Abstract
The implantation of cardiac implantable electronic devices (CIED) to prevent sudden cardiac death, ameliorate chronic congestive heart failure, and correct bradycardia has grown rapidly in the past decade. Bacterial infection of the device hardware (pacemaker or implantable cardioverter defibrillator [ICD] pulse generator, and intravascular wires or leads), including the tissue pocket, is rising at an even faster rate. The source infection inoculation typically occurs at the time the device is implanted, revised, or when a device generator replacement occurs. This risk is clearly related to patient and procedure-specific factors, and is reviewed in the present paper. Prophylactic preoperative antibiotics, strict sterile technique, and limiting procedure times may reduce infection risk. A novel Antibacterial Envelope (AIGISRx®, TYRX Inc., Monmouth Junction, NJ, USA) that is directly implanted with the device generator to provide site-specific therapy could significantly lower infection risk in certain high-risk cases. This may potentially reduce inpatient mortality related to endocarditis and sepsis, and lower healthcare costs related to CIED extraction and re-implantation, which can exceed $1 million per case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implantation of permanent cardiac implantable electronic devices (CIED) experienced an initial phase of rapid growth beginning in 1989 following the development of transvenous implantable cardioverter defibrillator (ICD) leads, allowing pectoral implants. Results of clinical trials and incorporation into heart care society guidelines for primary prevention of sudden death and cardiac resynchronization therapy (CRT) for heart failure, have resulted in >600,000 annual worldwide implants in 2010.1,2 A corresponding rise in CIED-related device infection has been noted, with a rate exceeding that of the increase in device use. The aggressive application of expanding indications for CIEDs has led to greater numbers of procedures in patients with significant comorbid conditions. Between 1993–2008 the annual rate of infection in a large inpatient registry was 1.6%, with a notable rise after 2004, parallel with the expansion of CRT pacing, and increases in the percentage of patients with diabetes and renal failure.2,3 Device generator replacements, CRT upgrades, abandoned hardware, chronic venous occlusion, and laser lead extraction for failed hardware have increased the complexity of implanted cardiac device cases, and potentially leads to longer case times, increasing infection risks.2,4 The rising trend of CIED-related infection will result in increases in morbidity, inpatient mortality risk, and the financial burden on the United States (US) healthcare system, to an estimated annual cost of >$250 million, and this is reviewed in the following paper.
Data published in 2006 demonstrated device infection rates were rising. A review of device implantation data from 1996–2003 showed a 49% rise in the number of new CIED implantations in the US, from 159,585 in 1996 to 237,720 in 2003. ICD insertions mainly accounted for the growth (rise of 160% for ICDs vs. 31% for pacemakers). During the same period, hospitalizations with CIED infection increased 3.1-fold (2.8-fold for pacemakers and sixfold for ICDs), showing numbers of CIED infection-related hospitalizations increased out of proportion to rates of new device implants. The mortality rate for inpatients with CIED-related infection was increased compared to pacemaker or ICD device implants without a hospital code for CIED infection. After correcting for age, gender, race, hospital size, presence of diabetes mellitus, or renal failure, CIED infection increased the risk of in-hospital death more than twofold (odds ratio [OR] 2.41, P=0.001). Predictors of death also included older age (OR 1.63 per 20-year increase in age, P=0.001) and the presence of renal failure (OR 1.76, P=0.001).5
The demographics of patients who received CRT-defibrillator (CRT-D) devices in 2011, as well as primary prevention ICDs, is pushing the boundaries of appropriateness, likely accounting for part of the rise in infection rates. Retrospective analysis of primary prevention patients with renal insufficiency or chronic kidney disease suggests an ICD confers no benefit to reducing mortality. Patients in whom baseline serum creatinine concentration was >2 mg/dL had a 62% 1 year survival despite an ICD in situ, yet the percentage of patients in ICD registries with renal insufficiency has risen dramatically since 2004.6,7 Cases requiring lead extraction in the setting of infection are associated with a high inpatient mortality rate, particularly in patients with end-stage renal disease.8 Given the necessity to completely remove or extract infected hardware, these cases comprise a heavy financial burden to the healthcare system.
An analysis of a retrospective cohort of 200,219 patients, including 5817 Medicare patients admitted for CIED infection in the setting of generator implantation, replacement, or revision from January 1, 2007 to December 31, 2007,4 demonstrated a 4.6%–11.3% in-hospital mortality rate (rate ratio of 4.8–7.7-fold increase). This represented an absolute in-hospital mortality increase of 4.3%–10.5% compared with CIED cases without infection. The inpatient length of stay (LOS) was longer at 9.6–15 days incremental stay related to the presence of device infection (LOS ratio 2.5–4.0-fold increase), and total cost was $28,676 to $53,349 per case (ranges represent difference between pacemaker, ICD, CRT-pacing [CRT-P] and CRT-D cases). Approximately 40% of the incremental cost of care was related to intensive care unit admissions, and the most extreme cases exceeded a cost of $1 million per case.4 Using a low estimated implantation volume of 400,000 annually for pacemaker or ICD systems with a 1.6% infection rate, the total estimated CIED infections would be 6400. If a case-cost to hospitals is $28,676 (pacemaker) to $53,349 (ICD) per case, and at least two-thirds of these 400,000 cases are ICD systems, then the annual cost of CIED infections in the US would be at a minimum $285 million US dollars.
Published randomized clinical trial data show infection may be reduced with the routine timely use of preoperative antibiotics, and choice of chlorhexidine skin prep over iodinebased solutions.9,10 A novel combined therapy device, the AIGISRx® Antibacterial Envelope (TYRX Inc., Monmouth Junction, NJ, USA), has been approved by the US Food and Drug Administration (FDA) for prevention of CRTdevice infection. Retrospective cohort studies suggest a significant reduction in infection rates, with potential for substantial cost savings, particularly if utilized in the highest risk cases.11 The increased need to reduce device-related infection is evident, yet few treatment options exist or have been rigorously studied. The present study examines the design and mechanism of action of the AIGISRx Antibacterial Envelope, and reviews available published clinical study results, ongoing trials, and practical experience of the authors with the use of this product.
Risk Factors for Infection, Microbiology, and Rationale for the AIGISRx Antibacterial Envelope
Patient-specific and procedure-based factors are known to be associated with a higher rate of peri-procedural infection including pulse generator change-out procedures, oral anticoagulant and steroid use, early re-intervention, temporary pacing, and systems with more than two leads.11 The presence of diabetes and chronic renal disease raise the expected rate of infection, with end-stage renal disease increasing the odds of infection by a factor of 4.6–12.12,13 The increasing rate of device infection from 2004–2008 may be accounted for in part by a rise in the comorbid illnesses of diabetes and renal failure as discussed above, and illustrated in Figure 1, adapted from Greenspon et al.2
Rate of cardiovascular implantable electronic device (CIED)-related infection and incidence of comorbidities in patients with CIED infection.2 Adapted from Greenspon AJ, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter defibrillators in the United States 1993–2008. J Am Coll Cardiol. 2011;58:1001–1006. Copyright (2011), with permission from Elsevier.
The rise in infection rate is also associated with a rapid expansion of CRT-P and CRT-D systems after results of clinical trials showed improved mortality and reduced heart failure morbidity with left ventricular or coronary sinus pacing in selected patients (chronic systolic heart failure [left ventricular ejection fraction {LVEF} <35%], QRS duration of >120 ms, and left bundle branch block, or ventricular dyssynchrony).14,15 CRT-P requires attention to placement of the left ventricular pacing lead in the optimal coronary sinus branch, to optimize clinical benefit. This may extend case times by ∼20–90 minutes typically, allowing more time for pocket or hardware contamination.
Outcomes for defibrillator patients within 90 days of device implant or revision are related to the procedural volume of the operating physician and procedure volume of the hospital. The majority of implanting physicians in the lowest volume subgroup have increased mechanical complications (OR 1.47, 95% CI 1.09–1.99) and infection risk (OR 2.47, 95% CI 1.18–5.17).16 A study of ICD implantations between January 2006 and December 2008 in the National Cardiovascular Data Registry (NCDR) ICD registry shows that the rate of any adverse event declined progressively with increasing procedure volume for all ICD subtypes (single chamber, dual chamber, and biventricular ICDs). Additionally, the highest volume, lowest complication quartile correspondingly had the highest percentage of cardiac electrophysiology board-certified implanting physicians.17 Regardless of operator volume, skill, or board certification, the rise in infection rates affects all implanting physicians and hospitals, and steps should be taken to reduce these risks.
The most common pathogens responsible for CIED infections are Staphylococcus aureus, and coagulase-negative staphylococcal species (CoNS).12,18 The combination of the antibiotics, rifampin and minocycline, are known to have in vitro activity against methicillin-resistant strains of S. aureus and CoNS. Clinical studies incorporating rifampin and minocycline into central venous catheters, cerebrospinal fluid drains, and hemodialysis catheters have demonstrated reduction in device-related infections.19–21
AIGISRx Design, Antibiotic, and Animal Study Results
The AIGISRx Antibacterial Envelope is an FDA-approved polypropylene mesh envelope that releases minocycline and rifampin from a bioresorbable polyarylate polymer over approximately 7 days, directly into the CIED generator pocket. The sterile prosthesis is designed to hold a pacemaker or ICD generator, and while in the device pocket, will release minocycline and rifampin locally.
In vivo studies using bacterial challenge by direct device pocket inoculation show protection against the CoNS strain Staphylococcus epidermidis, S. aureus, Escherichia coli, Hemophiliussp., and Corynebacteriumsp. 22 Initial in vivo pre-clinical studies showing potential efficacy for the reduction of device pocket infection provided the basis for a recently published large cohort study.23
The Cooperative Multicenter study Monitoring a CIED Antimicrobial Device (COMMAND) retrospective cohort study published by Bloom et al. in 2010 analyzed AIGISRx device implantation success, and resultant CIED infection rates, in 624 eligible patients over a study period from June 2008 to June 2009 with a mean follow-up of 1.9 months. Nearly half the patients had at least three predefined risk factors for CIED infection. Infection was confirmed in three of the 624 patients (0.48%).11 All three infections occurred in patients undergoing a revision or generator replacement procedure, known to be associated with higher risk. There were no infections in the initial or fresh pacemaker, ICD, or CRT system implant cases. Device replacement or revision procedures have been previously shown to have higher rates of infection (OR 2.24–3.67).11 The relative avascularity of the remnant fibrous capsule may contribute to this risk by reducing delivery of systemic intravenous antibiotic prophylaxis to the device generator pocket. More than two-thirds (68%) of the procedures in the COMMAND study were for replacement or revision. In addition, several other known risk factors for CIED infection were common in the COMMAND study population, including congestive heart failure, greater than two leads in place (CRT systems or abandoned hardware), oral anticoagulant use, and renal insufficiency.11 Limitations of the COMMAND study include its retrospective, nonrandomized nature, no direct control group set to compare infection rates internally (used prior published ICD registry data), and a short follow-up period. Two ongoing clinical prospective multicenter registry studies are currently enrolling patients; these are funded by TYRX, Inc., but are nonrandomized without a corresponding control group: (1) the AIGISRx Antibacterial Envelope and custom mesh for prevention of infection following cardiac rhythm management device replacement with an ICD (CITADEL), and (2) the AIGISRx Antibacterial Envelope and custom mesh for prevention of infection following cardiac rhythm management device replacement with a CRT device (CENTURION). To date, no randomized, controlled data are available with the use of this product to reduce CIED-related infection.
Authors Experience and Preliminary Data from Vanderbilt Heart And Vascular Institute
Beginning in August 2009, the AIGISRx Antibacterial Envelope became available for use at Vanderbilt University Hospital, Nashville, TN, USA. In accordance with institutional policy on new product use, restrictions were placed on utilization, based on available clinical studies, and expert opinion. The use of the device has since been limited to patients with two or more of the following high-risk features for devicerelated infection: diabetes (type I or II), renal insufficiency (creatinine ≥1.5 mg/dL), systemic anticoagulation, chronic daily steroid use, fever ≥38°C or leukocytosis .≥1,000 WBC/μL within 24 hours of the time of implantation, prior CIED infection, three or more leads (CRT systems or abandoned hardware), pacemaker dependence, or early pocket re-entry within 72 hours.
Retrospective case-control data will be submitted from Vanderbilt Heart and Vascular Institute for publication, with matched internal controls derived from the Institute’s computerized patient record database. The time period for controls will be CIED infection rates in high-risk patients for the 2 year period prior to availability of the antibacterial envelope, compared to infection rates over the ensuing 24 months (no added institutional changes occurred to operating room space, operating physicians, or operating room protocols during this time period). This will be the first study with a corresponding internal control group to evaluate the effect of the AIGISRx Antibacterial Envelope on high-risk device infection rates at one institution, over a time period of 4 years. A cost-effectiveness calculation will be included with the submission. A randomized, prospective trial designed to assess the efficacy of the AIGISRx Antibacterial Envelope should be pursued.
Practical Issues of Concern Including Implantation Technique
A learning curve is apparent with the use of the AIGISRx Antibacterial Envelope. The device sleeve comes in two sizes, one for typical pacemaker generators, and a second intended for use with ICD systems. The device has a 3–4 mm seam along three of its four edges (rectangular shaped), which is the fusion of two flat sheets of polypropylene mesh. A pre-formed opening, which extends roughly 80% of the length of one of the shorter sides, is intended for insertion of the device generator. The mesh has a high coefficient of friction, and will require upsizing the subcutaneous device pocket 10%–15% by volume to allow ease of entry. During device implantation, the sleeve should house the CIED generator and pacemaker or ICD leads, which may need to be altered depending on the CIED manufacturer. Inverting the AIGISRx device to turn the seam to the inside of the device allows easier entry of the generator, and extending the preformed edge to open the remaining sealed portion may facilitate implantation.
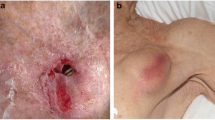
The device should not be left submerged in saline or antibacterial solution, as the minocycline-rifampin granules may dissolve off the envelope and reduce efficacy (will see orange discoloration of fluid in which the device is left soaking). Gently moistening the sleeve immediately prior to insertion in the pocket with saline will facilitate entry; however, the more important consideration is making the pocket larger by more extensive dissection. Routine practice involves aggressive irrigation of the subcutaneous device pocket once all hardware is inserted (often with polymixin-bacitracin solution). This should be avoided after the envelope is placed, as granules will also be irrigated or aspirated away in the flush. The practice of the present authors has been to insert the device leads fully in the pocket, then irrigate, wet, and invert the AIGISRx Antibacterial Envelope, place the generator in the envelope, connect leads to the device, and then place the generator and AIGISRx above the device leads in the subcutaneous pocket. With this technique, implantation will maximize the efficacy of the antibiotic granules and facilitate insertion (Figure 2Figure 2).
Extraction or removal of the AIGISRx upon device generator change, or revision after 2–3 months from initial implant, will require careful dissection. The recommendation from TYRX, Inc., is to dissect the envelope and device generator together as a unit, if necessary, as the device itself may be very difficult to see, as it is white and opaque, within a fibrotic capsule. Ideally to remove avascular tissue and the original sleeve, the entire system may need to be resected free. In underweight patients (body mass index <20) this may not be possible, and if there is adequate space a new AIGISRx may be implanted inside a prior device, though this may be challenging. Newer generation AIGISRx Antibacterial Envelopes with a thinner skeleton, or potentially bioabsorbable sleeves, which are currently under FDA review, may alleviate this concern.
Potential Cost-Effectiveness for the AIGISRx Antibacterial Envelope
The rate of device-related infection is steadily rising, and given the large volume and cost of CIED-related infection hospitalizations, the estimated minimum cost burden to the US healthcare system exceeds $285 million. The estimated absolute risk reduction associated with the use of the AIGISRx device is 2.5%, and would generate a number needed to treat to prevent one infection of 40. At a unit price of $895 for an AIGISRx ICD envelope, a cost to hospitals for a single CIED-related infection of $35,800 for ICDs would be the break-even point. Data from Sohail et al. suggest that US hospitals could be expected to recoup $17,549 dollars for each ICD-related infection that was prevented.4 This does not take into account the possibility that if applied appropriately, mortality from device-related infection could be reduced, though large, multicenter, randomized data would likely be needed to support this hypothesis.
Conclusions
CIED-related infections, typically resulting from Staphylococcus spp., are rising at a rate faster than the growth in new device implants. The rise correlates with increased numbers of CRT systems, laser lead extractions, and the growing prevalence of patients with diabetes or renal insufficiency undergoing procedures. The cost and morbidity of device-related infection is of great concern. Greater still is the mortality risk associated with a complicated hospital course for an infection related to a seemingly minor operation, such as a device generator change. Randomized data support the use of chlorhexidine surgical prep and preoperative antibiotic prophylaxis to reduce this risk. The AIGISRx Antibacterial Envelope offers a novel, cost-effective, locally delivered solution to further reduce this risk, particularly in the highest risk patient populations. Randomized trial data are required, and may be expected to demonstrate improved safety, cost, and potentially a reduction in mortality as it relates to a growing healthcare issue.
References
Kurtz SM, Ochoa JA, Lau E et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol. 2010;33:705–711.
Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter defibrillators in the United States 1993–2008. J Am Coll Cardiol. 2011;58:1001–1006.
Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter defibrillators: calendar year 2005: an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202–1212.
Sohail MR, Henrickson, CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011; Sep 12 [Epub ahead of print].
Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:586–597.
Turakhia MP, Varosy PD, Lee K, et al. Impact of renal function on survival in patients with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 2007;30:377–384.
Cuculich PS, Sanchez JM, Kerzner R, et al. Poor prognosis for patients with chronic kidney disease despite ICD therapy for the primary prevention of sudden death. Pacing Clin Electrophysiol. 2007;30:207–213.
Wazni O, Epstein LM, Carillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions [erratum: J Am Coll Cardiol. 2010;55:1055]. J Am Coll Cardiol. 2010;55:579–586.
Darouiche RO, Wall Jr. MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-Iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26.
De Oliveira JC, Martinelli M, Nishoka SADO, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter defibrillators: results of a large prospective randomized double-blinded placebo controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–24.
Bloom HL, Constantin L, Dan D, et al. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol. 2011;34:133–142.
Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715–720.
Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006;29:142–145.
Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure (CARE-HF). N Engl J Med. 2005;352:1539–1549.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure (COMPANION). N Engl J Med. 2004;350:2140–2150.
Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol. 2005;46:1536–1540.
Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2010;56:1133–1139.
Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemaker and cardioverter-defibrillators: results of a large prospective registry. Circulation. 2007;116:1349–1355.
Hockenhull JC, Dwan K, Boland A, et al. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1–154.
Chatzinikolaou I, Finkel K, Hanna H, et al. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter-related infections: a prospective, randomized study. Am J Med. 2003;115:352–357.
Zabramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725–730.
Agostinho A, James G, Wazni O, Citron M, Wilkoff BD. Inhibition of Staphylococcus aureus biofilms by a novel antibacterial envelope for use with implantable cardiac devices. Clin Transl Sci. 2009;2:193–198.
Hansen LK, Brown M, Johnson D, Palme Ii DF, Love C, Darouiche D. In vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing and Clin Electrophysiol. 2009;32:898–907.
Acknowledgments
C.R.E. has received speaking fees and consultant fees or honoraria from Navigant Consulting, Boston Scientific, St. Jude Medical, and TYRX Pharmaceuticals. M.J.K. has no conflicts of interest. C.R.E. is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. Prior to peer review, TYRX, Inc. was offered the opportunity to review this paper for scientific accuracy. No writing assistance, other editorial involvement, or financial support was provided by the manufacturer in the production of this manuscript.
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.combitherapy-open.com
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ellis, C.R., Kolek, M.J. Rising infection rate in cardiac electronic device implantation; the role of the AIGISRx® antibacterial envelope in prophylaxis. comb.prod.ther. 1, 3 (2011). https://doi.org/10.1007/s13556-011-0003-6
Received:
Published:
DOI: https://doi.org/10.1007/s13556-011-0003-6