Abstract
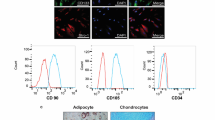
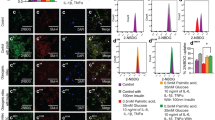
Obesity poses a significant risk factor for the onset of metabolic syndrome with allied complications, wherein mesenchymal stem cell therapy is seen as a promising treatment for obesity-induced metabolic syndrome. In the present study, we aim to explore the beneficial effects of the human placental mesenchymal stromal cells (P-MSCs) on obesity-associated insulin resistance (IR) including inflammation. To understand this, we have analyzed the peripheral blood glucose, serum insulin levels by ELISA, and the glucose uptake capacity of skeletal muscle by a 2-NBDG assay using flow cytometry in WNIN/GR-Ob rats treated with and without P-MSCs. Also, we have studied insulin signaling and cytokine profile in the skeletal muscle by western blotting, dot blotting, and Multiplex-ELISA techniques. The skeletal muscle of WNIN/GR-Ob rats demonstrates dysregulation of cytokines, altered glucose uptake vis-a-vis insulin signaling. However, P-MSCs’ treatment was effective in WNIN/GR-Ob rats as compared to its control, to restore HOMA-IR, re-establishes dysregulated cytokines and PI3K-Akt pathway in addition to enhanced Glut4 expression and glucose uptake studied in skeletal muscle. Overall, our data advocate the beneficial effects of P-MSCs to ameliorate inflammatory milieu, improve insulin sensitivity, and normalize glucose homeostasis underlining the Ob-T2D conditions, and we attribute for immunomodulatory, paracrine, autocrine, and multipotent functions of P-MSCs.




Similar content being viewed by others
References
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. https://doi.org/10.1210/er.2008-0024.
Harishankar N, Vajreswari A, Giridharan NV. WNIN/GR-Ob—an insulin-resistant obese rat model from inbred WNIN strain. Indian J Med Res. 2011;134(3):320–9.
Giridharan NV, Lakshmi CN, Raghuramulu N. Identification of impaired-glucose-tolerant animals from a Wistar inbred rat colony. Lab Anim Sci. 1997;47(4):428–31.
Kalashikam RR, Battula KK, Kirlampalli V, Friedman JM, Nappanveettil G. Obese locus in WNIN/obese rat maps on chromosome 5 upstream of leptin receptor. PLoS ONE. 2013;8(10): e77679. https://doi.org/10.1371/journal.pone.0077679.
Pragasam SSJ, Venkatesan V. Metabolic syndrome predisposes to osteoarthritis: lessons from model system. Cartilage. 2020. https://doi.org/10.1177/1947603520980161.
Singh H, Ganneru S, Malakapalli V, Chalasani M, Nappanveettil G, Bhonde RR, et al. Islet adaptation to obesity and insulin resistance in WNIN/GR-Ob rats. Islets. 2014;6(5–6): e998099. https://doi.org/10.1080/19382014.2014.998099.
Madhira SL, Challa SS, Chalasani M, Nappanveethl G, Bhonde RR, Ajumeera R, et al. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS ONE. 2012;7(10): e48061. https://doi.org/10.1371/journal.pone.0048061.
Giridharan NV. Glucose & energy homeostasis: lessons from animal studies. Indian J Med Res. 2018;148(5):659–69. https://doi.org/10.4103/ijmr.IJMR_1737_18.
Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, Vajreswari A. Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab. 2009;6:27. https://doi.org/10.1186/1743-7075-6-27.
Jeyakumar SM, Sheril A, Vajreswari A. Vitamin A improves hyperglycemia and glucose-intolerance through regulation of intracellular signaling pathways and glycogen synthesis in WNIN/GR-Ob obese rat model. Prev Nutr Food Sci. 2017;22(3):172–83. https://doi.org/10.3746/pnf.2017.22.3.172.
Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65(2):193–207. https://doi.org/10.33549/physiolres.932993.
Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Investig. 2017;127(1):43–54. https://doi.org/10.1172/jci88880.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Investig. 2006;116(7):1793–801. https://doi.org/10.1172/jci29069.
Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2018;9(1):1–58. https://doi.org/10.1002/cphy.c170040.
Mourkioti F, Rosenthal N. NF-kappaB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med (Berl). 2008;86(7):747–59. https://doi.org/10.1007/s00109-008-0308-4.
Proto JD, Tang Y, Lu A, Chen WC, Stahl E, Poddar M, et al. NF-κB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015;6(4): e1730. https://doi.org/10.1038/cddis.2015.66.
da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8(19): e14607. https://doi.org/10.14814/phy2.14607.
Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–4. https://doi.org/10.2337/diabetes.48.6.1270.
Marrocco V, Fiore P, Madaro L, Crupi A, Lozanoska-Ochser B, Bouché M. Targeting PKCθ in skeletal muscle and muscle diseases: good or bad? Biochem Soc Trans. 2014;42(6):1550–5. https://doi.org/10.1042/bst20140207.
Pereira S, Cline DL, Glavas MM, Covey SD, Kieffer TJ. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr Rev. 2021;42(1):1–28. https://doi.org/10.1210/endrev/bnaa027.
Muoio DM, Dohm GL, Fiedorek FT Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46(8):1360–3. https://doi.org/10.2337/diab.46.8.1360.
Pratipanawatr W, Pratipanawatr T, Cusi K, Berria R, Adams JM, Jenkinson CP, et al. Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation. Diabetes. 2001;50(11):2572–8. https://doi.org/10.2337/diabetes.50.11.2572.
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. https://doi.org/10.1146/annurev-immunol-031210-101322.
Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor Perspect Biol. 2014. https://doi.org/10.1101/cshperspect.a009191.
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–61. https://doi.org/10.1038/ni.1836.
Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–20. https://doi.org/10.1038/ni.2639.
Wang M, Song L, Strange C, Dong X, Wang H. Therapeutic effects of adipose stem cells from diabetic mice for the treatment of type 2 diabetes. Mol Ther. 2018;26(8):1921–30. https://doi.org/10.1016/j.ymthe.2018.06.013.
Yaochite JN, Caliari-Oliveira C, de Souza LE, Neto LS, Palma PV, Covas DT, et al. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res Ther. 2015;6(1):31. https://doi.org/10.1186/s13287-015-0017-1.
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61(6):1616–25. https://doi.org/10.2337/db11-1141.
Venkatesan V, Chalsani M, Nawaz SS, Bhonde RR, Challa SS, Nappanveettil G. Optimization of condition(s) towards establishment of primary islet cell cultures from WNIN/Ob mutant rat. Cytotechnology. 2012;64(2):139–44. https://doi.org/10.1007/s10616-011-9409-y.
Venkatesan V, Gopurappilly R, Goteti SK, Dorisetty RK, Bhonde RR. Pancreatic progenitors: the shortest route to restore islet cell mass. Islets. 2011;3(6):295–301. https://doi.org/10.4161/isl.3.6.17704.
Kotikalapudi N, Sampath SJP, Sukesh Narayan S, Ramesh RB, Nemani H, Mungamuri SK, et al. The promise(s) of mesenchymal stem cell therapy in averting preclinical diabetes: lessons from in vivo and in vitro model systems. Sci Rep. 2021;11(1):16983. https://doi.org/10.1038/s41598-021-96121-0.
Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res Ther. 2020;11(1):401. https://doi.org/10.1186/s13287-020-01865-7.
Mathew SA, Chandravanshi B, Bhonde R. Hypoxia primed placental mesenchymal stem cells for wound healing. Life Sci. 2017;182:85–92. https://doi.org/10.1016/j.lfs.2017.06.016.
Mathew SA, Bhonde RR. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol Res. 2018;132:90–8. https://doi.org/10.1016/j.phrs.2018.04.002.
Roman EA, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA, et al. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol. 2010;314(1):62–9. https://doi.org/10.1016/j.mce.2009.08.007.
Wong N, Fam BC, Cempako GR, Steinberg GR, Walder K, Kay TW, et al. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology. 2011;152(10):3690–9. https://doi.org/10.1210/en.2011-0288.
Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: a case-control study. Diabetes Care. 2006;29(5):1096–101. https://doi.org/10.2337/diacare.2951096.
Mungamuri SK, Qiao RF, Yao S, Manfredi JJ, Gu W, Aaronson SA. USP7 enforces heterochromatinization of p53 target promoters by protecting SUV39H1 from MDM2-mediated degradation. Cell Rep. 2016;14(11):2528–37. https://doi.org/10.1016/j.celrep.2016.02.049.
Chen Q, Rong P, Zhu S, Yang X, Ouyang Q, Wang HY, et al. Targeting RalGAPα1 in skeletal muscle to simultaneously improve postprandial glucose and lipid control. Sci Adv. 2019;5(4):eaav4116. https://doi.org/10.1126/sciadv.aav4116.
TeSlaa T, Teitell MA. Techniques to monitor glycolysis. Methods Enzymol. 2014;542:91–114. https://doi.org/10.1016/b978-0-12-416618-9.00005-4.
Mungamuri SK, Wang S, Manfredi JJ, Gu W, Aaronson SA. Ash2L enables P53-dependent apoptosis by favoring stable transcription pre-initiation complex formation on its pro-apoptotic target promoters. Oncogene. 2015;34(19):2461–70. https://doi.org/10.1038/onc.2014.198.
Li Z. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol. 2013;2(1):17. https://doi.org/10.1186/2162-3619-2-17.
Paprocka M, Krawczenko A, Dus D, Kantor A, Carreau A, Grillon C, et al. CD133 positive progenitor endothelial cell lines from human cord blood. Cytom Part A. 2011;79(8):594–602. https://doi.org/10.1002/cyto.a.21092.
Nishimura-Sakurai Y, Sakamoto N, Mogushi K, Nagaie S, Nakagawa M, Itsui Y, et al. Comparison of HCV-associated gene expression and cell signaling pathways in cells with or without HCV replicon and in replicon-cured cells. J Gastroenterol. 2010;45(5):523–36. https://doi.org/10.1007/s00535-009-0162-3.
Zha K, Li X, Yang Z, Tian G, Sun Z, Sui X, et al. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regen Med. 2021;6(1):14. https://doi.org/10.1038/s41536-021-00122-6.
Koyama N, Okubo Y, Nakao K, Osawa K, Fujimura K, Bessho K. Pluripotency of mesenchymal cells derived from synovial fluid in patients with temporomandibular joint disorder. Life Sci. 2011;89(19–20):741–7. https://doi.org/10.1016/j.lfs.2011.09.005.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. https://doi.org/10.2337/diacare.27.6.1487.
Venkatesan V, Madhira SL, Malakapalli VM, Chalasani M, Shaik SN, Seshadri V, et al. Obesity, insulin resistance, and metabolic syndrome: a study in WNIN/Ob rats from a pancreatic perspective. Biomed Res Int. 2013;2013: 617569. https://doi.org/10.1155/2013/617569.
Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet (London, England). 2010;375(9733):2267–77. https://doi.org/10.1016/s0140-6736(10)60408-4.
DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–63. https://doi.org/10.2337/dc09-S302.
Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017;315:18–26. https://doi.org/10.1016/j.cellimm.2017.03.001.
Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305–11. https://doi.org/10.1038/nrendo.2009.62.
Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–16. https://doi.org/10.1038/nrendo.2012.114.
Frydrych LM, Bian G, O’Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104(3):525–34. https://doi.org/10.1002/jlb.5vmr0118-021rr.
Bandaru P, Rajkumar H, Nappanveettil G. Altered or impaired immune response to hepatitis B vaccine in WNIN/GR-Ob rat: an obese rat model with impaired glucose tolerance. ISRN Endocrinol. 2011;2011: 980105. https://doi.org/10.5402/2011/980105.
Bandaru P, Rajkumar H, Nappanveettil G. Altered or impaired immune response upon vaccination in WNIN/Ob rats. Vaccine. 2011;29(16):3038–42. https://doi.org/10.1016/j.vaccine.2011.01.107.
Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells (Dayton, Ohio). 2014;32(6):1408–19. https://doi.org/10.1002/stem.1681.
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286(28):25007–15. https://doi.org/10.1074/jbc.M110.213108.
Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–97. https://doi.org/10.1146/annurev-pathol-011110-130138.
Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells (Dayton, Ohio). 2016;34(3):627–39. https://doi.org/10.1002/stem.2238.
Sun X, Hao H, Han Q, Song X, Liu J, Dong L, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8(1):241. https://doi.org/10.1186/s13287-017-0668-1.
Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–16. https://doi.org/10.1038/ni.3002.
von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells (Dayton, Ohio). 2012;30(7):1575–8. https://doi.org/10.1002/stem.1118.
Mäkelä T, Takalo R, Arvola O, Haapanen H, Yannopoulos F, Blanco R, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392–402. https://doi.org/10.1016/j.jcyt.2014.12.004.
Hamidian Jahromi S, Davies JE. Concise review: skeletal muscle as a delivery route for mesenchymal stromal cells. Stem Cells Transl Med. 2019;8(5):456–65. https://doi.org/10.1002/sctm.18-0208.
Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008;17(12):1295–304. https://doi.org/10.3727/096368908787648119.
Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18(10):1407–16. https://doi.org/10.1089/scd.2009.0164.
Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23(9):1075–85. https://doi.org/10.3727/096368913x665576.
Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, et al. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5(2):57. https://doi.org/10.1186/scrt446.
Bhansali S, Kumar V, Saikia UN, Medhi B, Jha V, Bhansali A, et al. Effect of mesenchymal stem cells transplantation on glycaemic profile & their localization in streptozotocin induced diabetic Wistar rats. Indian J Med Res. 2015;142(1):63–71. https://doi.org/10.4103/0971-5916.162116.
Kong D, Zhuang X, Wang D, Qu H, Jiang Y, Li X, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. 2014;60(12):1969–76. https://doi.org/10.7754/clin.lab.2014.140305.
Skyler JS, Fonseca VA, Segal KR, Rosenstock J. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. 2015;38(9):1742–9. https://doi.org/10.2337/dc14-2830.
Wu Z, Cai J, Chen J, Huang L, Wu W, Luo F, et al. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy. 2014;16(2):258–65. https://doi.org/10.1016/j.jcyt.2013.10.004.
Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, et al. Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: a randomized, placebo-controlled comparative study. Stem Cells Dev. 2017;26(7):471–81. https://doi.org/10.1089/scd.2016.0275.
Acknowledgements
The authors would like to acknowledge Dr. R. Hemalatha, Director, ICMR-NIN, for providing the necessary infrastructure to conduct the current research and sanction intramural funding. We wish to thank the Animal Facility for all its support in carrying out these experiments. We also thank ICMR for the SRF manpower support.
Funding
The present study has been supported by ICMR-NIN intramural fundings (15-BS03 and 18-BS10) to Dr. VV, KN received a Senior Research Fellowship (5/3/8/31/ITR-F/2018-ITR) from ICMR for the present study.
Author information
Authors and Affiliations
Contributions
KN carried out most of the animal experimentation, cell, and molecular work including characterization and injection of P-MSCs. SJPS was also associated in preparation, characterization, and injection of P-MSCs into rats. SNS and SKM were involved in designing some experiments, configuration of figures, and preparation of the manuscript. RRB was involved in human placenta collection, human ethical approval, as well as isolation of P-MSCs. VV coordinated the overall team, including project design and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this article.
Ethics statement
The authors confirm that the journal's ethical policies, as noted on the journal's author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received. Institutional Animal Ethics Committees (IAECs): P35F/IAEC/NIN/11/2012/VV/WNIN. Institutional Human Ethics Committees: MHB/SCR/021.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kotikalapudi, N., Sampath, S.J.P., Sinha, S.N. et al. Human placental mesenchymal stromal cell therapy restores the cytokine efflux and insulin signaling in the skeletal muscle of obesity-induced type 2 diabetes rat model. Human Cell 35, 557–571 (2022). https://doi.org/10.1007/s13577-021-00664-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00664-3