Abstract
Introduction
Lupus nephritis (LN) in pediatric systemic lupus erythematosus (pSLE) requires treatment with corticosteroids (CS) and mycophenolate mofetil (MMF) or cyclophosphamide (CYC). However, some patients fail standard therapy leaving physicians with few options. Although two recent phase II/III randomized controlled trials using abatacept (ABA) with and without MMF in adult SLE did not meet their endpoints, we examined if this combination therapy may have a therapeutic benefit in pSLE patients with refractory class IV and V LN.
Methods
We performed a retrospective chart analysis of five pSLE patients with class IV and V LN. All patients were treated with ABA + MMF after previous failure to CYC and MMF. CS doses and SLE Disease Activity Index (SLEDAI) score were assessed at diagnosis and after 12 and 24 weeks of each treatment.
Results
Patient age at diagnosis was 9–15 years (mean 12.6 ± 2.3). Average disease duration before initiation of ABA + MMF therapy was 22–97 months (mo) (mean 52.8 ± 30.8). Treatment with ABA + MMF resulted in an improvement in SLEDAI score and reduction in CS dose by 12 weeks in all five patients. One patient achieved complete remission and three patients were weaned off steroids after 7–20 mo (mean 13 ± 6.5). Repeated measures analysis of variance showed significant change from SLEDAI at time of diagnosis (baseline) over 24 weeks of treatment with CYC (P = 0.0013) and with ABA + MMF (P < 0.0001). Paired comparison to baseline SLEDAI scores showed some decrease after 12 weeks of treatment with MMF monotherapy (P = 0.0520).
Conclusion
The data suggest that combination therapy with ABA + MMF may be an alternative option in refractory pSLE nephritis. Additional studies are needed in pSLE to further assess the efficacy of this combination treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric systemic lupus erythematosus (pSLE) with class IV/V lupus nephritis (LN) often requires treatment with corticosteroids (CS) and other agents such as azathioprine (AZA), methotrexate (MTX), mycophenolate mofetil (MMF), or cyclophosphamide (CYC) [1, 2]. Although the 5-year survival rate exceeds 95%, the disease may remain intractable in some children [3] and new treatment modalities must sometimes be explored.
Abatacept (ABA) is a recombinant fusion protein that acts by competing with CD28 for binding to CD80/CD86, and inhibits its engagement on T cells and plasma cells [4]. Because both T and B cells play an active role in SLE, it was postulated that ABA could be beneficial in SLE. Several studies in murine models of SLE have shown improvement in both SLE and nephritis activity either with CTLA-4Ig alone or in combination with CYC [5, 6]. Although recent controlled trials of ABA, with and without combination treatment with MMF for adult SLE, did not meet their primary or secondary endpoints, exploratory analyses suggested efficacy in some subgroups [7, 8]. ABA is currently FDA approved for treatment of juvenile idiopathic arthritis (JIA) and rheumatoid arthritis, however, its use in pSLE has not been reported [9]. This small case series examines ABA in conjunction with MMF as a potentially effective treatment for pSLE in a subset of children who have failed other medications.
Materials and Methods
We performed a retrospective analysis of children with pSLE, with class IV and V LN diagnosed by pathology at Children’s Hospital Los Angeles, Los Angeles, CA, USA. Patients met at least 4 of the 11 American College of Rheumatology (ACR) criteria for a diagnosis of SLE [10]. All patients received and/or failed treatment with CYC at a daily oral dose of 2 mg/kg/dose, bi-weekly dose infusion of 10 mg/kg/dose or monthly infusion of 1,000 mg/m2, MMF monotherapy at a dose of 600–1,000 mg/m2/dose given twice daily (bid) or rituximab (RTX) 750 mg/m2 two doses given 2 weeks apart. Some patients received additional therapeutic interventions with azathioprine (AZA) and cyclosporine (CSA). After an inadequate response to the above therapy, combination therapy with ABA and MMF (ABA + MMF) was started. Using the SLEDAI scoring system which assigns an index score based on the presence or absence of 24 abnormalities associated with SLE (in 9 organ systems) with scores assigned as follows; 8 for central nervous system involvement and vasculitis, 4 for renal disease and musculoskeletal, 2 for serosal, dermal and immunologic and 1 for constitutional and hematologic. The higher the score, the more disease involvement [11]. The analyzed data points were assigned a (SLEDAI) score at time of diagnosis (referred to as baseline) and then 12 and 24 weeks into each treatment. These data points are separated in time by several years in some cases with minimal overlap of RTX or CYC treatment with the combination therapy. All patients received at least 4 doses of ABA (10 mg/kg/dose every 2 weeks for 3 doses, then every 4 weeks) in addition to CS. Patients with hypogammaglobulinemia also received replacement IVIG at 0.5 g/kg monthly at the discretion of the treating physician. Institutional review board approval and informed consent were obtained as appropriate. All procedures followed were in accordance with the ethics standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Statistical Analysis
Data collected included demographics, medications, laboratories, clinical findings, and adverse events. The pattern of change in SLEDAI scores from time of diagnosis, after 12 and 24 weeks of each treatment, was evaluated with repeated measures analysis of variance (ANOVA), for each drug, using SAS/STAT® v 9.2 statistical software (SAS institute Inc., SAS Campus Drive, Cary, NC 27513, USA) with P < 0.05 considered statistically significant.
Case Presentation
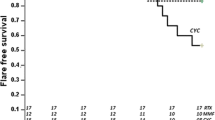
Patient 1 is a white male diagnosed with pSLE at age 14 years based on the positive antinuclear antibodies (ANA), double-stranded DNA (dsDNA), antiphospholipid antibodies (APL), glomerulonephritis (GN) (class IV/V), cytopenias, arthritis, vasculitis and malar rash. SLEDAI score at diagnosis was 32 (Fig. 1), and initial steroid dose was 90 mg daily. He had several complications, including pulmonary embolus and hemorrhage, and splenic infarction for which he was on subcutaneous enoxaparin. The treatment included 12 monthly infusions of CYC 1,000 mg/m2, AZA, 2 RTX doses (750 mg/m2 every 2 weeks for two doses), tacrolimus and MMF 600 mg/m2/dose bid. Owing to persistent disease activity, he remained on CS 30 mg daily for 12 mo until it was increased to 60 mg daily because of disease flare prior to initiating ABA + MMF. ABA + MMF were started 25 mo after diagnosis due to active GN and cytopenias. At 12 weeks of combination therapy, ABA + MMF, SLEDAI score decreased to 8 with resolution of urinary casts, hematuria, rash and thrombocytopenia, negative dsDNA (20–8 IU/ml), though CS dose remained unchanged at 60 mg daily. At 24 weeks of combination therapy, SLEDAI decreased to 6 with normalization of complement C3 (C3) (42–103 mg/dl), improved creatinine (4.2–1.8 mg/dl), and urine protein to creatinine ratio (UPC) (2.6–0.7); urine with no casts or active sediment and no clinically active disease. At this time, CS dose was 40 mg daily. The patient then transitioned to adult rheumatology care, at which time he was clinically stable.
Patient 2 is a Hispanic female diagnosed with pSLE at age 15 years based on the positive ANA, dsDNA, GN (class IV/V), cytopenias, and arthritis. SLEDAI score at diagnosis was 19. She was initially treated with CS 30 mg daily and MMF 750 mg/m2 bid. MMF monotherapy was stopped and daily CYC 2 mg/kg/dose was started at 37 mo after diagnosis due to new onset interstitial lung disease and worsening GN as evidenced by increasing proteinuria and urinary casts. RTX 750 mg/m2 every 2 weeks for 2 doses were given in addition to CYC 60 mo after diagnosis due to continued disease activity; however, because of persistent cytopenia and recurrent infections, CYC was replaced with MMF 750 mg/m2/dose bid. Although on MMF her disease flared and CS dose was increased to 90 mg daily. ABA + MMF was then started 63 mo after diagnosis due to active GN, cytopenias, and low complements. At 12 weeks of ABA + MMF her UPC decreased (57.7–0.7), thrombocytopenia resolved (64,000–238,000 k/µl), and C3 normalized (60–149 mg/dl). She also had improvement in white blood cells (WBC) from 3.4–11.6 k/µl and hemoglobin (HgB) from 6.9 to 8.6 g/dl. At 12 and 24 weeks of ABA + MMF combination therapy her SLEDAI score was 4 due to mild proteinuria. CS dose was decreased to 30 mg daily by 24 weeks. At this time, she was clinically stable and transitioned into adult rheumatology care.
Patient 3 is an African American female diagnosed with pSLE at age 13 years based on positive ANA, dsDNA, GN (IV), serositis, malar rash, cytopenias, arthritis, and photosensitivity. At diagnosis SLEDAI score was 28 and CS dose 60 mg daily. She had a complicated course with recalcitrant disease that included recurrent pleural effusions requiring pleurodesis. Several therapies including MTX, CSA, MMF 1,000 mg/m2/dose bid, RTX 750 mg/m2 for 2 doses, CYC 2 mg/k/day and nitrogen mustard were ineffective as evidenced by persistent hypocomplementemia, arthritis, and elevated UPC with urine casts. At 12 weeks of MMF 1,000 mg/m2/dose monotherapy, CS dose had been increased to 90 mg. Owing to persistent SLE activity; ABA was started 57 mo after diagnosis in conjunction with MMF. By 12 weeks of this therapy, her SLEDAI score had decreased to 10, UPC improved (23.2–10) and C3 normalized (39–80 mg/dl). In addition, her abnormal urinary sediment (cast, WBC, RBC), alopecia and pleurisy resolved and CS had been weaned to 40 mg daily. At 24 weeks, she had further improvement with a SLEDAI score of 6, C3 of 134, dsDNA of 46 IU/ml (decreased from >200 IU/ml), resolution of arthritis, stabilized GN with UPC of 2.5, and CS dose of 30 mg daily.
Patient 4 is a Hispanic female who was diagnosed with pSLE at age 12 years based on positive ANA, dsDNA, GN (IV), cytopenias, arthritis, and malar rash. SLEDAI score at diagnosis was 26 and initial CS dose 90 mg daily. In addition, she had menorrhagia with factors II and VIII antibodies. She was treated with daily oral CYC 2 mg/kg/dose for 18 mo, RTX 750 mg/m2 every 2 weeks for two doses due to persistent disease and MMF 750 mg/m2/dose bid monotherapy. She had progressive GN and persistent arthritis, rash, low complements, and hypogammaglobulinemia. ABA was added to MMF monotherapy 22 mo after diagnosis. At 12 weeks of combination therapy ABA + MMF, her SLEDAI score had decreased to 8, dsDNA was negative, UPC markedly decreased (6.38–0.69), C3 normalized (70–106 mg/dl) and she had resolution of arthritis, rash, oral ulcers, cytopenia, hematuria and casts. CS dose at this time was 20 mg daily. Data were not available at 24 weeks as she had been transitioned to another provider.
Patient 5 is an Asian female diagnosed with pSLE at age 9 years based on the positive ANA, dsDNA, GN (V), and cytopenias. SLEDAI score at diagnosis was 26 and initial CS dose was 60 mg daily. She was treated with monthly CYC 1,000 mg/m2 monthly infusion for 21 mo, RTX 750 mg/m2 for two doses, AZA, and then MMF 750 mg/m2/dose bid, but without sustained improvement. Owing to persistent proteinuria and low complements, ABA was started 97 mo after diagnosis in conjunction with MMF; by 12 weeks of ABA + MMF therapy her SLEDAI decreased to 12, C3 increased from 40 to 80 mg/dl, dsDNA decreased from 657 to 280 IU/ml, and CS decreased to 10 mg daily. By 24 weeks of ABA + MMF therapy, SLEDAI score was 2 with resolution of proteinuria, pyuria and normalization of C3, although the dsDNA was persistently positive at 146 IU/ml. Her clinical condition remains stable to date.
Results
Patient age at diagnosis was 9–15 years (mean 12.6 ± 2.3). Average disease duration prior to ABA + MMF therapy was 22–97 mo (mean 52.8 ± 30.8). Two patients had class IV LN, one patient had class V LN and two patients had class IV/V LN. All patients had tried and failed therapy with several other immunosuppressive agents, including CYC and MMF. Four of the five patients were on a minimum of 2 mg/kg/day of CS at time of diagnosis (Table 1).
Mean SLEDAI scores at time of diagnosis (baseline) and after 12 and 24 weeks of each therapy are shown in Table 2. Baseline SLEDAI scores were significantly elevated with the lowest score being 19 (Fig. 1). Mean dose of steroids, levels of dsDNA, C3 level and UPC are also listed in Table 2.
At 12 weeks of ABA + MMF all 5 patients had a decrease in their SLEDAI scores (Figs. 1, 2) and successfully had their CS dose reduced (Table 1; Fig. 3). Data were not available at 24 weeks for two of the five patients after taking MMF monotherapy and for one patient after taking ABA + MMF combination therapy, due to transition of care. One patient achieved complete remission and three patients were able to discontinue steroids after 7–20 mo (mean 13 ± 6.5) (Table 1) on ABA + MMF combination therapy.
Repeated measures ANOVA for patients with data at all three time points revealed significant decreases in SLEDAI scores for five patients after taking CYC (P = 0.0013) and for four patients after taking ABA + MMF (P < 0.0001). Post hoc pairwise comparisons to baseline at 12 and 24 weeks were significant for both drugs (P = 0.02 and P = 0.006, respectively, for CYC; P = 0.002 and P = 0.0006, respectively, for ABA + MMF). Since only three patients had SLEDAI scores after 24 weeks on MMF, repeated measures ANOVA was not used for this drug; however, five patients showed some decrease in scores after 12 weeks on MMF (paired t test P = 0.0520).
Discussion
The use of ABA with MMF resulted in improvement in complement (Fig. 4), dsDNA levels (Fig. 5) and blood counts (CBC) in all five patients despite the previous failure with CYC and MMF monotherapy. Furthermore, it permitted the reduction in the CS dose. UPC improved with all three therapeutic interventions (Fig. 6) which is consistent with previous studies evaluating the use of CYC and MMF in lupus nephritis [12]. When comparing mean UPC at 12 weeks, the response with CYC was more notable than at 12 weeks with MMF monotherapy or ABA + MMF, but by 24 weeks MMF monotherapy was comparable to ABA + MMF combination therapy and better than CYC monotherapy (Table 2). All patients responded with significant decrease in their SLEDAI scores while on ABA + MMF. Replacement IVIG was given to two patients for hypogammaglobulinemia. No significant adverse events were observed while on ABA + MMF therapy over a median of 15.5 (interquartile range 7.5–19.25) doses.
All patients had recalcitrant disease that had been aggressively treated with several other medications without adequate response prior to ABA + MMF treatment. The influence of noncompliance with MMF producing these findings is difficult to determine. However, mycophenolic acid levels and mycophenolic acid glucoronide levels (normal range 1–3.5 and 35–100 mcg/ml, respectively) measured in four of the five patients during MMF monotherapy were within therapeutic range. Although MMF alone has been shown to be as effective as CYC in adult studies [12], these studies have not been replicated in children. This study demonstrated that combination therapy with ABA + MMF was more effective than that of CYC and MMF monotherapy for this group of patients. ABA, particularly in combination with MMF, may be efficacious in treatment-refractory pSLE, including those with glomerulonephritis. Further prospective studies of ABA combo treatment in pSLE are needed to substantiate these findings.
References
Klein-Gitelman M, Reiff A, Silverman ED. Systemic lupus erythematosus in childhood. Rheum Dis Clin N Am. 2002;28:561–77.
Silverman E, Eddy A. Systemic lupus erythematosus. In: Cassidy JT, Petty RE, Laxer R, Lindsley C, editors. Textbook of pediatric rheumatology. Philadelphia: WB Saunders; 2011. p. 315–43.
Hashkes PJ, Wright BM, Lauer MS, Worley SE, Tang AS, Roettcher PA, et al. Mortality outcomes in pediatric rheumatology in the US. Arthritis Rheum. 2010;62:599–608.
Wofsy D, Daikh DI. Opportunities for future biological therapy in SLE. Baillieres Clin Rheumatol. 1998;12:529–41.
Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLAIg. Science. 1994;265:1225–7.
Daikh DI, Wofsy D. Reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol. 2001;165:2913–6.
Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace J, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus. Arthritis Rheum. 2010;62:3077–87.
Furie R, Nicholls K, Cheng T–T, Houssiau F, Burgos-Vargas R, Chen S-L, et al. Efficacy and safety of abatacept over 12 months in patients with lupus nephritis: results from a multicenter, randomized, double-blind, placebo-controlled phase II/III study. Arthritis Rheum. 2011;63(Suppl 10):2469.
Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792–802.
Hochberg MC, For the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (letter). Arthritis Rheum. 1997;40:1725.
Bombardier C, Gladman D, Urowitz M, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 2009;35(6):630–40.
Appel G, Contreras G, Dooley MA, Ginzler E, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12.
Acknowledgments
Statistical support is granted through an NIH grant to Children’s Hospital Los Angeles Biostatistics Department (NIH/NCRR SC-CTSI Grant Number UL1 RR031986). The content of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr Castillo is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflicts of interest
Rhina D Castillo, Suhas M. Radhakrishna, Andreas O Reiff, Colleen Azen, and Katherine AB Marzan declare no conflicts of interest.
Compliance with Ethics Guidelines
Institutional review board approval and informed consent were obtained as appropriate. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Castillo, R.D., Radhakrishna, S.M., Reiff, A.O. et al. Abatacept and Mycophenolate Mofetil Combination Therapy in Refractory Pediatric Systemic Lupus Erythematosus: A Case Series. Comb Prod Ther 3, 53–61 (2013). https://doi.org/10.1007/s13556-013-0002-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13556-013-0002-x