Abstract
The human monkeypox virus (MPV), a zoonotic illness that was hitherto solely prevalent in Central and West Africa, has lately been discovered to infect people all over the world and has become a major threat to global health. Humans unintentionally contract this zoonotic orthopoxvirus, which resembles smallpox, when they come into contact with infected animals. Studies show that the illness can also be transferred through frequent proximity, respiratory droplets, and household linens such as towels and bedding. However, MPV infection does not presently have a specified therapy. Smallpox vaccinations provide cross-protection against MPV because of antigenic similarities. Despite scant knowledge of the genesis, epidemiology, and ecology of the illness, the incidence and geographic distribution of monkeypox outbreaks have grown recently. Polymerase chain reaction technique on lesion specimens can be used to detect MPV. Vaccines like ACAM2000, vaccinia immune globulin intravenous (VIG-IV), and JYNNEOS (brand name: Imvamune or Imvanex) as well as FDA-approved antiviral medications such as brincidofovir (brand name: Tembexa), tecovirimat (brand name: TPOXX or ST-246), and cidofovir (brand name: Vistide) are used as therapeutic medications against MPV. In this overview, we provide an outline of the MPV’s morphology, evolution, mechanism, transmission, diagnosis, preventative measures, and therapeutic approaches. This study offers the fundamental information required to prevent and manage any further spread of this emerging virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monkeypox virus (MPV), a member of the Poxviridae family and the Orthopoxvirus (OPV) genus, is causative for the unusual zoonotic disease referred to as monkeypox. Genus OPV includes variola virus, cowpox, camelpox, vaccinia virus, and MPV, which can infect humans and cause serious diseases [1]. An unprecedented and abrupt epidemic of monkeypox disease in various countries around the world has suddenly arisen as a cause for alarm. Earlier cases of “monkey smallpox” were especially prevalent in Central and West Africa with similar symptoms to smallpox, but a milder infection that primarily presents as a high fever, headache, lymphadenopathy, and systemic pustules and blisters. Case mortality rates range from 1 to 10%. The current MPV outbreak is the biggest and most widespread non-endemic outbreak ever recorded. In contrast to prior outbreaks, there is no definite evidence tying the afflicted people to a common factor of virus exposure, including visiting an endemic region or getting into touch with infected animals. The vast majority of people affected are younger or middle-aged men who have sex with men (MSM) and have recently engaged in sexual activity with new or multiple partners. The virus tends to spread through intimate physical contact [2], albeit the transmission dynamics and route of infection are yet unknown. Patients who have been diagnosed are recommended to isolate, and their close contacts are tracked down. As of July 12, 2022, there have been no MPV-related fatalities reported in the non-endemic zones [3]. Given the unprecedented extent of human-to-human transmission and the sharp rise in cases, World Health Organization (WHO) raised the risk level of MPV to moderate, with a high-risk level in the European Region, which is home to more than 80% of all new MPV infections. In 110 countries, there are more than thousands of instances of monkeypox virus infection that have been verified as a result of this outbreak, which is rapidly spreading and affecting humans.
An international public health community-wide response has been sparked by these incidents [4]. Although a monkeypox infection can cause illness that is clinically identical to smallpox, it is thought to travel slowly, reducing the probability of a widespread, quickly spreading pandemic [5].
The major part of the 1960s was devoted to the academic study of MPV. As soon as it was known that MPV could infect people in areas that were believed to be free of smallpox, attitudes drastically changed. Worries that MPV would take over the area left by the variola virus (VARV) resulted from this. However, a WHO-led effort suggested that this was unlikely. Before the eradication of VARV, it was commonly accepted that human MPV infections existed, but were unrecognized because of smallpox. Although West African countries have historically seen attenuated human infections, the Congo Basin region of Africa has recorded the most serious human MPV infections. Despite indications of human-to-human transmission, interaction with animals infected with the MPV virus triggers the majority of human diseases (such as bush meat). An MPV outbreak occurred in 2003 as a result of imported West African rats carrying the MPX virus infecting native prairie dogs afterwards intended for sale as pets [6]. While human infections can begin in a variety of ways, and these distinct ways appear to affect how the disease presents clinically, no one has died from the virus and it is a less harmful West African variant. However, this incident demonstrated how quickly MPV can transcend the interspecies divide [7].
The ongoing MPV epidemic has drawn considerable interest from all across the world and is thought to pose a risk to larger populations. Despite evidence that the smallpox vaccination protects against MPV by 85%, inoculation against the smallpox virus has not been offered since the WHO declared the smallpox virus extinct in 1980 [7, 8]. Additionally, MPV-specific medications and vaccinations are lacking. Therefore, in this review article, we focused on different prospects of MPV including its morphology, evolution, mechanism, transmission, diagnosis, preventative measures, and therapeutic approaches.
Methodology
Using the terms “evolution of monkeypox virus”, “diagnosis of monkeypox virus”, “curative therapy of monkeypox virus”, “herbal-based antiviral characteristics”, and “computational research”, published literature over the past few decades on the monkeypox virus was collected from a variety of Internet-based sources including PubMed, SpringerLink, Wiley online library, Web of Science, ScienceDirect, and Google Scholar. This literature study includes book chapters, research papers, and reviews that were published.
History of MPV
Due to outbreaks of a pox-like infection in macaque monkeys housed at a Danish research facility in 1958, MPV was initially identified as the disease known as “monkeypox” [9]. A smallpox-like infection that hospitalized a 9-month-old boy in the Democratic Republic of the Congo on September 1, 1970, led to the isolation of a virus related to MPV [10]. In medical history, this represents the first instance of MPV in a person. The patient showed typical MPX signs, such as fever and a rash resembling chicken pox that developed into hemorrhagic lesions that crusted over and healed over the course of the next 2 weeks. Despite making an early recovery, the patient passed away in the hospital as a result of a secondary infection [11].
The first initial MPV case was reported in Nigeria in 1971, and ten MPV instances were documented from 1971 to 1978 [12]. In Central and West Africa, an increasing number of human cases have since been documented. Since 1970, monkeypox instances have been documented in people in 11 different African countries: Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, Cote d'Ivoire, Liberia, Nigeria, the Republic of the Congo, Sierra Leone, and the Democratic Republic of the Congo [13]. Monkeypox’s potential effects are unknown. As an illustration, the Democratic Republic of the Congo reported an epidemic in 1996–1997 that had a lower case–mortality ratio and a higher strike rate than usual [14]. In Nigeria, since 2017, there have been over 500 suspected cases, 200 confirmed cases, and a cumulative number of fatalities of roughly 3% [15]. MPX has recently been observed throughout North America and Europe. In at least 20 non-African countries since the first MPX cases were reported in Europe in early May 2022, there have been over 400 confirmed or suspected cases [16]. The relevance of monkeypox to global public health is demonstrated by the fact that it affects not only nations in West and Central Africa, but also the rest of the world. Monkeypox originally appeared outside of Africa in the USA. Dormice and pouched rats from Gambia were housed alongside these animals in Ghana. This outbreak caused approximately 70 cases of monkeypox to be documented in the USA [17]. Moreover, reports of monkeypox have been made in Singapore (May 2019), the USA (July and November 2021), the UK (September 2018, December 2019, May 2021, and May 2022), Israel (September 2018), and the USA (December 2019) [14]. The epidemiology, origins of infection, and mechanisms of transmission are the subject of current research.
Morphology and genome organization of MPV
Similar to other orthopoxviruses, MPV virions are architecturally structured lipoprotein transmembrane domains enclosing ovoid or brick-shaped particles. The known MPV size is about 200 by 250 nm [18], and therefore can be easily observable under a light microscope, while electron microscopy is needed to resolve ultra-resolution. The MPV virion comprised four major components—outer lipoprotein envelope, outer membrane, central core, and lateral bodies. The outer membrane which has several surface tubules encloses lateral bodies, palisade layer and the core [19]. The central core consists of core fibril and viral double-stranded DNA (dsDNA) and is surrounded by the palisade layer, a tightly arranged rod-shaped structure (Fig. 1). The outer lipoprotein envelope is often present on spontaneously released virions, while it is absent in viruses released through cellular disruption. Therefore, two infectious viral particles are formed during replication—the intracellular mature virus (IMV) and the extracellular enveloped virus (EV) [20]. The lipoprotein envelope that covers the surface of the intracellular mature virus encases the viral core and peripheral body, which contain specific proteins [21]. A mature virion has about 80 viral proteins [22]. The MPV genome (Fig. 2) comprises a linear double-stranded deoxyribose nucleic acid (dsDNA) with a size of about 197 kb [23]. A set of short tandem repeats and terminal hairpin loops are found at each end of each genome with identical, but oppositely oriented, terminal inverted repetition of about 6 kbp in size [24]. The genome contains approximately 190 non-overlapping open-reading frames (ORFs), hairpin termini [25] with at least 60 amino acid residues [24]. MPV DNA has a minimal concentration of guanine and cytosine, around 31.1% [25]. MPV has been classified into two distinct genetic clades: Central African and West African.
Genomic structure of MPV. The genome consists of approx. 196,858 bp, where the central genomic region is 101,476 bp. Both terminal variable end includes a 6379 bp terminal inverted repetition (ITR) with hairpin loop of ⁓ 80 bp short tandem repeats, coding region, and unique ITR sequences NR1 and NR2 (Prepared in Procreate® App.)
The whole genome sequencing of orthopoxviruses showed high level of homology between centrally located genes, and high degree of variability among terminally situated genes on either side of the genome. The conserved orthopoxvirus genes play an important role in viral replication and virion assembly, while orthopoxvirus genes with variable terminal may facilitate their virulence [19].
The worldwide outbreak of MPV
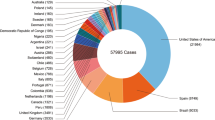
MPV was first discovered in 1958 in captive Macaca fascicularis monkey species in Copenhagen, Denmark. Although there is still some debate about the actual origin, because the monkeys that were originally shipped to Denmark in 1958 were not from Africa, but rather from Singapore [9, 26, 27]. Over the years, MPV is capable of infecting and causing disease in a wide variety of mammalian species across the world [26]. The first case of MPV in humans was reported in 1970 in the Basankusus District, Zaire. From 1970 to 1979, the MPV virus cases were reported from tropical rain forests of Central and West Africa, including Zaire, Liberia, Nigeria, Sierra Leone, and Ivory Coast [28]. Before 2003, MPV infection was geographically confined to some endemic countries in Central and West Africa and had never been reported outside Africa, but zoonotic transmission became the leading cause of MPV outbreaks in humans outside Africa [13, 29,30,31]. It has been found that Cynomys infected with rodents brought to Ghana are responsible for the spread of the disease to humans [9, 32]. Recently between 2003 and 2021, sporadic cases of MPV have been reported from different parts of the world including Europe, North America, the Middle East, etc. [13, 30]. Meanwhile in the Democratic Republic of Congo, thousands of cases per year with high fatality rates have been reported [33]. In 2003, around 53 cases of MPV human infection were reported in the USA [30]. In 2019, Singapore reported one suspect of MPV in a traveler returning from Nigeria [34]. United Kingdom (UK) 2021 confirmed three cases of MPV with a travel history of Nigeria [35]. Further, several MPV-infected cases from the USA with a travel history of Nigeria have been confirmed [36, 37]. The global re-emergence of MPV cases with a high infection rate in non-endemic countries was observed in 2022. Belgium, Sweden, Australia, and Italy also confirmed their first MPV cases (WHO Report) [38]. In the middle of May 2022, around 13, 14 and 17 MPV cases were reported from Canada, Portugal, and Spain (WHO Report). Till June 2022, there were around 1475 cases of MPV in different countries including the UK (366), Spain (275), Portugal (209), Germany (165), Canada (112), France (91), Netherlands (60), USA (45), Italy (32), Belgium (24), Switzerland (14), UAE (13), Ireland (9), Australia (8), Czech Republic (6), Slovenia (6), Ghana (5), Denmark (4), Israel (4), Finland (3), Hungary (3), Argentina (2), Mexico (2), Norway (2), Latvia (2), Australia (1), Malta (1), Greece (1), Gibraltar (1), Morocco (1), Brazil (1), and Poland (1) [33]. In July, India also confirmed three MPV-positive cases [39]. According to the Centre for Disease Control and Prevention (CDC), in November, 9, 2022 (at 5:00 PM EDT), data from more than 50,000 people around the globe show infection by MPV (Table 1 and Fig. 3).

(Source: WHO, European CDC, US CDC, and Ministries of Health) [40]
A worldwide epidemic of monkeypox virus
Evolution of MPV
A limited number of phylogenetic analyses of viral genomes in specific or endemic regions have restricted the preventive and control measures of the infection. The recent global outbreak of MPV again shows the importance of evaluating its phylogenetics. Few phylogenetic evaluations of MPV have been done, which suggests that there are two distinct clades of MPV: (1) the Central African or the Congo Basin clade and (2) the West African clade [19, 41,42,43], and the phylogenetic and ecological possibilities of the MPV outbreak in Southern Sudan in 2005 were also studied. The findings suggested that the virus, belonging to the clade identified in the Congo Basin, adapted to the different ecological environments and caused outbreaks at geographically discrete locations in Sudan [40]. Berthet et al. [44] investigated the phylogenetic relationships among MPV lineages prior identified in Central Africa and West Africa during 2001–2018. The result indicated that the ten isolates from the Central African Republic belong to the three lineages, which are closely linked to those found in the Democratic Republic of Congo. The phylogenetic pattern showed that all isolates originated from the rainforest section of the Congo Basin [44]. However, in recent phylogenetic studies, it has been observed that the current MPV 2022 in Europe showed significant divergence from its related viral predecessors [41, 42]. Luna et al. [45] analyzed the phylogenetic pattern of 337 MPV genomes collected from NCBI and GISAID and grouped MPV into three monophyletic lineages—two previously known lineages and one newly classified lineage from the 2022 global outbreak of MPV infection. The genome includes hMPV-1A lineage and A.1, A1.1, A.2, and B.1 as newly identified lineages, where B.1 lineage is mainly responsible for the outbreak in 2022 [45]. The phylogenetic analysis also discloses the preliminary signs of microevolution of MPV during inter-human transmission.
Mechanism of action of MPV
Monkeypox virus replication is explained by the poxvirus replication cycle. Poxviruses, like all other viruses, have proteins that enable and promote the virus’s adherence to a cell, fusion with a cell’s membrane, and penetration into the host cell. In the case of the poxvirus, the extracellular enveloped virion (EV), which has a second outer membrane, as well as the mature virion (MV), which has a single membrane, are both destroyed prior to fusion [46]. By adhering to laminin or glycosaminoglycans on the cell surface, the four viral proteins that are related to the MV cooperate to aid the virus in adhering to a host cell. Regardless of whether the MV or EV initiates invasion, 11–12 non-glycosylated transmembrane proteins with sizes ranging from 4 to 43 kDa are necessary for virus attachment to the host cell. While MVs have a relatively stable outer membrane and are thought to mediate transmission among host animals, EVs have a fragile outer membrane and are notably specialized for departing the intact cell and proliferating within the host [47].
Guarnieri bodies, which are now more often known as factories, are cytoplasmic structures where the monkeypox virus DNA replication process takes place [48]. Each factory originates from a single infectious particle, and during the initial stages of infection, they are each little DNA-containing structures surrounded by membranes that resemble the rough endoplasmic reticulum (RER) of the cell. As DNA synthesis continues, these factories will become larger and gradually start to take on an irregular shape as cavities filled with viral mRNA and host translation factors develop [45]. A complex of late gene products and a group of viral membrane assembly proteins work together in the later stages of the replication cycle to break down the endoplasmic reticulum membranes surrounding them and produce crescent-shaped structures as substrates for the formation of the immature virions (IV). The most prevalent infectious species, MV, are created from the IV next [49]. These MPVs will fuse with the cytoplasmic membrane and leave the cell, thus completing the cycle. The mechanism of MPV action is presented in Fig. 4.
Transmission and prevention of MPV
MPV may spread in two different ways: primary transmission (from animal to human) and secondary transmission (from human to human). Monkeypox is transmitted through direct contact with an animal’s body secretions or animal bites. Additionally, human pox virus (HPV) may be transmitted by breathing droplets during a close, prolonged face-to-face encounter, by direct exposure to an infected patient’s body fluids, or by highly contagious particulate items [10]. Research on macaque monkeys treated with airborne droplets of MPV revealed that the virus primarily infects the lower airway epithelium, and then travels to lymph nodes before entering the bloodstream through monocytes. Then, MPV lesions may develop in the dermis, oral epithelium, gastrointestinal and reproductive system, thymus, spleen, and other organs [50].
Zoonoses transmission can happen directly through an encounter with either blood or bodily fluids, inoculation through the mucocutaneous lesions of an infected animal, ingestion of one of the indigenous viral hosts, or both [15]. Additionally, there have been cases of nosocomial transmission [51,52,53]. Nosocomial MPV transmission between patients and medical personnel is still a major worry in epidemics for both endemic and non-endemic areas. Nosocomial infections of smallpox were linked to it, and healthcare settings had the highest incidence of transmission [54]. Hospital-related MPV epidemics are also particularly severe and persistent. This is probably the consequence of several variables, including diseases in susceptible populations, healthcare cleanliness standards, and the usage of aerosol-generating medical procedures [55]. In one case in the UK, a medical professional who had encountered the clothes and mattresses of an MPV patient became diagnosed with MPV [56]. As evidence of MPV’s propensity to transmit in hospital environments, extended human-to-human transmission of monkeypox was recorded in a hospital in Impfondo, Republic of Congo [57]. To reduce the risk of MPV transmission inside hospitals, preventive measures including the use of proper personal protective equipment (PPE), efficient waste disposal techniques, and isolation of the infected patient must be implemented.
Monkeypox is being considered a sexually transmitted disease in the recent outbreak [58]. With almost 95% of reported cases in young men (under 40 years old), the sex distribution of patients in the recent outbreak exhibits a substantial skew. There has been an increase in the number of males who have sex with men (MSM) [59], and heterosexual relationships should also be taken into account. In a recent report, individuals with vaginal and groin lesions who are infected have been suspected of contracting the disease through sexual contact [60]. Pregnant mothers who have the virus can transmit it to their unborn, as per reports. Although research studies of miscarriage and fetal mortality exist, there is little evidence available on the effects of human MPV transmission on birth outcomes with vertical transmission [61]. Different modes of transmission of the monkeypox virus are presented in Fig. 5.
According to the Centre of Disease Control and Prevention (CDC) guidelines, the preventive measures for MPV are as follows:
-
1.
Avoid skin-to-skin contact (hug, cuddle, kiss, or sex) with people who have a skin condition suspected to be monkeypox (skin with rashes, pimples, scabs or blisters) on hands, feet, face, chest, genitals, or mouth. Prevent the touching of rashes or scabs.
-
2.
Avoid close contact with the materials used by the person suffering from monkeypox: the sharing of utensils with the person with MPV should be avoided and also prevent the handling and touching of the towels, bedding, or clothes of the infected person.
-
3.
Proper hand sanitization: before eating or touching unhygienic surfaces, wash hands properly with soap and water or use hand sanitizer.
-
4.
Vaccinate yourself: JYNNEOS vaccine, used against smallpox is also approved for the prevention of MPV. The vaccine was used in the USA during the outbreak.
-
5.
If you are from Central or West Africa, avoid animals, especially rodents and primates that can transmit MPV
It is important to provide proper care and respect that preserve privacy, confidentiality and dignity of the MPV-infected patients.
Clinical signs and symptoms
Lymph node enlargement, particularly in the submental, submandibular, cervical and inguinal regions, is the most reliable clinical sign differentiating monkeypox from smallpox and chickenpox [56]. Pharyngeal, conjunctival, and vaginal mucosae inflammation and nonspecific lesions have been linked to enanthema. Exanthema stage lesions progress synchronously over 14–21 days in a particular body location, resembling smallpox lesions [62]. However, unlike smallpox, crops can cause skin sores. In contrast to smallpox, the lesions’ distribution is not very centrifugal. Umbilication, crusting, and desquamation follow the course of lesions from macules to papules to vesicles and pustules. The majority of lesions are 3–15 mm in diameter. Affected areas include the head, face, trunk, and limbs. Both covered and uncovered parts have lesions. The soles and palms have lesions. Necrosis, petechiae, and ulceration are potential signs [63]. When pain does arise, it generally results from a subsequent bacterial infection. Pain is infrequent. Pruritus may occur. Patients who have received the smallpox vaccine experience a milder version of the illness. Lesions in youngsters may manifest as nonspecific, erythematous papules ranging in width from 1 to 5 mm, indicative of arthropod bite responses. A faint umbilication may be observed. Twenty percent of unvaccinated patients in the African outbreaks had a confluent, erythematous eruption on the face and upper torso, which some writers refer to as the septicemic rash of monkeypox. The hemorrhagic and flat forms of smallpox, which have been documented in individuals with smallpox, have not been observed in patients with monkeypox [64]. As lesions heal, they may leave behind deep pockmarks. During the monkeypox epidemic in 2022, a substantial percentage of sufferers were guys who had sex with other men. The perianal and vaginal regions were the initial sites of lesion appearance in a few patients. Clinicians should have a strong suspicion that a patient has monkeypox when they find typical lesions in these areas, especially if they have recently traveled. Several clinical signs and symptoms caused by MPV are presented in Fig. 6
Diagnosis of MPV
It is necessary to quickly identify, diagnose, isolate, and manage affected patients in both sporadic and endemic regions in order to control the spread of monkeypox in the community [65]. A precise diagnosis is essential to control natural illness or in the early identification of a potential bioterrorism incident since the clinical presentation of monkeypox and those of chickenpox and smallpox are remarkably similar. Since there is no appropriate, approved antiviral medication for monkeypox, isolation and quick ring vaccination are the sole viable healthcare preventative measures once the infection agent is detected. Scabs as well as other cutaneous tissues must be handled carefully and gathered in aseptic conditions while taking pulmonary protections as a result of transmission by physical interaction and atmospheric emissions.
Biological characteristics have traditionally been utilized to distinguish and classify orthopoxviruses. Clinically, MPV infection can be identified by the presence of cutaneous conditions like syphilis, measles, chickenpox, microbial dermatitis, infestations, and medication-related sensitivities. The better diagnostic specimens include those that originate directly from rashes, such as cutaneous, fluids, scabs, or, in certain situations, a biopsy. Although it is still the first-line method today, orthopoxviruses cannot be distinguished using electron microscopy [66, 67]. The sole technique that can distinguish between various orthopoxviruses is polymerase chain reaction (PCR), being the preferred technique for confirming MPV infections. Additionally, novel diagnostic techniques diagnosing orthopoxviruses infections, including immunohistochemistry (IHC) and serological testing, are available. When determining if encephalitis caused by an orthopoxvirus has occurred, the anti-orthopoxvirus IgM response in the cerebrospinal fluid (CSF) may be helpful, through seroconversion from the anti-orthopoxvirus IgM response to the anti-orthopoxvirus IgG response, accelerating the process [68]. Serologic diagnostic techniques like enzyme-linked immunosorbent assay (ELISA) are highly helpful for retrospective analysis when there is no virologic material available.
Antiviral medications and vaccines used to prevent orthopoxvirus infections
For the time being, the monkeypox zoonotic disease has no particular treatment. There are, however, several antiviral drugs and vaccines that are administered to combat smallpox as well as other ailments that may benefit individuals with monkeypox infection. The fact that population vaccination coverage against smallpox is diminishing globally is one of the most critical factors driving the present outbreak of monkeypox. Monkeypox risk is reduced by roughly 85% following smallpox immunization. The MPV may have a chance of occupying the ecological and immunological niche that the smallpox virus previously filled, 40 years after smallpox vaccinations were discontinued [69]. The first research study, which was published in 1968, reported that vaccination against the smallpox virus might be effective against MPV [70]. Another study was conducted to determine that smallpox vaccination, as shown by the existence of vaccination scars, provided 85% immunity against MPV [7]. Due to the similarity between smallpox and monkeypox viruses, the smallpox vaccination can also safeguard individuals from contracting the latter disease [71, 72]. The approved FDA and known antiviral drugs like tecovirimat (brand name: TPOXX or ST-246) [73,74,75], cidofovir (brand name: Vistide) [76,77,78], and brincidofovir (brand name: Tembexa) [77, 79] and vaccines like ACAM2000 [80], Vaccinia immune globulin intravenous (VIG-IV) [81], and JYNNEOS (brand name-Imvamune or Imvanex) [82] are used as a therapeutic medication against MPV. Information on antiviral medications and vaccinations, as well as their mechanisms of action against viruses, is included in Table 2. The ongoing clinical trials and their phases in different countries are mentioned in Table 3.
The number of cases of monkeypox is rising globally, which raises worries that the virus, similar to SARS-CoV-2, might spread globally and have severe effects on the already overburdened medical system. ACAM2000 and JYNNEOS are two licensed smallpox vaccinations that offer cross-protection against MPV. Second-generation vaccinations, including ACAM2000, are known as replication-competent vaccinia virus vaccines because they can replicate the virus in mammalian cells. ACAM2000 has been granted approval by the US FDA for use in the population at increased risk of contracting smallpox through active immunization [90]. To develop it and determine the presence of recognized adventitious agents, Vero kidney cells from African green monkeys are employed [91]. Research indicates that LC16m8 can prevent deadly monkeypox in monkeys and may produce resistance mechanisms toward smallpox [92]. Under different research, immunization with a highly attenuated vaccinia virus vaccine (LC16m8) might provide long-lasting protection from MPV infection [93]. As the antiviral medication impacts the efficacy of ACAM2000 if delivered concurrently, a recent study revealed that the incidence of monkeypox infection was greater among the monkeys that administered the ACAM2000 vaccine and concurrent tecovirimat treatment [94]. Concerning the smallpox vaccination, the most often reported adverse reactions were myalgia, lymphadenitis, malaise, lethargy, headaches, and fever. The following uncommon, but significant side effects have been linked to replication-competent smallpox vaccinations: conjunctivitis, eczema vaccinatum (EV), cephalitis, encephalomyelitis, erythema multiforme major, and fetus mortality [90].
The nucleotide analog cidofovir (CDV) prevents the poxvirus’s DNA polymerase from functioning by converting to a cytidine triphosphate analog within the cell [95]. On the effectiveness of CDV in the treatment of human monkeypox infection, there are no reliable data available. The effectiveness of CDV medication in treating patients with severe monkeypox infection is yet unknown. In addition, CDV is utilized to treat human infections with the molluscum contagiosum poxvirus [96]. Brincidofovir (BCV) is a CDV-derived lipid conjugate. Compared to CDV, BCV has better cellular absorption and a higher rate of conversion to the active form [97]. In comparison to CDV, BCV has a superior renal safety profile [79, 98]. According to research, giving individuals with monkeypox infection 200 mg of BCV orally once per week may increase their liver enzyme levels. Comparing BCV to CDV, the safety profile of the former may be improved. The use of BCV to treat cytomegalovirus infection did not result in any severe side effects. In addition, BCV is effective against the human molluscum contagiosum poxvirus [99, 100].
Tecovirimat, another antiviral drug, is a strong inhibitor of the orthopoxvirus protein VP37, which is involved in the development and ejection of enveloped virions (CDC 2022). In a previous investigation, it was discovered that TCV suppresses both the variola and monkeypox viruses by lowering the generation and release of encapsulated orthopoxvirus in systemic inflammatory infections [101]. The use of it to treat MPV was authorized in Europe in 2022 [102]. In combination with ACAM2000, tecovirimat may harm immunogenicity [94].
Therapeutic approaches, particularly those derived from medicinal plants, are becoming more and more popular. As potentially antiviral drugs, a range of medicinal herbs is being investigated. According to traditional medicinal studies, several species of plants may have potent antiviral efficacy against a variety of viruses. With the aid of developing technologies, a thorough examination of the antiviral properties of several plant extracts has been conducted. To find novel antiviral drugs aimed against the resurgence of the poxvirus, it is necessary to use the information from traditional medicines. Alchemilla vilgaris ethanol aerial extracts and ethyl acetate root extracts were used in an investigation to analyze the bioactive compounds’ toxicity and antiviral activity. In this investigation, the orthopoxviruses vaccinia virus (strain L-IVP) and ectromelia virus (strain K-1) from the mouse smallpox virus was employed. Extracts from the roots and aerial sections of A. vilgaris were found to possess antiviral properties against the orthopoxviruses tested in the investigation [103]. Sarracenia purpurea was identified as the first efficient inhibitor of poxvirus replication at the level of early viral transcription in in vitro research. The findings suggest S. purpurea may operate as another therapeutic approach against orthopoxviruses such as MPV (monkeypox virus) and VARV (variola virus) in light of the resurgence of diseases caused by poxviruses [104]. The most effective plants may be processed for the extraction and characterization of putative antiviral natural compounds that can act as solid candidates in the development of novel antiviral drugs.
Recent findings of potent hit compounds against MPV using computational methods
To keep track of the current outbreak in the lack of a specific medication, it is urgently necessary to develop novel efficient and secure treatments. Drug discovery is a laborious, costly, and time-consuming approach with a high rate of failures. These constraints can be resolved by computer-aided drug identification and drug repositioning. A potential antiviral agent can be immediately discovered among medications previously endorsed by the US Food and Drug Administration (FDA) employing a variety of in silico approaches that have reported on multiple targeted MPV proteins.
Recent computational research identified promising MPV crucial protein inhibitors using a variety of computational techniques, such as computational modeling, molecular docking, molecular dynamic (MD) simulation, and molecular mechanics generalized born surface area (MM-GBSA). In this investigation, three significant MPV proteins—D8L (host cell entry), F13L (catalysis of the envelopment of intracellular mature virus particles), and A6R (viral replication)—were employed as virtual targets to analyze the potential efficacy of FDA-approved medications such as adrenor, azacitidine, cidofovir, cladribine, epivir, fludarabine, and zolmitriptan. According to the report, fludarabine, an anticancer medication, exhibited the strongest in silico activity on the crucial protein A6R [105].
According to a previous study, there is currently no known structure for the MPV E8 protein or treatment for MPV. With the help of computational methods, this investigation aimed to discover potent compounds that block the MPV E8. In this study, homology modeling with the AlphaFold deep learning server was implemented to obtain a high-quality 3D structure of the MPV E8 protein. Diosmin and FAD (flavin adenine dinucleotide) were reported to have potential as hit compounds against the MPV E8 protein, which might aid in restricting viral entrance and suppress the virus in subsequent fusion events [106].
A comprehensive computational analysis of betulin, a pentacyclic naturally occurring triterpene that serves as the precursor of a large extended family of natural bioactive derivatives, was conducted. The possible inhibitory effects of betulin on one of the proteins involved in monkeypox, i.e., A42R profilin-like protein (strain Zaire-96-I-16) were investigated using in silico molecular docking. The examined MPV protein (PDB code: 4QWO) was demonstrated to be responsive to betulin's functionality, and betulin links with THR126 and ARG129 via a hydrogen bond and an alkyl interaction, respectively [107].
A molecular study was conducted to examine the potential for repurposing FDA-approved medications as an antagonist against the MPV cysteine proteinase. According to the outcomes of this in silico investigation, the antibiotic tetracycline may be able to prevent the MPV cysteine proteinase from functioning. This result, however, is not definitive because there is a dearth of crystallization information for this proteinase enzyme [108].
Another computational drug repurposing study was conducted to determine which currently recommended medications would be capable of inhibiting the essential MPV proteins, thymidylate kinase, and D9 decapping enzyme. Using molecular docking and molecular dynamics simulations, four possible inhibitors, namely tipranavir, cefiderocol, doxorubicin, and dolutegravir, were detected as potential candidates for repurposing against the MPV from a library of US FDA-approved drugs [109].
MPV surveillance
The MPV outbreak in 2022 is unlike any other seen before outside Africa, as it has shown a unique pattern with more cases and greater transmission rate than any other prior outbreaks. Given the seriousness of the situation, proper surveillance is essential to protect communities most vulnerable to the disease and is also an important factor in preventing the zoonotic transmission and spread of monkeypox [110]. The inter-human transmission of MPV occurs through direct contact with the mucous membranes, or infected skin, or body fluids from those lesions through respiratory droplets, oropharynx and saliva, anorectum, semen, urine, and ocular fluid [111, 112]. In MPV infection, there is no definitive infectious period, but patients are generally considered infectious from the time symptoms develop until crusted lesions have fallen off and a new skin has formed. However, few studies have indicated that in some patients, the infection is detected prior to the emergence of symptoms. In 2017, ten suspected cases of MPV in the Republic of the Congo initiated the investigation and aimed to educate and create awareness of the community on strengthening the surveillance [113]. To identify cases, clusters, and sources of infections as soon as possible, the WHO proposed surveillance strategy which aims to: offer optimal clinical care, classify and manage contacts, prevent further transmission by isolating the infected cases, save frontline health workers, and deliver preventive measures based on the identified pathways of transmission. The unexpected rise in the MPV cases suggests the prevalence rate of infection in humans; therefore, it is important to study different approaches to prevent and control the global occurrence of the disease. Branda et al. built the EpiMPX surveillance system for researchers and policymakers to observe the epidemic trends of MPV in Europe and Italy [110]. Infectious disease dynamics can be tracked from a community level to a building level using wastewater from surface waters, sewers, wastewater treatment plants, or point sources [114, 115]. During COVID, wastewater surveillance has been widely used to study the degree of the cases circulating in the communities. Tiwari et al. suggested the perspective of wastewater- and environment-based surveillance to monitor MPV caseloads [116, 117]. Several other pathogens, including the other members of poxviridae family, hepatitis A and E virus, adenovirus, rotavirus, influenza A virus, dengue virus, etc., have been successfully monitored at population level using the same approach. Additionally, it is imperative to develop epidemiological and laboratory surveillance systems to effectively target the risk of monkeypox disease prevention, control, and transmission globally. Though clinical surveillance has limitations, including social stigma, access and cost of diagnostic testing, inability to reach asymptomatic individuals, local, regional, and global outbreak response can be strengthened by combining clinical and environmental laboratory surveillance capabilities [116]. There exist numerous challenges for consistent MPV reporting; therefore, efforts should be made to improve disease surveillance during the outbreak.
Conclusion
The MPV pandemic has migrated to almost 110 different nations. According to a WHO investigator, monkeypox may not evolve into an outbreak since MPV’s viral load is rather modest. Yet, this pandemic infection with a very significant thousands of cases alarmed global health officials as the largest and also most pervasive MPV pandemic outside of Africa up to this point. For monkeypox infection, there are no evidence-based preventative or treatment measures available. The current analysis assumes that smallpox immunizations and antiviral medications may manage monkeypox epidemics. As a result, the present monkeypox outbreak strongly suggests the use of these smallpox immunizations and antiviral drugs. Additionally, to fully understand their precise roles in human monkeypox infections, we advise further research into smallpox vaccinations and orthopoxvirus inhibitors in an in vivo study. Monkeypox outbreaks may be controlled in contrast to the repositioning of anti-smallpox medications by promoting awareness, implementing effective precautionary measures, quickly identifying and isolating patients, tracing contacts, and providing appropriate treatment. Although finding a compound’s efficacy for a novel disease by virtual screening has been a time- and cost-effective method, subsequent testing (in vivo and in vitro) is required to validate the hit compound’s effectiveness before being administered to patients.
Abbreviations
- MPV:
-
Monkeypox virus
- OPV:
-
Orthopoxvirus
- WHO:
-
World Health Organization
- VARV:
-
Variola virus
- EV:
-
Enveloped virion
- MV:
-
Mature virion
- RER:
-
Rough endoplasmic reticulum
- HPV:
-
Humanpox virus
- PPE:
-
Personal protective equipment
- PCR:
-
Polymerase chain reaction
- CSF:
-
Cerebrospinal fluid
- ELISA:
-
Enzyme-linked immunosorbent assay
- CVD:
-
Cidofovir
- BCV:
-
Brincidofovir
- FDA:
-
Food and Drug Administration
References
Marennikova SS, Moyer RW. Classification of poxviruses and brief characterization of the genus orthopoxvirus. In: Orthopoxviruses pathogenic for humans. New York: Springer-Verlag; 2005. p. 11–8.
Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022. https://doi.org/10.7759/cureus.26531.
Di Gennaro F, Veronese N, Marotta C, Shin JI, Koyanagi A, Silenzi A, Antunes M, Saracino A, Bavaro DF, Soysal P, et al. Human monkeypox: a comprehensive narrative review and analysis of the public health implications. Microorganisms. 2022;10:1633. https://doi.org/10.3390/microorganisms10081633.
Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, Wu J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol. 2022. https://doi.org/10.1002/jmv.27931.
Chadha J, Khullar L, Gulati P, Chhibber S, Harjai K. Insights into the monkeypox virus: making of another pandemic within the pandemic? Environ Microbiol. 2022;24:4547–60. https://doi.org/10.1111/1462-2920.16174.
Hutson CL, Carroll DS, Gallardo-Romero N, Weiss S, Clemmons C, Hughes CM, Salzer JS, Olson VA, Abel J, Karem KL, et al. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS ONE. 2011;6:e28295. https://doi.org/10.1371/journal.pone.0028295.
Fine PEM, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–50. https://doi.org/10.1093/ije/17.3.643.
Abdelaal A, Reda A, Lashin BI, Katamesh BE, Brakat AM, AL-Manaseer BM, Kaur S, Asija A, Patel NK, Basnyat S, et al. Preventing the next pandemic: is live vaccine efficacious against monkeypox, or is there a need for killed virus and MRNA vaccines? Vaccines. 2022;10:1419. https://doi.org/10.3390/vaccines10091419.
von Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 2009;46:156–76. https://doi.org/10.1111/j.1699-0463.1959.tb00328.x.
Ligon BL. Monkeypox: a review of the history and emergence in the western hemisphere. Semin Pediatric Infect Dis. 2004;15:280–7. https://doi.org/10.1053/j.spid.2004.09.001.
Awan UA, Riasat S, Naeem W, Kamran S, Khattak AA, Khan S. Monkeypox: a new threat at our doorstep! J Infect. 2022;85:e47–8. https://doi.org/10.1016/j.jinf.2022.05.027.
Foster SO, Brink EW, Hutchins DL, Pifer JM, Lourie B, Moser CR, Cummings EC, Kuteyi OE, Eke RE, Titus JB, et al. Human monkeypox. Bull World Health Organ. 1972;46:569–76.
Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16:e0010141. https://doi.org/10.1371/journal.pntd.0010141.
Patel P. Monkeypox is declared as a global health emergency. are we prepared? Natl J Community Med. 2022;13:417–8. https://doi.org/10.55489/njcm.130720222314.
Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. https://doi.org/10.3390/v12111257.
Pal M, Mengstie F, Kandi V. Epidemiology, diagnosis, and control of monkeypox disease: a comprehensive review. Am J Infect Dis Microbiol. 2017;7:94.
Sale TA, Melski JW, Stratman EJ. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J Am Acad Dermatol. 2006;55:478–81. https://doi.org/10.1016/j.jaad.2006.05.061.
Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973;37:1–18. https://doi.org/10.1128/br.37.1.1-18.1973.
Sklenovská N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. https://doi.org/10.3389/fpubh.2018.00241.
Appleyard G, Hapel AJ, Boulter EA. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. https://doi.org/10.1099/0022-1317-13-1-9.
Pickup DJ. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11:e1004904. https://doi.org/10.1371/journal.ppat.1004904.
Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. Protein composition of the vaccinia virus mature virion. Virology. 2007;358:233–47. https://doi.org/10.1016/j.virol.2006.08.025.
Kugelman JR, Johnston SC, Mulembakani PM, Kisalu N, Lee MS, Koroleva G, McCarthy SE, Gestole MC, Wolfe ND, Fair JN, et al. Genomic variability of monkeypox virus among humans, democratic Republic of the Congo. Emerg Infect Dis. 2014. https://doi.org/10.3201/eid2002.130118.
Shchelkunov SN, Totmenin AV, Safronov PF, Mikheev MV, Gutorov VV, Ryazankina OI, Petrov NA, Babkin IV, Uvarova EA, Sandakhchiev LS, et al. Analysis of the monkeypox virus genome. Virology. 2002;297:172–94. https://doi.org/10.1006/viro.2002.1446.
Shchelkunov SN, Totmenin AV, Babkin IV, Safronov PF, Ryazankina OI, Petrov NA, Gutorov VV, Uvarova EA, Mikheev MV, Sisler JR, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. https://doi.org/10.1016/S0014-5793(01)03144-1.
Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–57. https://doi.org/10.2217/fvl.12.130.
Bonilla-Aldana DK, Rodriguez-Morales AJ. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet Q. 2022;42:148–50. https://doi.org/10.1080/01652176.2022.2088881.
Breman JG, Kalisa-Ruti N, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58:165–82.
Petersen E, Abubakar I, Ihekweazu C, Heymann D, Ntoumi F, Blumberg L, Asogun D, Mukonka V, Lule SA, Bates M, et al. Monkeypox—enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78–84. https://doi.org/10.1016/j.ijid.2018.11.008.
Farahat RA, Abdelaal A, Shah J, Ghozy S, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ, McHugh TD, Leblebicioglu H. Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022;21:26. https://doi.org/10.1186/s12941-022-00518-2.
Mohapatra RK, Tuli HS, Sarangi AK, Chakraborty S, Chandran D, Chakraborty C, Dhama K. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: another potential global threat? Int J Surg. 2022;103:106705. https://doi.org/10.1016/j.ijsu.2022.106705.
Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342–50. https://doi.org/10.1056/NEJMoa032299.
Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855. https://doi.org/10.1016/j.jaut.2022.102855.
Yong SEF, Ng OT, Ho ZJM, Mak TM, Marimuthu K, Vasoo S, Yeo TW, Ng YK, Cui L, Ferdous Z, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26:1826–30. https://doi.org/10.3201/eid2608.191387.
Hobson G, Adamson J, Adler H, Firth R, Gould S, Houlihan C, Johnson C, Porter D, Rampling T, Ratcliffe L, Russell K, Shankar AG, Wingfield T. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32):2100745. https://doi.org/10.2807/1560-7917.ES.2021.26.32.2100745.
Costello V, Sowash M, Gaur A, Cardis M, Pasieka H, Wortmann G, Ramdeen S. Imported monkeypox from international traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28:1002–5. https://doi.org/10.3201/eid2805.220292.
Rao AK, Schulte J, Chen T-H, Hughes CM, Davidson W, Neff JM, Markarian M, Delea KC, Wada S, Liddell A, et al. Monkeypox in a traveler returning from Nigeria—Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022;71:509–16. https://doi.org/10.15585/mmwr.mm7114a1.
Zumla A, Valdoleiros SR, Haider N, Asogun D, Ntoumi F, Petersen E, Kock R. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis. 2022;22:929–31. https://doi.org/10.1016/S1473-3099(22)00354-1.
Sah R, Mohanty A, Siddiq A, Singh P, Abdelaal A, Alshahrani NZ, Dhama K. Monkeypox reported in India—South East Asia region: health and economic challenges. Lancet Reg Health Southeast Asia. 2022;4:100063. https://doi.org/10.1016/j.lansea.2022.100063.
https://www.Cdc.Gov/Poxvirus/Monkeypox/Response/2022/World-Map.Html. Accessed 15 Nov 2022
Sadeuh-Mba SA, Yonga MG, Els M, Batejat C, Eyangoh S, Caro V, Etoundi A, Carniel E, Njouom R. Monkeypox virus phylogenetic similarities between a human case detected in cameroon in 2018 and the 2017–2018 outbreak in Nigeria. Infect Genet Evol. 2019;69:8–11. https://doi.org/10.1016/j.meegid.2019.01.006.
León-Figueroa DA, Bonilla-Aldana DK, Pachar M, Romaní L, Saldaña-Cumpa HM, Anchay-Zuloeta C, Diaz-Torres M, Franco-Paredes C, Suárez JA, Ramirez JD, et al. The never-ending global emergence of viral zoonoses after COVID-19? the rising concern of monkeypox in Europe, North America and Beyond. Travel Med Infect Dis. 2022;49:102362. https://doi.org/10.1016/j.tmaid.2022.102362.
Nakazawa Y, Emerson GL, Carroll DS, Zhao H, Li Y, Reynolds MG, Karem KL, Olson VA, Lash RR, Davidson WB, et al. Phylogenetic and ecologic perspectives of a monkeypox outbreak, Southern Sudan, 2005. Emerg Infect Dis. 2013;19:237–45. https://doi.org/10.3201/eid1902.121220.
Berthet N, Descorps-Declère S, Besombes C, Curaudeau M, Nkili Meyong AA, Selekon B, Labouba I, Gonofio EC, Ouilibona RS, Simo Tchetgna HD, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11:13085. https://doi.org/10.1038/s41598-021-92315-8.
Luna N, Ramírez AL, Muñoz M, Ballesteros N, Patiño LH, Castañeda SA, Bonilla-Aldana DK, Paniz-Mondolfi A, Ramírez JD. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: emergence of a novel viral lineage? Travel Med Infect Dis. 2022;49:102402. https://doi.org/10.1016/j.tmaid.2022.102402.
Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4:688–707. https://doi.org/10.3390/v4050688.
Moss B. Poxvirus DNA replication. Cold Spring Harb Perspect Biol. 2013;5:a010199–a010199. https://doi.org/10.1101/cshperspect.a010199.
Kieser Q, Noyce RS, Shenouda M, Lin Y-CJ, Evans DH. Cytoplasmic factories, virus assembly, and DNA replication kinetics collectively constrain the formation of poxvirus recombinants. PLoS ONE. 2020;15:e0228028. https://doi.org/10.1371/journal.pone.0228028.
Katsafanas GC, Moss B. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe. 2007;2:221–8. https://doi.org/10.1016/j.chom.2007.08.005.
Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab Invest. 2001;81:1581–600. https://doi.org/10.1038/labinvest.3780373.
Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, Doty J, Hughes CM, Kabamba J, Malekani J, et al. Extended human-to-human transmission during a monkeypox outbreak in the democratic Republic of the Congo. Emerg Infect Dis. 2016;22:1014–21. https://doi.org/10.3201/eid2206.150579.
Yinka-Ogunleye A, Aruna O, Ogoina D, Aworabhi N, Eteng W, Badaru S, Mohammed A, Agenyi J, Etebu EN, Numbere T-W, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–51. https://doi.org/10.3201/eid2406.180017.
Ihekweazu C, Yinka-Ogunleye A, Lule S, Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev Anti Infect Ther. 2020;18:389–92. https://doi.org/10.1080/14787210.2020.1735361.
Kiang KM, Krathwohl MD. Rates and risks of transmission of smallpox and mechanisms of prevention. J Lab Clin Med. 2003;142:229–38. https://doi.org/10.1016/S0022-2143(03)00147-1.
Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11:940. https://doi.org/10.3390/v11100940.
Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, Hardman A, Harper N, Jarvis R, Mawdsley S, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–5. https://doi.org/10.3201/eid2604.191164.
Learned LA, Reynolds MG, Wassa DW, Li Y, Olson VA, Karem K, Stempora LL, Braden ZH, Kline R, Likos A, et al. Extended Interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–34.
Vallée A, Farfour E, Zucman D. Monkeypox virus: a novel sexually transmitted disease? A case report from France. Travel Med Infect Dis. 2022;49:102394. https://doi.org/10.1016/j.tmaid.2022.102394.
Heskin J, Belfield A, Milne C, Brown N, Walters Y, Scott C, Bracchi M, Moore LS, Mughal N, Rampling T, et al. Transmission of monkeypox virus through sexual contact—a novel route of infection. J Infect. 2022;85:334–63. https://doi.org/10.1016/j.jinf.2022.05.028.
Ogoina D, Izibewule JH, Ogunleye A, Ederiane E, Anebonam U, Neni A, Oyeyemi A, Etebu EN, Ihekweazu C. The 2017 human monkeypox outbreak in nigeria—report of outbreak experience and response in the niger delta university teaching hospital, Bayelsa State, Nigeria. PLoS ONE. 2019;14:e0214229. https://doi.org/10.1371/journal.pone.0214229.
Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, Martin JW, Muyembe J-JT. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the democratic Republic of Congo. J Infect Dis. 2017;216:824–8. https://doi.org/10.1093/infdis/jix260.
Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82:957–63. https://doi.org/10.1007/s40265-022-01742-y.
Damon IK. Status of human monkeypox: clinical disease. Epidemiol Res Vaccine. 2011;29:D54–9. https://doi.org/10.1016/j.vaccine.2011.04.014.
Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43:1503–10. https://doi.org/10.1016/j.jacc.2003.11.053.
Jeyaraman M, Selvaraj P, Halesh MB, Jeyaraman N, Nallakumarasamy A, Gupta M, Maffulli N, Gupta A. Monkeypox: an emerging global public health emergency. Life. 2022;12:1590. https://doi.org/10.3390/life12101590.
Kurth A, Nitsche A. Fast and reliable diagnostic methods for the detection of human poxvirus infections. Future Virol. 2007;2:467–79. https://doi.org/10.2217/17460794.2.5.467.
Barreto-Vieira DF, Barth OM. Negative and positive staining in transmission electron microscopy for virus diagnosis. In: Shah MM, editor. Microbiology in agriculture and human health. London: InTech; 2015.
Tyler KL. Emerging viral infections of the central nervous system: part 1. Arch Neurol. 2009. https://doi.org/10.1001/archneurol.2009.153.
Quarleri J, Delpino MV, Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022;44:2095–103. https://doi.org/10.1007/s11357-022-00611-6.
McConnell S, Hickman RL, Wooding WL, Huxsoll DL. Monkeypox: experimental infection in chimpanzee (Pan Satyrus) and immunization with vaccinia virus. Am J Vet Res. 1968;29:1675–80.
Downie AW, McCARTHY K. The viruses of variola, vaccina, cowpox and ectromelia. Neutralization tests on the chorio-allantois with unabsorbed and absorbed immune sera. Br J Exp Pathol. 1950;31:789–96.
De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. https://doi.org/10.1016/S0166-3542(02)00008-6.
Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol. 2005;79:13139–49. https://doi.org/10.1128/JVI.79.20.13139-13149.2005.
Jordan R, Leeds JM, Tyavanagimatt S, Hruby DE. Development of ST-246® for treatment of poxvirus infections. Viruses. 2010;2:2409–35. https://doi.org/10.3390/v2112409.
Grosenbach DW, Jordan R, Hruby DE. Development of the small-molecule antiviral ST-246 ® as a smallpox therapeutic. Future Virol. 2011;6:653–71. https://doi.org/10.2217/fvl.11.27.
Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. https://doi.org/10.1016/S0166-3542(02)00196-1.
Magee WC, Aldern KA, Hostetler KY, Evans DH. Cidofovir and (S)-9-[3-Hydroxy-(2-Phosphonomethoxy)Propyl]Adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob Agents Chemother. 2008;52:586–97. https://doi.org/10.1128/AAC.01172-07.
MacNeil A, Reynolds MG, Braden Z, Carroll DS, Bostik V, Karem K, Smith SK, Davidson W, Li Y, Moundeli A, et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis. 2009;48:e6–8. https://doi.org/10.1086/595552.
Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by DsDNA viruses. Expert Rev Anti Infect Ther. 2014;12:1171–8.
Nalca A, Zumbrun EE, ACAM2000&trade. The new smallpox vaccine for united states strategic national stockpile. DDDT. 2010. https://doi.org/10.2147/DDDT.S3687.
Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, Gerber SI, Garcia-Houchins S, Lederman E, Hruby D, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–61. https://doi.org/10.1086/587668.
Petersen BW, Kabamba J, McCollum AM, Lushima RS, Wemakoy EO, Muyembe Tamfum J-J, Nguete B, Hughes CM, Monroe BP, Reynolds MG. Vaccinating against monkeypox in the democratic republic of the congo. Antiviral Res. 2019;162:171–7. https://doi.org/10.1016/j.antiviral.2018.11.004.
Bauer DJ, Vincent LS, Kempe CH, Young PA, Downie AW. Prophylaxis of smallpox with methisazone1. Am J Epidemiol. 1969;90:130–45. https://doi.org/10.1093/oxfordjournals.aje.a121057.
Marquez VE, Hughes SH, Sei S, Agbaria R. The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antiviral Res. 2006;71:268–75. https://doi.org/10.1016/j.antiviral.2006.04.012.
Jahrling PB, Zaucha GM, Huggins JW. Countermeasures to the reemergence of smallpox virus as an agent of bioterrorism. In: Michael Scheld W, Craig WA, Hughes JM, editors. Emerging infections 4. Washington: ASM Press; 2014. p. 187–200.
Duraffour S, Drillien R, Haraguchi K, Balzarini J, Topalis D, van den Oord JJ, Andrei G, Snoeck R. KAY-2-41, a novel nucleoside analogue inhibitor of orthopoxviruses in vitro and in vivo. Antimicrob Agents Chemother. 2014;58:27–37. https://doi.org/10.1128/AAC.01601-13.
Smee DF, Bray M, Huggins JW. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in Vitro. Antivir Chem Chemother. 2001;12:327–35. https://doi.org/10.1177/095632020101200602.
Mazurkov OYu, Kabanov AS, Shishkina LN, Sergeev AA, Skarnovich MO, Bormotov NI, Skarnovich MA, Ovchinnikova AS, Titova KA, Galahova DO, et al. New effective chemically synthesized anti-smallpox compound NIOCH-14. J Gen Virol. 2016;97:1229–39. https://doi.org/10.1099/jgv.0.000422.
Delaune D, Iseni F. Drug development against smallpox: present and future. Antimicrob Agents Chemother. 2020;64:e01683-e1719. https://doi.org/10.1128/AAC.01683-19.
Petersen BW, Damon IK, Pertowski CA, Meaney-Delman D, Guarnizo JT, Beigi RH, Edwards KM, Fisher MC, Frey SE, Lynfield R, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep. 2015;64:1–26.
Greenberg RN, Kennedy JS. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008;17:555–64. https://doi.org/10.1517/13543784.17.4.555.
Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Ogata M, Fukushi S, Mizutani T, Sata T, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R. Protects Monkeys from Monkeypox J Virol. 2006;80:5179–88. https://doi.org/10.1128/JVI.02642-05.
Iizuka I, Ami Y, Suzaki Y, Nagata N, Fukushi S, Ogata M, Morikawa S, Hasegawa H, Mizuguchi M, Kurane I, et al. A single vaccination of nonhuman primates with highly attenuated smallpox vaccine, LC16m8, provides long-term protection against monkeypox. Jpn J Infect Dis. 2017;70:408–15. https://doi.org/10.7883/yoken.JJID.2016.417.
Russo AT, Berhanu A, Bigger CB, Prigge J, Silvera PM, Grosenbach DW, Hruby D. Co-administration of tecovirimat and ACAM2000™ in non-human primates: effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644–54. https://doi.org/10.1016/j.vaccine.2019.10.049.
Duraffour S, Andrei G, Topalis D, Krečmerová M, Crance J-M, Garin D, Snoeck R. Mutations conferring resistance to viral DNA polymerase inhibitors in camelpox virus give different drug-susceptibility profiles in vaccinia virus. J Virol. 2012;86:7310–25. https://doi.org/10.1128/JVI.00355-12.
Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652–4. https://doi.org/10.1001/archdermatol.2011.20.
Hostetler KY, Beadle JR, Trahan J, Aldern KA, Owens G, Schriewer J, Melman L, Buller RM. Oral 1-O-Octadecyl-2-O-Benzyl-Sn-Glycero-3-Cidofovir targets the lung and is effective against a lethal respiratory challenge with ectromelia virus in mice. Antiviral Res. 2007;73:212–8. https://doi.org/10.1016/j.antiviral.2006.10.009.
Tippin TK, Morrison ME, Brundage TM, Momméja-Marin H. Brincidofovir is not a substrate for the human organic anion transporter 1: a mechanistic explanation for the lack of nephrotoxicity observed in clinical studies. Ther Drug Monit. 2016;38:777–86. https://doi.org/10.1097/FTD.0000000000000353.
Cohen JI, Davila W, Ali MA, Turk S-P, Cowen EW, Freeman AF, Wang K. Detection of molluscum contagiosum virus (MCV) DNA in the Plasma of an immunocompromised patient and possible reduction of MCV DNA With CMX-001. J Infect Dis. 2012;205:794–7. https://doi.org/10.1093/infdis/jir853.
Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–62. https://doi.org/10.1016/S1473-3099(22)00228-6.
Smith SK, Olson VA, Karem KL, Jordan R, Hruby DE, Damon IK. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob Agents Chemother. 2009;53:1007–12. https://doi.org/10.1128/AAC.01044-08.
Thakur V, Thakur P, Srivastava S, Kumar P. Monkeypox virus (MPX) in humans a concern: trespassing the global boundaries—correspondence. Int J Surg. 2022;104:106703. https://doi.org/10.1016/j.ijsu.2022.106703.
Filippova EI. Antiviral activity of lady’s mantle (Alchemilla Vulgaris L.) extracts against orthopoxviruses. Bull Exp Biol Med. 2017;163:374–7. https://doi.org/10.1007/s10517-017-3807-x.
Arndt W, Mitnik C, Denzler KL, White S, Waters R, Jacobs BL, Rochon Y, Olson VA, Damon IK, Langland JO. In vitro characterization of a nineteenth-century therapy for smallpox. PLoS ONE. 2012;7:e32610. https://doi.org/10.1371/journal.pone.0032610.
Altayb HN. Fludarabine, a potential DNA-dependent RNA polymerase inhibitor, as a prospective drug against monkeypox virus: a computational approach. Pharmaceuticals. 2022;15:1129. https://doi.org/10.3390/ph15091129.
Lam T-P, Tran V-H, Mai TT, Lai NV-T, Dang B-TN, Le M-T, Tran T-D, Trinh D-TT, Thai K-M. Identification of diosmin and flavin adenine dinucleotide as repurposing treatments for monkeypox virus: a computational study. IJMS. 2022;23:11570. https://doi.org/10.3390/ijms231911570.
Burkhanova TM, Krysantieva AI, Babashkina MG, Konyaeva IA, Monina LN, Goncharenko AN, Safin DA. In silico analyses of betulin: DFT studies, corrosion inhibition properties, ADMET prediction, and molecular docking with a series of SARS-CoV-2 and monkeypox proteins. Struct Chem. 2022. https://doi.org/10.1007/s11224-022-02079-8.
Odhar HA. Computational repurposing of fda approved drugs against monkeypox virus cysteine proteinase: a molecular docking and dynamics simulation study. Charlottesville: OSF Preprints; 2022.
Sahoo AK, Augusthian PD, Muralitharan I, Vivek-Ananth RP, Kumar K, Kumar G, Ranganathan G, Samal A. In silico identification of potential inhibitors of vital monkeypox virus proteins from FDA approved drugs. Mol Divers. 2022. https://doi.org/10.1007/s11030-022-10550-1.
Branda F, Pierini M, Mazzoli S. Monkeypox: EpiMPX surveillance system and open data with a special focus on European and Italian Epidemic. J Clin Virol Plus. 2022;2:100114. https://doi.org/10.1016/j.jcvp.2022.100114.
Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos MÁ, Vilella A, Navarro M, Rodriguez-Elena L, Riera J, Català A, Martínez MJ, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance. 2022. https://doi.org/10.2807/1560-7917.ES.2022.27.28.2200503.
Poxviruses. In Hunter’s tropical medicine and emerging infectious disease; Elsevier, 2013; pp. 256–262
Doshi RH, Guagliardo SAJ, Dzabatou-Babeaux A, Likouayoulou C, Ndakala N, Moses C, Olson V, McCollum AM, Petersen BW. Strengthening of surveillance during monkeypox outbreak, Republic of the Congo, 2017. Emerg Infect Dis. 2018;24:1158–60. https://doi.org/10.3201/eid2406.180248.
Wannigama DL, Amarasiri M, Hongsing P, Hurst C, Modchang C, Chadsuthi S, Anupong S, Phattharapornjaroen P, Fernandez SMAHRS, et al. Multiple traces of monkeypox detected in non-sewered wastewater with sparse sampling from a densely populated metropolitan Area in Asia. Sci Total Environ. 2023;858:159816. https://doi.org/10.1016/j.scitotenv.2022.159816.
Levy JI, Andersen KG, Knight R, Karthikeyan S. Wastewater surveillance for public health. Science. 2023;379:26–7. https://doi.org/10.1126/science.ade2503.
Tiwari A, Adhikari S, Kaya D, Islam MdA, Malla B, Sherchan SP, Al-Mustapha AI, Kumar M, Aggarwal S, Bhattacharya P, et al. Monkeypox outbreak: wastewater and environmental surveillance perspective. Sci Total Environ. 2023;856:159166. https://doi.org/10.1016/j.scitotenv.2022.159166.
Hemati S, Mohammadi-Moghadam F. A systematic review on environmental perspectives of monkeypox virus. Rev Environ Health. 2023. https://doi.org/10.1515/reveh-2022-0221.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, SB, PS and RS; writing—original draft preparation, SB, LN, PS, SP; writing—review and editing, PS, RS and AG; supervision, AG; project administration, PS and RS All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Begum, J.P.S., Ngangom, L., Semwal, P. et al. Emergence of monkeypox: a worldwide public health crisis. Human Cell 36, 877–893 (2023). https://doi.org/10.1007/s13577-023-00870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00870-1