Abstract
Introduction
Acne remains one of the most common inflammatory dermatoses seen worldwide. There are significant challenges when managing acne relating to a variety of factors, including (1) lack of consensus on the use of the numerous available grading systems and outcome measures, (2) appreciation of the numerous areas that relate to severity, (3) the chronic nature of acne which requires a longitudinal approach to management (including both facial and truncal disease), and (4) the need to target acne early to avoid physical and psychosocial scarring. Consideration of these aspects when managing acne should result in improved outcomes. Acne guidelines review the available evidence based on robust clinical trials and are usually supplemented with some expert opinion when evidence is not available.
Methods
In this paper, the UK Acne Working Group reflects on the latest National Institute for Health and Care Excellence (NICE) acne guidelines with a goal of providing additional practical insights.
Conclusion
The group have identified areas where new evidence has now become available since the formulation of the NICE acne guidelines. This publication considers newly approved acne medications in the UK, guidance on assessing acne severity, approaches to managing truncal acne, acne sequelae, and adult female acne with hormonal therapies.
Plain Language Summary
The National Institute for Health and Care Excellence (NICE) produced acne guidelines in June 2021 for clinicians and patients. New evidence and information on practical aspects of acne management have emerged since this time. A panel of clinicians with expertise in acne discuss herein some areas of interest that may support acne management, some of which could be considered in a second iteration of NICE acne guidelines. These areas include how to assess acne, the medical approach to truncal acne, how clinicians may manage the long-lasting acne sequelae of scarring and darkly pigmented spots, and the use of medical hormonal therapies for women (such as birth control pills) to manage acne that may have a causative contribution of hormone imbalances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Acne is one of the most common inflammatory dermatoses seen worldwide and can result in significant physical and psychological sequelae. |
While high-quality acne guidelines exist, further areas of research and investigation have been identified in many guidelines including recognition and treatment of truncal acne as well as clinical sequelae. |
What was learned from this study? |
This paper addresses some of the areas not currently included in acne guidelines. |
Novel treatment options for acne and evidence for their use have been considered since publication of previous guidelines. |
New approaches with medical therapies for sequelae such as scarring and pigmentation have also been included. |
Introduction: Current Acne Management
The National Institute for Health Care and Excellence (NICE) published the first guideline for the management of acne vulgaris for the UK in June 2021 [1]. The goal was to provide advice for the management of acne directed toward healthcare professionals and patients and family members [1]. The primary focus of the guideline treatment approaches included topical and oral medications and the use of physical modalities. The overall impact of acne on patients’ health and wellbeing was also considered [1]. The guidance encourages British healthcare practitioners to optimize management of acne by considering the individual needs, preferences, and values of their patients.
A well-rounded approach is important in managing acne as this common inflammatory skin disease can be self-limited but is frequently a chronic condition [2]. The World Health Organization and US Centers for Disease Control and Prevention both identify diseases as chronic when they are long-term, relapsing, and not self-limited [3]. Chronic diseases can be characterized by acute outbreaks or wave-like courses and frequently have a profound psychosocial impact. It has long been accepted that the psychosocial and quality of life (QOL) impairments associated with acne may be significant and can persist over decades [4, 5]. As such acne fits with the definition as a chronic disease [2]. As early as 1999, Mallon et al. used validated questionnaires to assess morbidity in 111 patients with moderate to severe acne and when comparing with patients with other chronic diseases found patients with acne had functional impairments similar to those in sufferers of asthma or epilepsy [6]. The impact of acne may be secondary to acne itself or to the long-term sequelae such as scarring [7,8,9]. Acne-associated pigmentation (acne-induced hyperpigmentation, AIH) is also associated with significant reductions in QOL scores and poor body image [4]. Further, acne, scarring, and AIH can lead to stigmatization and less favorable perceptions compared to those with clear skin by the general population [10,11,12,13,14,15]. Life decisions relating to relationships and study and work choices in teenage years can be influenced by the effects of acne and may have life-long implications [4, 16]. Tan et al. expanded the scope of early QOL research by studying the impact of truncal acne, reporting that truncal acne either alone or with facial acne had an independent negative effect on QOL [17]. Conversely, effective treatment of acne can profoundly affect patients’ QOL and patient treatment satisfaction [16, 18]. This can be particularly important in acne, where adherence to treatment is often poor [17, 19].
Table 1 presents an overview of current acne management recommendations. The NICE guidance suggests a 12-week course of one of the following as first-line acne therapy: fixed combinations of adapalene/benzoyl peroxide (BPO), tretinoin/clindamycin (acne of any severity), BPO or BPO/clindamycin (mild-moderate acne), and addition of an oral antibiotic to topical therapy (moderate-severe acne). A further discussion of the pros and cons of these options as well as alternatives is presented in the NICE guidance [1].
Finally, it is increasingly recognized that the inclusion of a good skincare regimen (cleanser, treatment, moisturizer, photoprotection [CTMP]) can enhance patient outcomes and satisfaction [20].
In this publication, the UK Acne Working Group reflects on the latest NICE acne guidelines with the goal of providing additional practical insights for practicing clinicians who manage patients with acne.
Methods
Individually and via online communications, the UK Acne Working Group carefully studied the first UK acne NICE guideline, to identify potential gaps, key areas, and practical aspects that could potentially support acne management. The UK acne panel consists of allied healthcare professionals (AHPs) selected for their acne expertise embracing primary care, pharmacy, primary secondary care, and academia. Roles within these areas were different (e.g. the group included a pharmacist, a general practitioner, a nurse, and consultant dermatologists). This was done to provide a broad opinion from several perspectives that we felt could help to inform acne practice and management across the whole patient pathway. The panel conducted workshops led by the chair to identify areas pertinent to their own clinical practice where they deemed there was lack of evidence or an unmet need to address. The members then reconvened for discussion. Whereas there was some variation across the different roles, there were common and recurring themes which were then all included in this review. As a result of the full inclusions, voting on these themes was not required. After the group carefully reviewed the guidelines, a live meeting was held to discuss topics identified in the guidelines and come to a consensus about areas that could use additional input from healthcare practitioners with experience in acne management. In line with these themes and where evidence was lacking at the time of the NICE guidance, further pragmatic review of the literature was done which identified novel evidence from robust clinical trials which were assessed for quality and risk of bias and then included in the paper. This publication reports on some novel clinical trials and expert opinion with the aim of supporting improved outcomes for patients with acne. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Moving Forward: Suggestions for Areas Not Included in the NICE Acne Guidelines 2.0
Authors observed several topics where additional attention could be beneficial for acne management and possible consideration to update in future acne guidelines because there was not a substantial evidence base supporting recommendations for these potentially challenging aspects of acne management at the time of the NICE guidelines (and, as such, these topics were not covered in depth in that publication). These include:
-
1.
Assessment of acne
-
2.
Truncal acne management
-
3.
Addressing acne-induced sequelae
-
4.
Use of hormonal therapies in adult female patients
The methodology of NICE has been acknowledged by the European Dermatology Forum (EDF) to the degree that EDF decided not to repeat the work done by NICE. However, the heterogeneity of outcome measures and different grading scales does challenge the ability to compare results and conduct robust meta-analyses which would further inform recommendations.
Assessment of Acne
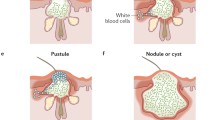
The first NICE acne guideline included a well-rounded discussion about the use of lesion counts to assess acne [1, 21]. There were many different grading assessments used (more than 15 plus variations in lesion counts used in definitions) and the evidence-based studies included in the guidance embraced differing definitions for mild-moderate acne which included the following examples among others: Leeds score 0.25 to 1.0; 10–50 inflammatory lesions and 10–100 non-inflammatory lesions; grades 1 and 2 comedonal and papulopustular acne; Leeds severity grade 2–5 on Leeds revised scale and 15–50 inflammatory lesions; Evaluator Global Severity Score of 2; and mild-moderate on Combined Acne Severity Classification with a lesion count ≥ 10 [1]. Based on review of the studies included, NICE guidance defined mild to moderate acne as “people who have one or more of: any number of non-inflammatory lesions (comedones), up to 34 inflammatory lesions (with or without non-inflammatory lesions), up to two nodules.” [1] Further suggestions were also defined for moderate-to-severe acne as 35 or more inflammatory lesions and three or more nodules.
The NICE guidance did not examine other aspects which are potentially influential in defining acne severity. These include acne location, lesion size, and psychosocial impact resulting from acne. The visibility of acne could impact perception of severity [21]. Stein Gold et al. note that “a single parameter such as lesion count does not capture the complexity of acne presentation,” and discussed the inclusion of Investigator Global Assessments (IGAs)—which were developed to give a more general gestalt impression of acne severity—in clinical trials [21]. Scarring and markers of poor prognosis have also been proposed as an important part of acne assessment [22]. The Personalising Acne: Consensus of Experts (PACE) publications, in generating recommendations for a comprehensive, long-term approach to acne, emphasized that “risks (or fear about) acne sequelae are highlighted as particularly important” and encourage regular discussions of these aspects of acne with patients [22]. We recognize that acne severity variables other than lesion counts have not been well studied, but believe they have importance for optimizing patient management.
Management of Truncal Acne
The NICE acne guidance focused primarily on facial acne and did not have specific recommendations for managing truncal acne [1]. Approximately half of patients with acne have truncal involvement (acne on the chest, back, or shoulders), concurrent with or, more rarely, separate from facial acne. The severity or grade of truncal acne does not always match that of facial acne [23, 24]. Truncal acne is often underdiagnosed since patients discuss facial acne and fail to voluntarily report truncal acne [23, 24]. Tan et al. reported that patients with both facial and truncal acne were twice as likely to report their disease had a large/very large impact on their life compared to those with just facial acne [17].
The Comprehensive Acne Severity Scale (CASS) was one of the earliest grading scales validated for both facial and truncal acne [25]. Alternatively, a facial IGA can be combined with a physician global assessment of the trunk (PGA) [24]. As noted by Stein Gold and others, international working groups have been formed to standardize acne tools, including the Acne Core Outcomes and Research Network (ACORN), the PACE group, and the European Academy of Dermatology and Venereology Task Force [21, 22]. These groups, along with the UK Acne Working Group, have been actively involved in improving the tools available for researchers and clinicians to manage acne and potential knowledge gaps.
While truncal acne is generally considered to have a pathophysiology that is similar to that of facial acne, there are some unique considerations in management of truncal disease—including that a large skin surface area may be affected [24, 26, 27]. Additionally, some medications, like benzoyl peroxide (BPO), have bleaching effects which cause alterations in clothing and linens, and may not be as desirable for truncal acne as for facial acne [26]. Finally, truncal scarring commonly includes unsightly hypertrophic and keloid scarring, macular atrophic scarring, and perifollicular elastolysis, which is a type of scarring almost exclusively found on the trunk [26]. Liu and Tan have recommended that, similar to facial acne, treatment of truncal acne should target several pathophysiologic mechanisms at the same time [27].
Topical retinoids have been used for truncal acne, but only trifarotene has been rigorously studied for both facial and truncal acne [28]. Trifarotene has been approved by regulatory bodies for the treatment of both facial and truncal acne for patients aged ≥ 9 years (USA) and 12 years (EU). It has higher selectivity for retinoic acid receptor gamma in the skin compared to other retinoids [29]. In two large-scale phase 3 studies (n = 1214 trifarotene and n = 1206 vehicle), trifarotene demonstrated rapid efficacy with reductions in lesions that were significantly superior to vehicle on the face at week 1 and on the trunk at week 2 [28]. Additionally, trifarotene-treated patients were more likely to achieve IGA success by week 12 on both the face and trunk (P < 0.001 for all comparisons) [28]. Trifarotene was well tolerated but had a higher incidence of mild cutaneous irritation compared to vehicle, an effect that was greater on the face than on the trunk. Irritation typically peaked early in therapy (week 1 for face and week 2 for trunk) and gradually reduced with improved tolerability over time [28]. Use of a good skin care regime should address this problem. Figure 1 presents a patient with truncal acne treated with trifarotene.
Woo et al. note that BPO has been used for treatment of truncal acne, particularly in wash-off cleansers [30]. Woo further suggests that clascoterone, a topical androgen receptor inhibitor, may be an option for truncal acne as it was studied during an open-label extension of phase III clinical trials for treatment of facial and truncal acne [30].
Procedural treatments like lasers, photodynamic therapy, and peels have been used for truncal acne but are not well supported by clinical trial evidence [30]. In addition, these procedures are not generally available in the English National Health Service (NHS), can be costly for patients as they are often not covered by insurers, and may require multiple office visits.
How to Address Acne-Induced Sequelae
Atrophic acne scarring and AIH are the most notable acne sequelae; both present management challenges and are long-lasting. This highlights the importance of prevention, and treatment approaches currently rest upon the foundation of early and effective acne management [31]. Sequelae can occur with acne of any severity—even mild disease—but scarring correlates with inflammation and can affect more skin areas as acne severity increases [31]. Risk factors for acne-related scarring are thought to include acne inflammation, time to effective treatment, relapsing course, and family history of acne and scarring [32, 33]. The most clearly linked risk factor for AIH is skin tone, with more highly pigmented skin prone to AIH lesions. Similar to truncal acne, experts recommend discussing the risk for acne sequelae (both scarring and pigmentation) at the first consultation and regularly thereafter [13]. Reducing risk for acne sequelae is also a recommended goal of maintenance therapy [13]. The NICE guidance notes that a referral to a dermatologist is important for individuals who experience psychological distress from acne or acne-related scarring, and called for more research into risk factors for scarring [1].
This gap in the NICE guidance was due to lack of information from current studies demonstrating effectiveness of treatments for acne scarring. The PACE group highlighted a need for high-quality evidence to support individual treatments for acne sequelae [13]. In 2023, Schleicher et al. published results of a randomized, split-face, double-blind phase 4 study in subjects aged 17 to 35 (n = 121) with moderate-to-severe acne and acne scarring [34]. Patients received 6 months treatment with either trifarotene or its vehicle once daily [34]. At 6 months, there was a significantly greater reduction in mean total atrophic scar count from baseline: − 5.9 for trifarotene vs − 2.7 for vehicle, P < 0.0001 [34]. Differences in favor of trifarotene in reducing scarring vs vehicle were apparent as early as week 2 (− 1.5 vs − 0.7 for vehicle, P < 0.01) [34]. By week 12, scar success rates were approximately threefold higher in the trifarotene group; at week 24, the difference was even greater (63.6% trifarotene vs 31.3% vehicle, P < 0.001) [34]. Dreno et al. reported that adapalene 0.3%/BPO 2.5% (A/BPO) applied once daily for 48 weeks (24-week randomized split-face vs vehicle phase followed by a 24-week open-label phase) significantly reduced atrophic acne scars [35]. During the 24-week split-face phase, scar counts decreased by 21.7% on A/BPO-treated sides while counts increased by 16.7% on the vehicle-treated side. Improvements after week 24 were also apparent, with continued improvements on sides originally treated with A/BPO and a 22.7% reduction in scar counts on the side originally treated with vehicle [35]. These results underscore the importance of early and effective acne treatment in preventing and reducing acne scar formation [35].
Authors note it is important to establish how long acne has been present and to be aware of the degree and duration of clinical inflammation, since this influences resultant scarring (Fig. 2). Clinicians should select therapy to reduce inflammatory acne, and patients should be educated to avoid excoriation of acne lesions and be made aware of the potential for improvement of acne scars over time. NICE guidance suggests if acne scarring is severe and persists a year after their acne has cleared, it is also reasonable to refer to a consultant-led team for consideration of CO2 laser treatment (alone or after a session of punch elevation) or glycolic acid peel although these are still not freely available in NHS departments [36].
Acne scarring is more likely if treatment is delayed. From Schleicher with open access creative commons license permission [34]. Randomized, split-face, 24-week trial of trifarotene or vehicle applied once daily in patients with moderate-to-severe acne and acne scarring
Interesting information has emerged from Aubert et al., who found that trifarotene has both depigmenting and anti-pigmenting activity [29]. Results of a phase IV double-blind, vehicle-controlled randomized trial of trifarotene in patients with mild-moderate acne and AIH showed that trifarotene was associated with greater improvements from baseline in AIH at week 12 [37]. While differences between the trifarotene and vehicle groups were not statistically significant at week 24 (primary endpoint), trifarotene achieved a significantly more rapid improvement vs vehicle with visible differences at week 12 [37]. There was also a greater reduction in post acne hyperpigmentation index (PAHPI) total facial score in the trifarotene vs vehicle group at week 24 (− 18.9% vs − 11.3%, P < 0.01) [37].
Use of Hormonal Treatments in Adult Female Patients
The NICE guidance recommends that women with acne who also require a hormonal contraceptive could receive the combined oral contraceptive in combination with a first-line acne treatment option [1]. However in contrast to other guidelines, NICE did not recommend combined oral contraceptives as treatment for acne as suggested evidence to support them in this context was lacking. The European Medicines Agency (EMA) and American Academy of Dermatology (AAD) both recommend combined oral contraceptives for women with acne in the context of requiring contraception or menstrual irregularities if there are no contraindications [38]. The AAD supports the use of several combined oral contraceptives for the treatment of acne in female patients but recommends avoiding oral contraceptives for patients younger than 14 years or within 2 years of first starting menstruation unless clinically warranted to avoid osteopenia or decreased bone mineral densities [38].
There is no strong evidence to identify the most effective treatment of acne in women with polycystic ovarian syndrome (PCOS). However, NICE suggest that if first-line anti-acne treatments are not effective in patient with PCOS and acne then it is reasonable to trial a hormonal treatment with either a combined oral contraceptive pill or ethinylestradiol with cyproterone acetate (co-cyprindiol) with a 6-month review of the latter.
Although not mentioned in the NICE guidance (unlike the AAD guidelines), orally administered spironolactone acting as an antiandrogen has been used off license for many years as treatment for female patients with papulopustular acne. A hybrid systematic review suggested a paucity of evidence for its use but recent research has shown that spironolactone may be a useful alternative to antibiotics in adult women with acne—an important feature in this era of increasing antibiotic resistance [39]. In an independent UK study supported by an National Institute for Health and Care Research Health Technology Assessment grant, adult women had a greater improvement on the acne-specific QOL symptom subscale compared to placebo at 24 weeks [40]. Treatment success on IGA at week 12 was 19% in those treated with spironolactone compared to 6% of those in the placebo group [40]. Barbieri et al. reported that spironolactone had similar clinical effectiveness compared with oral tetracyclines [41]. Further, the risk of being prescribed a different systemic agent among patients treated with spironolactone and tetracycline antibiotics was similar (odds ratio 1.07) in a retrospective cohort [41]. Spironolactone is generally well tolerated, but can be associated with breast tenderness, irregular menstrual cycles, dizziness, headaches, and nausea/vomiting [42]. In addition, a baseline check of potassium levels and renal function is recommended before initiating spironolactone for acne to rule out renal impairment. However, ongoing monitoring is unnecessary for most young women [43, 44].
Areas for Future Research
Having identified core outcomes domains, ACORN, the Acne Core Outcome Research Network, is now working to validate, standardize, and harmonize tools for use in assessing acne in clinical practice and trials. A James Lind Alliance acne priority setting partnership (AcnePSP) has highlighted research questions that are important to stakeholders by securing responses from 4363 stakeholders interested in acne. The AcnePSP identified the top 10 areas of research many of which align with research priorities identified in the NICE acne guidelines. These include:
-
Risk factors for acne scarring
-
Appropriate skin care advice for people with acne (including cleansers, moisturizers, and sunscreens)
-
Effects of diet in acne
-
Better understanding of the effectiveness of physical/procedural treatments for acne (light devices, chemical peels)
-
Management options for refractory acne
-
Management for people with acne and PCOS
It would also be helpful to have more research into how to optimize maintenance therapies in acne given the chronic nature of the disease.
The NICE acne guidance recognizes the potential for significant psychosocial impact of both acne and acne sequelae; in this context, an assessment tool to help determine ‘significant psychological distress’ is an area of interest.
Conclusions
It is well recognized that harmonizing outcome measures will allow for better comparisons between treatments and also support translation of research outcomes to the real-world setting. Standardized outcomes also facilitate meta-analyses of data across studies. Since the publication of the latest NICE acne guidance, novel approaches to manage acne sequelae with the newest fourth-generation topical retinoid trifarotene for both acne and sequelae have been reported. In addition, evidence for spironolactone as an alternative to oral antibiotics has been demonstrated for adult women with acne which importantly addresses the global concern of antimicrobial resistance and need to deliver antibiotic stewardship.
Data Availability
This is an opinion-based article, no new data were generated to create this publication, and no data sharing is available.
References
National Institute for Health and Care Excellence. Acne vulgaris: management. 2021.
Gollnick HP, Finlay AY, Shear N, Global Alliance to Improve Outcomes in Acne. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9:279–84.
Centers for disease control and prevention. National center for health statistics. Classifications of diseases and functioning and disability. http://www.cdc.gov/nchs/about/otheract/icd9/icfhome.htm. Accessed 30 July 2024.
Akinboro AO, Ezejiofor OI, Olanrewaju FO, et al. The impact of acne and facial post-inflammatory hyperpigmentation on quality of life and self-esteem of newly admitted Nigerian undergraduates. Clin Cosmet Investig Dermatol. 2018;11:245–52.
Eichenfield DZ, Sprague J, Eichenfield LF. Management of acne vulgaris: a review. JAMA. 2021;326:2055–67.
Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672–6.
Chuah SY, Goh CL. The impact of post-acne scars on the quality of life among young adults in Singapore. J Cutan Aesthet Surg. 2015;8:153–8.
Hayashi N, Miyachi Y, Kawashima M. Prevalence of scars and “mini-scars”, and their impact on quality of life in Japanese patients with acne. J Dermatol. 2015;42:690–6.
Tan JK, Li Y, Fung K, et al. Divergence of demographic factors associated with clinical severity compared with quality of life impact in acne. J Cutan Med Surg. 2008;12:235–42.
Schuster B, Gallinger J, Philipp-Dormston WG, Vasel M, Layton AM. Less confident, successful and happy: patients with post-acne hyperpigmentation are stigmatized. Br J Dermatol. 2023;188:682–4.
Pathmarajah P, Peterknecht E, Cheung K, Elyoussfi S, Muralidharan V, Bewley A. Acne vulgaris in skin of color: a systematic review of the effectiveness and tolerability of current treatments. J Clin Aesthet Dermatol. 2022;15:43–68.
Tan J, Beissert S, Cook-Bolden F, Chavda R, Harper J, Hebert A, et al. Impact of facial atrophic acne scars on quality of life: a multi-country population-based survey. Am J Clin Dermatol. 2022;23:115–23.
Layton A, Alexis A, Baldwin H, et al. Identifying gaps and providing recommendations to address shortcomings in the investigation of acne sequelae by the personalising acne: consensus of experts panel. JAAD Int. 2021;5:41–8.
Anaba EL, Oaku RI. Cross-sectional study of beliefs, perceptions, knowledge of treatment modalities and willingness to pay for acne scar treatment in acne patients. West Afr J Med. 2020;37:625–30.
Dreno B, Tan J, Kang S, et al. How people with facial acne scars are perceived in society: an online survey. Dermatol Ther (Heidelb). 2016;6:207–18.
Gieler U, Gieler T, Kupfer JP. Acne and quality of life - impact and management. J Eur Acad Dermatol Venereol. 2015;29(Suppl 4):12–4.
Tan J, Beissert S, Cook-Bolden F, et al. Impact of facial and truncal acne on quality of life: a multi-country population-based survey. JAAD Int. 2021;3:102–10.
Brodell RT, Schlosser BJ, Rafal E, et al. A fixed-dose combination of adapalene 0.1%-BPO 2.5% allows an early and sustained improvement in quality of life and patient treatment satisfaction in severe acne. J Dermatolog Treat. 2012;23:26–34.
Jones-Caballero M, Pedrosa E, Penas PF. Self-reported adherence to treatment and quality of life in mild to moderate acne. Dermatology. 2008;217:309–14.
Goh CL, Wu Y, Welsh B, et al. Expert consensus on holistic skin care routine: focus on acne, rosacea, atopic dermatitis, and sensitive skin syndrome. J Cosmet Dermatol. 2023;22:45–54.
Stein Gold L, Tan J, Kircik L. Evolution of acne assessments andimpact on acne medications: an evolving, imperfect paradigm. J Drugs Dermatol. 2016;15:79–86.
Tan J, Alexis A, Baldwin H, et al. The personalised acne care pathway-recommendations to guide longitudinal management from the personalising acne: consensus of experts. JAAD Int. 2021;5:101–11.
Tan J, Del Rosso JQ, Weiss JS, et al. Prevalence and demographics of truncal involvement among acne patients: survey data and a review of the literature. J Clin Aesthet Dermatol. 2022;15:62–7.
Tan J, Alexis A, Baldwin H, et al. Gaps and recommendations for clinical management of truncal acne from the personalising acne: consensus of experts panel. JAAD Int. 2021;5:33–40.
Tan JK, Tang J, Fung K, et al. Development and validation of a comprehensive acne severity scale. J Cutan Med Surg. 2007;11:211–6.
Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77:285–9.
Liu C-W, Tan J. Understanding truncal acne: a practical guide to diagnosis and management. Skin Therapy Letter. http://www.skintherapyletter.com/conditions/acne/truncal-diagnosis-management/. Accessed 16 July 2018.
Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691–9.
Aubert J, Piwnica D, Bertino B, et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-gamma agonist trifarotene. Br J Dermatol. 2018;179:442–56.
Woo YR, Kim HS. Truncal acne: an overview. J Clin Med. 2022;11:3660.
Thiboutot D, Dreno B, Abanmi A, et al. Practical management of acne for clinicians. An international consensus from the global alliance to improve outcomes in acne. J Am Acad Dermatol. 2018. https://doi.org/10.1016/j.jaad.2017.09.078.
Tan J, Kang S, Leyden J. Prevalence and risk factors of acne scarring among patients consulting dermatologists in the Unites States. J Drugs Dermatol. 2017;16:97–102.
Tan J, Thiboutot D, Gollnick H, et al. Development of an atrophic acne scar risk assessment tool. J Eur Acad Dermatol Venereol. 2017. https://doi.org/10.1111/jdv.14325.
Schleicher S, Moore A, Rafal E, et al. Trifarotene reduces risk for atrophic acne scars: results from a phase 4 controlled study. Dermatol Ther (Heidelb). 2023. https://doi.org/10.1007/s13555-023-01042-7.
Dreno B, Bissonnette R, Gagne-Henley A, et al. Long-term effectiveness and safety of up to 48 weeks’ treatment with topical adapalene 0.3%/benzoyl peroxide 2.5% gel in the prevention and reduction of atrophic acne scars in moderate and severe facial acne. Am J Clin Dermatol. 2019;20:725–32.
Tan JK, Tang J, Fung K, et al. Development and validation of a scale for acne scar severity (SCAR-S) of the face and trunk. J Cutan Med Surg. 2010;14:156–60.
Alexis A, Del Rosso J, Forman S, et al. Efficacy and safety of trifarotene cream 50µg/g for the treatment of acne-induced post-inflammatory hyperpigmentation in subjects with Fitzpatrick skin types I-VI: results from a phase IV trial (LEAP). Berlin Germany: European Academy of Dermatology and Venereology; 2023.
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(945–73):e33.
Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169–91.
Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:e074349.
Barbieri JS, Choi JK, Mitra N, Margolis DJ. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010–2016. J Drugs Dermatol. 2018;17:632–8.
Charny JW, Choi JK, James WD. Spironolactone for the treatment of acne in women, a retrospective study of 110 patients. Int J Womens Dermatol. 2017;3:111–5.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (research on adverse drug events and reports) program. Int J Women’s Dermatol. 2019;5:155–7.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941–4.
Acknowledgments
Medical Writing and Editorial Assistance
Editorial assistance was provided by Valerie Sanders of Sanders Medical Writing and funded by Galderma UK.
Funding
Editorial support and funding for the journal’s Rapid Service fee was provided by Galderma UK.
Author information
Authors and Affiliations
Contributions
Alison M Layton contributed to concept and design of the manuscript and reviewed/approved its submission. Drs Girish Gupta, Daron Seukeran, Thivi Maruthappu, Stephanie Gaillard, Heather Whitehouse, Faisal R. Ali, Angelika Razzaque, Firas Al-Niaimi, and Sarah Copperwheat reviewed and approved the manuscript and its submission. All authors reviewed the final manuscript and approved it for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Professor Alison Layton has acted as consultant advisor or received honoraria to deliver unrestricted educational events for Almirall, Alliance, Beiersdorf, Galderma, La Roche-Posay, LEO Pharma, L’Oréal, and Novartis over the last 3 years. Dr Girish Gupta has received honoraria for advisory board of Almirall and Galderma as well as lecture fees from Almirall, La Roche-Posay and Viatris. Professor Firas Al-Niami has received honoraria for advisory board of Galderma. Dr Sarah Copperwheat has received honoraria from Galderma, Leo, Derma UK and Incyte. Dr Heather Whitehouse has received honoraria for advisory board from Galderma. Dr Stephanie Gallard has received honoraria to deliver educational events and attend advisory boards as well as sponsorship for conference attendance from various companies. Dr Thivi Marruthapu has received honoraria for consulting for Galderma, Johnson and Johnson, Abbvie, Pfizer, Loreal, Procter and Gamble and Novartis. Dr Angelika Razzaque received sponsorship from Almirall, BMS, DermaUK, Galderma, LRP and UCB. Dr Faisal R Ali has received fees/honoraria for speaking and advisory work with Galderma and L’Oreal. Dr Daron Seukeran has received honoraria for advisory board from Galderma.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Layton, A.M., Gupta, G., Seukeran, D. et al. What’s New After NICE Acne Guidelines. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01275-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01275-0