUN, USF1, TP53 and TFAP2C. PPI analysis showed that LGALS3, LOXL1, S100A6 and SPP1 might interact with each other indirectly, but no interaction was found between them and LBH. The candidate genes and their interaction partners were enriched in focal adhesion, extracellular matrix organization and binding. Upregulation of LBH, S100A6 and SPP1 were further validated in TGF-β-treated LX-2 as well as in DDC or CCL4-treated mice models. Decreased LBH and SPP1 expression reduces the expression of HSC activation-related markers in TGF-β-treated LX-2. Our results indicated that LBH, LGALS3, LOXL1, S100A6 and SPP1 were candidate genes which may participate in the HSC activation during liver fibrosis.







Similar content being viewed by others
Availability of data and materials
The data analyzed in this study are available from the corresponding author upon reasonable request. All the datasets analyzed during the current study can be deposited in GEO, the detailed information can see in Supplementary Table 1.
Abbreviations
- HSCs:
-
Hepatic stellate cells
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially expressed genes
- PPI:
-
Protein–protein interaction
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- CCL4 :
-
Carbon tetrachloride
- DDC:
-
3,5-Diethoxycarbonyl-1,4-dihydrocollidine
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- LBH:
-
Limb bud and heart
- LGALS3:
-
Galectin 3
- LOXL1:
-
Lysyl oxidase like 1
- S100A6:
-
S100 calcium-binding protein A6
- SPP1:
-
Secreted phosphoprotein 1
References
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. https://doi.org/10.1016/j.mam.2018.09.002.
Dhar D, et al. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med (Maywood). 2020;245(2):96–108. https://doi.org/10.1177/1535370219898141.
Wang ZY, et al. Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis. Sci Rep. 2021;11(1):19396. https://doi.org/10.1038/s41598-021-98806-y.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–66. https://doi.org/10.1038/s41575-020-00372-7.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411. https://doi.org/10.1038/nrgastro.2017.38.
Kocabayoglu P, et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63(1):141–7. https://doi.org/10.1016/j.jhep.2015.01.036.
Yang L, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146(5):1339-50.e1. https://doi.org/10.1053/j.gastro.2014.01.061.
Guangcun H, et al. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci. 2012;17(7):2495–507.
Seki E, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–32. https://doi.org/10.1038/nm1663.
Meng F, et al. Interleukin-17 signaling in inflammatory, Kupffer Cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(3):765-776.e3. https://doi.org/10.1053/j.gastro.2012.05.049.
Tai Y, et al. Identification of miRNA-target gene regulatory networks in liver fibrosis based on bioinformatics analysis. PeerJ. 2021;9:e11910. https://doi.org/10.7717/peerj.11910.
Kim JY, et al. Induction of E6AP by microRNA-302c dysregulation inhibits TGF-beta-dependent fibrogenesis in hepatic stellate cells. Sci Rep. 2020;10(1):444. https://doi.org/10.1038/s41598-019-57322-w.
Chen L, et al. Delta-like ligand 4/DLL4 regulates the capillarization of liver sinusoidal endothelial cell and liver fibrogenesis. Biochim Biophys Acta Mol Cell Res. 2019;1866(10):1663–75. https://doi.org/10.1016/j.bbamcr.2019.06.011.
Guo Y, et al. TGF-beta/YB-1/Atg7 axis promotes the proliferation of hepatic progenitor cells and liver fibrogenesis. Biochim Biophys Acta Mol Basis Dis. 2022;1868(1):166290. https://doi.org/10.1016/j.bbadis.2021.166290.
Xia P, et al. Therapeutic effects of recombinant human S100A6 and soluble receptor for advanced glycation end products(sRAGE) on CCl(4)-induced liver fibrosis in mice. Eur J Pharmacol. 2018;833:86–93. https://doi.org/10.1016/j.ejphar.2018.05.030.
Ashad-Bishop K, et al. Loss of Limb-Bud-and-Heart (LBH) attenuates mammary hyperplasia and tumor development in MMTV-Wnt1 transgenic mice. Biochem Biophys Res Commun. 2019;508(2):536–42. https://doi.org/10.1016/j.bbrc.2018.11.155.
Deng M, et al. Limb-bud and heart attenuates growth and invasion of human lung adenocarcinoma cells and predicts survival outcome. Cell Physiol Biochem. 2018;47(1):223–34. https://doi.org/10.1159/000489801.
Ekwall AK, et al. The rheumatoid arthritis risk gene LBH regulates growth in fibroblast-like synoviocytes. Arthritis Rheumatol. 2015;67(5):1193–202. https://doi.org/10.1002/art.39060.
Krenkel O, et al. Single cell RNA sequencing identifies subsets of hepatic stellate cells and myofibroblasts in liver fibrosis. Cells. 2019;8(5):503. https://doi.org/10.3390/cells8050503.
Dong XH, et al. S100 calcium binding protein A6 and associated long noncoding ribonucleic acids as biomarkers in the diagnosis and staging of primary biliary cholangitis. World J Gastroenterol. 2021;27(17):1973–92. https://doi.org/10.3748/wjg.v27.i17.1973.
Zhao W, et al. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt A):1129–37. https://doi.org/10.1016/j.bbadis.2018.01.019.
Yang A, et al. Hepatic stellate cells-specific LOXL1 deficiency abrogates hepatic inflammation, fibrosis, and corrects lipid metabolic abnormalities in non-obese NASH mice. Hepatol Int. 2021;15(5):1122–35. https://doi.org/10.1007/s12072-021-10210-w.
Song Z, et al. Osteopontin takes center stage in chronic liver disease. Hepatology. 2021;73(4):1594–608. https://doi.org/10.1002/hep.31582.
Nardo AD, et al. Impact of osteopontin on the development of non-alcoholic liver disease and related hepatocellular carcinoma. Liver Int. 2020;40(7):1620–33. https://doi.org/10.1111/liv.14464.
Zeng F, et al. Predicting non-alcoholic fatty liver disease progression and immune deregulations by specific gene expression patterns. Front Immunol. 2020;11:609900. https://doi.org/10.3389/fimmu.2020.609900.
Cui G, et al. Thrombin cleavage of osteopontin controls activation of hepatic stellate cells and is essential for liver fibrogenesis. J Cell Physiol. 2019;234(6):8988–97. https://doi.org/10.1002/jcp.27571.
Tian J, et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016;30(12):4202–13. https://doi.org/10.1096/fj.201600392RR.
Jiang JX, et al. Galectin-3 modulates phagocytosis-induced stellate cell activation and liver fibrosis in vivo. Am J Physiol Gastrointest Liver Physiol. 2012;302(4):G439–46. https://doi.org/10.1152/ajpgi.00257.2011.
Traber PG, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8(10):e75361. https://doi.org/10.1371/journal.pone.0075361.
Zhang Y, Yao X. Role of c-Jun N-terminal kinase and p38/activation protein-1 in interleukin-1β-mediated type I collagen synthesis in rat hepatic stellate cells. APMIS. 2012;120(2):101–7. https://doi.org/10.1111/j.1600-0463.2011.02816.x.
Schulien I, et al. The transcription factor c-Jun/AP-1 promotes liver fibrosis during non-alcoholic steatohepatitis by regulating Osteopontin expression. Cell Death Differ. 2019;26(9):1688–99. https://doi.org/10.1038/s41418-018-0239-8.
Zhuang S, et al. Inhibition of GSK-3β induces AP-1-mediated osteopontin expression to promote cholestatic liver fibrosis. FASEB J. 2018;32(8):4494–503. https://doi.org/10.1096/fj.201701137R.
Guo Q, et al. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71(6):1193–205. https://doi.org/10.1016/j.jhep.2019.07.019.
Mi XJ, et al. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem. 2019;67(5):1392–401. https://doi.org/10.1021/acs.jafc.8b05943.
Zhao XK, et al. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci Rep. 2017;7(1):4032. https://doi.org/10.1038/s41598-017-04317-0.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81970528), Shanghai Municipal Science and Technology Commission Research Project (22ZR1449700), and Shanghai General Hospital Start-up Fund (02.06.01.20.01). The funders had no role in the study design, data analysis, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
XC, HD and LL designed and conceived the study. WD and YG conducted the experiments. WD, YG, ZS and JW analyzed the data and prepared figures. WD and YG wrote the original draft. XC, HD and LL reviewed and edited the paper. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Ethical approval
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Shanghai General Hospital (2020AW075). All methods were executed out in compliance with relevant guidelines and regulations and reported in consistent with the ARRIVE guidelines for reporting animal experiments.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, W., Guo, Y., Shen, Z. et al. Identification of LBH and SPP1 involved in hepatic stellate cell activation during liver fibrogenesis. Human Cell 36, 1054–1067 (2023). https://doi.org/10.1007/s13577-023-00889-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00889-4