Abstract
Background
Hypofractionation has been recently considered as an alternative to improve stereotactic radiosurgery treatments of lesions close to the optic pathways. To estimate the intrinsic benefit from fractionation versus single-dose radiosurgery for perioptic lesions, the value of the alpha/beta ratio of the optic pathways needs to be known. Based on the linear quadratic (LQ) model, hypofractionation versus single-fraction SRS can be justified in cases where parts of the optic apparatus necessarily receive the full therapeutic peripheral dose, if there is a positive difference between α/ß of the lesion and the α/ß of the surrounding organs at risk. Furthermore, the knowledge of α/ß ratios is required to calculate radiobiological dose parameters, such as the biologically effective dose (BED) and single fraction equivalent dose (SFED), and helps estimate normal tissue complication probability (NTCP), dose constraints, and retreatment doses. Only 3 alpha/beta ratios for the visual system have been published so far, varying between -0.6 and 3.06 Gy.
Material and methods
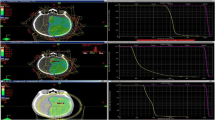
The alpha/beta ratio of the optic pathways was estimated from a fraction equivalent plot based on a meta-analysis of 429 studies published between 2000 and June 2018. We included 15 studies with fraction sizes between 1 and 31, considering the following inclusion criteria: at least one well-documented RION case with detailed dosimetric analysis for the visual system, follow-up period (FUP) of at least 24 months, no tumor progression, no prior radiation. Additionally, we included results from our center on 68 hypofractionated treatments and 161 single-fraction SRS treatments for perioptic lesions.
Results
The fraction equivalent (FE) plot method revealed an alpha/beta ratio of the optic pathway of 1.03 Gy, confidence interval [-0.38–1.60]. Well-documented RION cases are rare in the literature; there is still not enough data to distinguish between alpha/beta ratios of the optic chiasm, the nerves, and the tracts. Optimized hypofractionation schedules were calculated for the treatment of meningiomas, chordomas, and brain metastases.
Conclusion
Compared to single-fraction SRS, a significant intrinsic benefit from hypofractionation can be achieved, not only for perioptic malignant tumors, but for benign lesions as well, because of the very low alpha/beta ratio of the optic system of 1.03 Gy. An increased single fraction equivalent dose of up to 10% for perioptic meningiomas and of more than 25% for malignant tumors can be reached with optimized hypofractionated stereotactic radiosurgery schedules.


Similar content being viewed by others
Abbreviations
- BED:
-
Biologically effective dose
- SFED:
-
Single fraction equivalent dose
- NTCP:
-
Normal tissue complication probability
- TCP:
-
Tumor control probability
- RION:
-
Radiation-induced optical neuropathy
- FUP:
-
Follow-up period
- FE:
-
Fraction equivalent
- SRS:
-
Stereotactic radiosurgery
- HFSRS:
-
Hypofractionated stereotactic radiosurgery
- FSRT:
-
Fractionated stereotactic radiotherapy
- RT:
-
Radiotherapy
- OAR:
-
Organ at risk
- LQ:
-
Linear quadratic
- SBRT:
-
Stereotactic body radiotherapy
- Dmax:
-
Maximum point dose
- AVP:
-
Anterior visual pathway
- AVM:
-
Arteriovenous malformation
- GKRS:
-
Gamma Knife stereotactic radiosurgery
- FGATIR:
-
Fast gray matter acquisition T1 inversion recovery
References
Kondziolka D, Lunsford L, Maitz A, Flickinger J (1998) Radiobiologic considerations in gamma knife radiosurgery. In: Gamma knife brain surgery. Karger, Basel, pp 21–38
Adler JR, Gibbs IC, Puataweepong P, Chang SD (2006) Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesions. Neurosurgery 59:244–254. https://doi.org/10.1227/01.NEU.0000223512.09115.3E
Hiniker SM, Modlin LA, Choi CY, Atalar B, Seiger K, Binkley MS, Harris JP, Liao YJ, Fischbein N, Wang L, Ho A, Lo A, Chang SD, Harsh GR, Gibbs IC, Hancock SL, Li G, Adler JR, Soltys SG (2016) Dose-response modeling of the visual pathway tolerance to single-fraction and hypofractionated stereotactic radiosurgery. Semin Radiat Oncol 26:97–104. https://doi.org/10.1016/j.semradonc.2015.11.008
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J (2010) Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76:S28–S35. https://doi.org/10.1016/j.ijrobp.2009.07.1753
Milano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tomé WA, Sahgal A, Xue J, Ma L, Solberg TD, Kirkpatrick JP, Constine LS, Flickinger JC, Marks LB, el Naqa I (2018) Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2018.01.053
van den Bergh ACM, Hoving MA, Links TP, Dullaart RPF, Ranchor AV, ter Weeme CA, Canrinus AA, Szabó BG, Pott JWR (2003) Radiation optic neuropathy after external beam radiation therapy for acromegaly: report of two cases. Radiother Oncol 68:101–103. https://doi.org/10.1016/S0167-8140(03)00201-9
Danesh-Meyer HV (2008) Radiation-induced optic neuropathy. J Clin Neurosci 15:95–100. https://doi.org/10.1016/j.jocn.2007.09.004
Leavitt JA, Stafford SL, Link MJ, Pollock BE (2013) Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 87:524–527. https://doi.org/10.1016/j.ijrobp.2013.06.2047
Pollock BE, Link MJ, Leavitt JA, Stafford SL (2014) Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery 75:456–460. https://doi.org/10.1227/NEU.0000000000000457
Deng X, Yang Z, Liu R, Yi M, Lei D, Wang Z, Zhao H (2013) The maximum tolerated dose of gamma radiation to the optic nerve during γ knife radiosurgery in an animal study. Stereotact Funct Neurosurg 91:79–91. https://doi.org/10.1159/000343212
Gordon KB, Char DH, Sagerman RH (1995) Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys 31:1123–1139. https://doi.org/10.1016/0360-3016(95)00062-4
Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR (1994) Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys 30:755–763. https://doi.org/10.1016/0360-3016(85)90366-9
Hall EJ, Brenner DJ (1993) The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol 25:381–385. https://doi.org/10.1016/0360-3016(93)90367-5
Liu L, Bassano DA, Prasad SC, Hahn SS, Chung CT (2003) The linear-quadratic model and fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 57:827–832. https://doi.org/10.1016/S0360-3016(03)00634-5
Williams MV, Denekamp J, Fowler JF (1985) A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys 11:87–96. https://doi.org/10.1016/0360-3016(85)90366-9
Flickinger JC, Lunsford LD, Kondziolka D, Maitz AH, Epstein AH, Simons SR, Wu A (1992) Radiosurgery and brain tolerance: an analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol 23:19–26. https://doi.org/10.1016/0360-3016(92)90539-T
Bertrand R, Mongrain E, Thorin O (2000) In vitro response of human and porcine vascular cells exposed to high dose-rate γ-irradiation. Int J Radiat Biol 76:999–1007. https://doi.org/10.1080/09553000050051016
Carlson DJ, Stewart RD, Li XA, Jennings K, Wang JZ, Guerrero M (2004) Comparison of in vitro and in vivo alpha/beta ratios for prostate cancer. Phys Med Biol 49:4477–4491. https://doi.org/10.1088/0031-9155/49/19/003
Kondziolka D, Shin SM, Brunswick A, Kim I, Silverman JS (2015) The biology of radiosurgery and its clinical applications for brain tumors. Neuro-Oncol 17:29–44. https://doi.org/10.1093/neuonc/nou284
Kirkpatrick JP, Soltys SG, Lo SS et al (2017) The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro-Oncol 19:ii38–ii49. https://doi.org/10.1093/neuonc/now301
Shrieve DC, Hazard L, Boucher K, Jensen RL (2004) Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg 101(Suppl 3):390–395. https://doi.org/10.3171/jns.2004.101.supplement3.0390
Douglas BG, Fowler JF (1976) The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. Radiat Res 66:401–426. https://doi.org/10.2307/3574407
de Boer RW (1988) The use of the D versus dD plot to estimate the ratio from iso-effect radiation damage data. Radiother Oncol 11:361–367. https://doi.org/10.1016/0167-8140(88)90207-1
Tucker SL (1984) Tests for the fit of the linear-quadratic model to radiation isoeffect data. Int J Radiat Oncol 10:1933–1939. https://doi.org/10.1016/0360-3016(84)90274-8
Thames HD, Rozell ME, Tucker SL, Ang KK, Fisher DR, Travis EL (1986) Direct analysis of quantal radiation response data. Int J Radiat Biol Relat Stud Phys Chem Med 49:999–1009. https://doi.org/10.1080/09553008514553221
Kline LB, Kim JY, Ceballos R (1985) Radiation optic neuropathy. Ophthalmology 92:1118–1126. https://doi.org/10.1016/S0161-6420(85)33898-8
Astradsson A, Munck af Rosenschöld P, Feldt-Rasmussen U et al (2017) Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncol 56:415–421. https://doi.org/10.1080/0284186X.2016.1270466
Demizu Y, Murakami M, Miyawaki D, Niwa Y, Akagi T, Sasaki R, Terashima K, Suga D, Kamae I, Hishikawa Y (2009) Analysis of vision loss caused by radiation-induced optic neuropathy after particle therapy for head-and-neck and skull-base tumors adjacent to optic nerves. Int J Radiat Oncol 75:1487–1492. https://doi.org/10.1016/j.ijrobp.2008.12.068
Farzin M, Molls M, Kampfer S, Astner S, Schneider R, Roth K, Dobrei M, Combs S, Straube C (2016) Optic toxicity in radiation treatment of meningioma: a retrospective study in 213 patients. J Neuro-Oncol 127:597–606. https://doi.org/10.1007/s11060-016-2071-7
Grant RA, Whicker M, Lleva R, Knisely JPS, Inzucchi SE, Chiang VL (2014) Efficacy and safety of higher dose stereotactic radiosurgery for functional pituitary adenomas: a preliminary report. World Neurosurg 82:195–201. https://doi.org/10.1016/j.wneu.2013.01.127
Hasegawa T, Kobayashi T, Kida Y (2010) Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: evaluation in 100 patients with craniopharyngioma. Neurosurgery 66:688–694; discussion 694-695. https://doi.org/10.1227/01.NEU.0000367554.96981.26
Hauptman JS, Barkhoudarian G, Safaee M, Gorgulho A, Tenn S, Agazaryan N, Selch M, de Salles AAF (2012) Challenges in linear accelerator radiotherapy for chordomas and chondrosarcomas of the skull base: focus on complications. Int J Radiat Oncol 83:542–551. https://doi.org/10.1016/j.ijrobp.2011.08.004
Iwata H, Sato K, Tatewaki K, Yokota N, Inoue M, Baba Y, Shibamoto Y (2011) Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro-Oncol 13:916–922. https://doi.org/10.1093/neuonc/nor055
Park K-J, Kano H, Parry PV et al (2011) Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 69:1188–1199. https://doi.org/10.1227/NEU.0b013e318222afed
Ronson BB, Schulte RW, Han KP, Loredo LN, Slater JM, Slater JD (2006) Fractionated proton beam irradiation of pituitary adenomas. Int J Radiat Oncol 64:425–434. https://doi.org/10.1016/j.ijrobp.2005.07.978
Skeie BS, Enger PØ, Skeie GO, Thorsen F, Pedersen PH (2010) Gamma knife surgery of meningiomas involving the cavernous sinus. Neurosurgery 66:661–669. https://doi.org/10.1227/01.NEU.0000366112.04015.E2
Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ (2003) A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol 55:1177–1181. https://doi.org/10.1016/S0360-3016(02)04380-8
Weber DC, Momjian S, Pralong FP, Meyer P, Villemure JG, Pica A (2011) Adjuvant or radical fractionated stereotactic radiotherapy for patients with pituitary functional and nonfunctional macroadenoma. Radiat Oncol Lond Engl 6:169. https://doi.org/10.1186/1748-717X-6-169
Wenkel E, Thornton AF, Finkelstein D, Adams J, Lyons S, de la Monte S, Ojeman RG, Munzenrider JE (2000) Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol 48:1363–1370. https://doi.org/10.1016/S0360-3016(00)01411-5
Vernimmen FJAI, Slabbert JP (2010) Assessment of the α/ß ratios for arteriovenous malformations, meningiomas, acoustic neuromas, and the optic chiasma. Int J Radiat Biol 86:486–498. https://doi.org/10.3109/09553001003667982
Henderson FC, McCool K, Seigle J, Jean W, Harter W, Gagnon GJ (2009) Treatment of chordomas with CyberKnife: georgetown university experience and treatment recommendations. Neurosurgery 64:A44–A53. https://doi.org/10.1227/01.NEU.0000341166.09107.47
Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar A, Kondziolka D, Lunsford LD (2005) The impact of whole-brain radiation therapy on the long-term control and morbidity of patients surviving more than one year after gamma knife radiosurgery for brain metastases. Int J Radiat Oncol 62:1125–1132. https://doi.org/10.1016/j.ijrobp.2004.12.092
Hanin LG, Zaider M (2010) Cell-survival probability at large doses: an alternative to the linear-quadratic model. Phys Med Biol 55:4687–4702. https://doi.org/10.1088/0031-9155/55/16/005
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37:4078–4101. https://doi.org/10.1118/1.3438081
Leber KA, Berglöff J, Pendl G (1998) Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88:43–50. https://doi.org/10.3171/jns.1998.88.1.0043
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E III, Kooy HM, Flickinger JC (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27:215–221. https://doi.org/10.1016/0360-3016(93)90230-S
Xue J, Emami B, Grimm J, Kubicek GJ, Asbell SO, Lanciano R, Welsh JS, Peng L, Quon H, Laub W, Gui C, Spoleti N, Das IJ, Goldman HW, Redmond KJ, Kleinberg LR, Brady LW (2018) Clinical evidence for dose tolerance of the central nervous system in hypofractionated radiotherapy. J Radiat Oncol 7:293–305. https://doi.org/10.1007/s13566-018-0367-2
Goldsmith BJ, Rosenthal SA, Wara WM, Larson DA (1992) Optic neuropathy after irradiation of meningioma. Radiology 185:71–76. https://doi.org/10.1148/radiology.185.1.1523337
Jiang GL, Tucker SL, Guttenberger R, Peters LJ, Morrison WH, Garden AS, Ha CS, Ang KK (1994) Radiation-induced injury to the visual pathway. Radiother Oncol J Eur Soc Ther Radiol Oncol 30:17–25. https://doi.org/10.1016/0167-8140(94)90005-1
Flickinger JC, Kondziolka D, Lunsford LD (2003) Radiobiological analysis of tissue responses following radiosurgery. Technol Cancer Res Treat 2:87–92. https://doi.org/10.1177/153303460300200203
Speckter H, Bido J, Hernandez G et al (2018) Inversion recovery sequences improve delineation of optic pathways in the proximity of suprasellar lesions. J Radiosurgery SBRT 5:115–122
Guerrero M, Li XA (2004) Extending the linear–quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol 49:4825–4835. https://doi.org/10.1088/0031-9155/49/20/012
Park C, Papiez L, Zhang S, Story M, Timmerman RD (2008) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol 70:847–852. https://doi.org/10.1016/j.ijrobp.2007.10.059
Brown JM, Brenner DJ, Carlson DJ (2013) Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 85:1159–1160. https://doi.org/10.1016/j.ijrobp.2012.11.003
Brown JM, Carlson DJ, Brenner DJ (2014) The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol 88:254–262. https://doi.org/10.1016/j.ijrobp.2013.07.022
Kirkpatrick JP, Brenner DJ, Orton CG (2009) The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Med Phys 36:3381–3384. https://doi.org/10.1118/1.3157095
Song CW, Lee Y-J, Griffin RJ, Park I, Koonce NA, Hui S, Kim MS, Dusenbery KE, Sperduto PW, Cho LC (2015) Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol 93:166–172. https://doi.org/10.1016/j.ijrobp.2015.05.016
Sperduto PW, Song CW, Kirkpatrick JP, Glatstein E (2015) A hypothesis: indirect cell death in the radiosurgery era. Int J Radiat Oncol 91:11–13. https://doi.org/10.1016/j.ijrobp.2014.08.355
Anker CJ, Shrieve DC (2009) Basic principles of radiobiology applied to radiosurgery and radiotherapy of benign skull base tumors. Otolaryngol Clin N Am 42:601–621. https://doi.org/10.1016/j.otc.2009.04.001
Barazzuol L, Burnet NG, Jena R, Jones B, Jefferies SJ, Kirkby NF (2010) A mathematical model of brain tumour response to radiotherapy and chemotherapy considering radiobiological aspects. J Theor Biol 262:553–565. https://doi.org/10.1016/j.jtbi.2009.10.021
Jones B, Sanghera P (2007) Estimation of radiobiologic parameters and equivalent radiation dose of cytotoxic chemotherapy in malignant glioma. Int J Radiat Oncol 68:441–448. https://doi.org/10.1016/j.ijrobp.2006.12.025
Qi XS, Schultz CJ, Li XA (2006) An estimation of radiobiologic parameters from clinical outcomes for radiation treatment planning of brain tumor. Int J Radiat Oncol 64:1570–1580. https://doi.org/10.1016/j.ijrobp.2005.12.022
Santacroce A, Walier M, Régis J, Liščák R, Motti E, Lindquist C, Kemeny A, Kitz K, Lippitz B, Álvarez RM, Pedersen PH, Yomo S, Lupidi F, Dominikus K, Blackburn P, Mindermann T, Bundschuh O, van Eck ATCJ, Fimmers R, Horstmann GA (2012) Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 70:32–39; discussion 39. https://doi.org/10.1227/NEU.0b013e31822d408a
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Timmerman RD (2008) An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 18:215–222. https://doi.org/10.1016/j.semradonc.2008.04.001
Acknowledgments
The authors gratefully acknowledge Dr. Diego Almeida, Dr. George Lara, Dr. Alejandro Villanueva, Dr. Ricardo Domingo, Dr. Remberto Escoto, Dr. Luis Moreno Sanchez, Ms. Cathy Lebron, and Ms. Evelyn Borel and for valuable contributions to this study.
Author information
Authors and Affiliations
Contributions
Conception and design: Herwin Speckter
Data collection: Jairo Santana, Isidro Miches, Giancarlo Hernandez, Jose Bido, Diones Rivera, Luis Suazo, Santiago Valenzuela, Jazmin Garcia, Peter Stoeter, Herwin Speckter
Data analysis and interpretation: Jairo Santana, Isidro Miches, Giancarlo Hernandez, Jose Bido, Diones Rivera, Luis Suazo, Santiago Valenzuela, Jazmin Garcia, Peter Stoeter, Herwin Speckter
Manuscript writing: Herwin Speckter
Final approval of manuscript: Jairo Santana, Isidro Miches, Giancarlo Hernandez, Jose Bido, Diones Rivera, Luis Suazo, Santiago Valenzuela, Jazmin Garcia, Peter Stoeter, Herwin Speckter
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Speckter, H., Santana, J., Miches, I. et al. Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J Radiat Oncol 8, 279–289 (2019). https://doi.org/10.1007/s13566-019-00398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-019-00398-8