Abstract
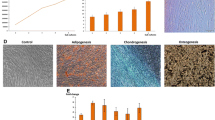
Among all the adult stem cells, odontogenic stem cells inherit the characterization of neurogenic potential of their precursor ones–the cranial crest cells. Dental follicle cells (DFCs), one of the special kind of odontogenic stem cells, are raising interest in applying to regenerative medicine for they possess multi-differentiation potential, relatively free access and ethic-friendly characteristic. Parkinson’s disease (PD), as one of the common neurodegenerative disorders, affects about 0.3% of the general population. Stem cell therapies are thought to be effective to treat it. Aiming at tackling ethical-concernings, confined sources and practically applicational limits, we made use of dopaminergic neurongenic differentiation potential of the DFCs and dedicated every effort to applying them as promising cell source for treating PD. Dental follicle cells were cultured from human dental follicle tissues collected from 12 to 18-year-old teenagers’ completely impacted third molars. Our data demonstrated that hDFCs were expressing mesenchymal stem cell-associated surface markers, and possessed the ability of osteogenic, adipogenic and neurogenic differentiation in vitro. Additionally, hDFCs formed neuron-like cells in vitro and in vivo, as well as expressing dopaminergic-neuronogenic marker–TH. Moreover, hDFCs survived in the transplanted areas of the Parkinson’s disease model of mouse over six weeks post-surgery, and the number of TH-positive DFCs in the DFCs-Grafted group surpassed its counterpart of the MPTP group with statistically significant difference. This study indicated that hDFCs might be a promising source of dopaminergic neurons for functional transplantation, and encouraged further detailed studies on the potential of hDFCs for treating PD.







Similar content being viewed by others
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- PD:
-
Parkinson’s disease
- DPSCs:
-
Dental pulp stem cells
- SHEDs:
-
Stem cells from exfoliated deciduous teeth
- DFCs:
-
Dental follicle cells
- SCAPs:
-
Stem cells from apical papilla
- DPLSCs:
-
Dental periodontal ligament stem cells
- BMSCs:
-
Bone marrow stromal cells
- NCCs:
-
Neural crest cells
- GFAP:
-
Glial fibrillary acidic protein
- ESCs:
-
Embryonic stem cells
- NSCs:
-
Neural stem cells
- PBS:
-
Phosphate buffer saline
- α-MEM:
-
Alpha type of modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- TH:
-
Tyrosine hydroxylase
- SNc:
-
Substantia nigra compacta
- GFP:
-
Green fluorescent protein
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
References
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J. 2011;22:91–8.
Tang Y, Han L, Bai X, et al. Intranasal delivery of bone marrow stromal cells preconditioned with Fasudil to treat a mouse model of Parkinson’s disease. Neuropsychiatr Dis Treat. 2020;16:249–62.
Park BN, Kim JH, Lee K, Park SH, An YS. Improved dopamine transporter binding activity after bone marrow mesenchymal stem cell transplantation in a rat model of Parkinson’s disease: small animal positron emission tomography study with F-18 FP-CIT. Eur Radiol. 2015;25:1487–96.
Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9.
Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12.
Morsczeck C, Vollner F, Saugspier M, et al. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig. 2010;14:433–40.
Yang C, Sun L, Li X, et al. The potential of dental stem cells differentiating into neurogenic cell lineage after cultivation in different modes in vitro. Cell Reprogram. 2014;16:379–91.
Chen G, Sun Q, Xie L, et al. Comparison of the odontogenic differentiation potential of dental follicle, dental papilla, and cranial neural crest cells. J Endod. 2015;41:1091–9.
Yang C, Li X, Sun L, Guo W, Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng. 2017;14:26005.
Sonntag KC, Song B, Lee N, et al. Pluripotent stem cell-based therapy for Parkinson’s disease: current status and future prospects. Prog Neurobiol. 2018;168:1–20.
Luo SX, Huang EJ. Dopaminergic neurons and brain reward pathways: from neurogenesis to circuit assembly. Am J Pathol. 2016;186:478–88.
Vasudevan S, Kajtez J, Bunea AI, et al. Leaky optoelectrical fiber for optogenetic stimulation and electrochemical detection of dopamine exocytosis from human dopaminergic neurons. Adv Sci (Weinh). 2019;6:1902011.
Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson’s disease. Nature. 1999;399:A32-39.
Parmar M. Towards stem cell based therapies for Parkinson's disease. Development. 2018; 145.
Chi H, Guan Y, Li F, Chen Z. The effect of human umbilical cord mesenchymal stromal cells in protection of dopaminergic neurons from apoptosis by reducing oxidative stress in the early stage of a 6-OHDA-induced Parkinson’s disease model. Cell Transplant. 2019;28:87S-99S.
Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164:215–26.
Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–9.
Alizadeh R, Kamrava SK, Bagher Z, et al. Human olfactory stem cells: as a promising source of dopaminergic neuron-like cells for treatment of Parkinson’s disease. Neurosci Lett. 2019;696:52–9.
Meligy FY, Elgamal DA, Abd AE, et al. Testing alternatives: the use of adipose-derived mesenchymal stem cells to slow neurodegeneration in a rat model of Parkinson’s disease. Mol Biol Rep. 2019;46:5841–58.
Lei P, Ayton S, Finkelstein DI, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–5.
Jo YY, Lee HJ, Kook SY, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–51.
Kiriyama K, Ohtaki H, Kobayashi N, et al. A nucleoprotein-enriched diet suppresses dopaminergic neuronal cell loss and motor deficit in mice with MPTP-induced Parkinson’s disease. J Mol Neurosci. 2015;55:803–11.
Cao Q, Qin L, Huang F, et al. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol Appl Pharmacol. 2017;319:80–90.
Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–8.
Kuribara H, Higuchi Y, Tadokoro S. Effects of central depressants on rota-rod and traction performances in mice. Jpn J Pharmacol. 1977;27:117–26.
Fan Y, Winanto, Ng SY. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl Neurodegener. 2020;9:2.
Du Y, Ling J, Wei X, et al. Wnt/beta-catenin signaling participates in cementoblast/osteoblast differentiation of dental follicle cells. Connect Tissue Res. 2012;53:390–7.
Chin S, Furukawa K, Ono A, et al. Immunohistochemical localization of mesenchymal stem cells in ossified human spinal ligaments. Biochem Biophys Res Commun. 2013;436:698–704.
Chang CC, Chang KC, Tsai SJ, Chang HH, Lin CP. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc. 2014;113:956–65.
Rezai-Rad M, Bova JF, Orooji M, et al. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy. 2015;17:1572–81.
Lin CY, Chin YT, Kuo PJ, et al. 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-glucoside potentiates self-renewal of human dental pulp stem cells via the AMPK/ERK/SIRT1 axis. Int Endod J. 2018;51:1159–70.
Ulusoy C, Zibandeh N, Yildirim S, et al. Dental follicle mesenchymal stem cell administration ameliorates muscle weakness in MuSK-immunized mice. J Neuroinflammation. 2015;12:231.
Topcu SL, Zibandeh N, Genc D, et al. Immunomodulatory and tissue-preserving effects of human dental follicle stem cells in a rat cecal ligation and perforation sepsis model. Arch Med Res. 2020;51:397–405.
Genc D, Zibandeh N, Nain E, et al. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin Exp Allergy. 2018;48:663–78.
Mahajan VS, Demissie E, Mattoo H, et al. Striking immune phenotypes in gene-targeted mice are driven by a copy-number variant originating from a commercially available C57BL/6 strain. Cell Rep. 2016;15:1901–9.
Li X, Yang C, Li L, et al. A therapeutic strategy for spinal cord defect: human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res Int. 2015;2015: 197183.
Mendes-Pinheiro B, Anjo SI, Manadas B, et al. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective effects in a Parkinson’s disease rat model. Front Bioeng Biotechnol. 2019;7:294.
Tan K, Lim W, Chai C, et al. Sequential application of discrete topographical patterns enhances derivation of functional mesencephalic dopaminergic neurons from human induced pluripotent stem cells. Sci Rep. 2018;8:9567.
Soheilifar MH, Javeri A, Amini H, Taha MF. Generation of dopamine-secreting cells from human adipose tissue-derived stem cells in vitro. Rejuvenation Res. 2018;21:360–8.
Suon S, Yang M, Iacovitti L. Adult human bone marrow stromal spheres express neuronal traits in vitro and in a rat model of Parkinson’s disease. Brain Res. 2006;1106:46–51.
Guo W, He Y, Zhang X, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708–23.
Zhu Z, Yichen W, Ziheng Z, et al. The loss of dopaminergic neurons in DEC1 deficient mice potentially involves the decrease of PI3K/Akt/GSK3beta signaling. Aging (Albany NY). 2019;11:12733–53.
Wu C, Xue LD, Su LW, et al. Magnesium promotes the viability and induces differentiation of neural stem cells both in vitro and in vivo. Neurol Res. 2019;41:208–15.
Munoz MF, Arguelles S, Medina R, Cano M, Ayala A. Adipose-derived stem cells decreased microglia activation and protected dopaminergic loss in rat lipopolysaccharide model. J Cell Physiol. 2019;234:13762–72.
Mendes-Pinheiro B, Teixeira FG, Anjo SI, Manadas B, Behie LA, Salgado AJ. Secretome of undifferentiated neural progenitor cells induces histological and motor improvements in a rat model of Parkinson’s disease. Stem Cells Transl Med. 2018;7:829–38.
L’Episcopo F, Tirolo C, Peruzzotti-Jametti L, et al. Neural stem cell grafts promote astroglia-driven neurorestoration in the aged Parkinsonian brain via Wnt/beta-catenin signaling. Stem Cells. 2018;36:1179–97.
Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91.
Giovanni A, Sonsalla PK, Heikkila RE. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 2: central administration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther. 1994;270:1008–14.
Sood A, Shukla J, Shree R, Vatsa R, Modi M, Mittal BR. Comparative performance of 99mTc-TRODAT-1 SPECT/CT and 18F-FDOPA PET/CT imaging in patients with Parkinson’s disease, parkinson-plus syndrome, and essential tremor. Clin Nucl Med. 2021;46:95–102.
Acknowledgements
This work is supported by National Key Research and Development Program of China (Nos. 2017YFA0104800), National Natural Science Foundation of China (31771062, 31971281), and Key Research and Development Program of Sichuan Province (2017SZ0031). The authors express their most grateful gratitude for the kindly support.
Author information
Authors and Affiliations
Contributions
BF, experiments performing, data collection and analyzing and manuscript drafting; XJ, HX and YC, experiments interpretation and data analyzing; LXH and CGQ, data analyzing; TWD and GWH, conception and design, supervision of project conduction; BF and GWH, critical revision of the manuscript. The authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial, personal, or other conflicts of interest to disclose. We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of this manuscript.
Ethics approval and consent to participate
Ethical approval to report this case was obtained from Ethics Committee of the State Key Laboratory of Oral Diseases, West China Hospital of Stomatology (WCHSIRB-D-2012-058). All animal experiments were conducted in accordance with the principles and procedures of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the State Key Laboratory of Oral Diseases, West China Hospital of Stomatology (Chengdu, Sichuan).
Consent for publication
There are no human subjects in this article and informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (WMV 16566 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bi, F., Xiong, J., Han, X. et al. Dental follicle cells show potential for treating Parkinson’s disease through dopaminergic-neuronogenic differentiation. Human Cell 35, 1708–1721 (2022). https://doi.org/10.1007/s13577-022-00774-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00774-6