Abstract
Introduction
Historically, patients with skin of color are underdiagnosed with psoriasis and underrepresented in clinical trials. In this study, we assess the efficacy and safety of risankizumab in patients with moderate-to-severe plaque psoriasis by race and ethnicity in the open label extension LIMMitless (NCT03047395).
Methods
Patients received continuous treatment with 150 mg risankizumab through their initial trial and the open label extension. Patients self-identified their race and ethnicity. Efficacy was assessed using Psoriasis Area Severity Index (PASI) and Dermatology Life Quality Index (DLQI). Safety is reported by events/100 patient-years.
Results
A total of 897 patients (race: 662 White, 196 Asian, 25 Black or African American, 14 Other; ethnicity: 98 Hispanic or Latino, 799 non-Hispanic or Latino) were included in this analysis. Compared to baseline, patients had a mean percent reduction in PASI between 94.6% (Asian) and 99.3% (Black or African American) and reported mean percent improvements in DLQI ranging from 87.1% (Asian and Black or African American) to 93.7% (Hispanic or Latino) at week 100.
Conclusion
While the data presented here comprise a small retrospective descriptive analysis and cannot detect statistical differences, efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis appears similar across the racial and ethnic groups studied and no new safety signals were detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with skin of color are often underdiagnosed and underrepresented in clinical trials. |
This study assesses the efficacy and safety of risankizumab in the treatment of moderate-to-severe plaque psoriasis across races and ethnicities in the open-label extension trial LIMMitless. |
At week 100, mean reduction in PASI from baseline ranged from 94.6% (Asian) to 99.3% (Black or African American), and patients reported a mean improvement in DLQI ranging from 87.1% (Asian and Black or African American) to 93.7% (Hispanic). |
No new safety signals were reported. |
Efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis appears similar across racial and ethnic groups. |
Introduction
Psoriasis is a chronic inflammatory disease with a variety of clinical manifestations, the most common of which is well demarcated scaly erythematous plaques on the skin. Psoriasis prevalence estimates range from 0.06 to 1.9% in the African American population to 0.225–3.6% in the White population [1,2,3]. Racial/ethnic variations in clinical characteristics and quality of life impact as well as disparities in research and access to care have been reported [4, 5]. Asians reported a significantly earlier age of onset compared to Caucasian patients, and non-White patients reported higher levels of skin involvement [1, 5,6,7]. Additionally, non-White patients report that psoriasis has a greater impact on their quality of life, even when controlling for baseline affected body surface area and Physician’s Global Assessment scores [5, 7, 8]. Additionally, symptoms such as erythema, a clinical hallmark of inflammation in psoriasis lesions, may appear less red in patients with darker pigmentation compared to those with lighter pigmentation. Erythema in skin of color may also have a variable range of color including violaceous, gray, and red-brown hues. Pigmentation alterations in the form of post inflammatory hyper- or hypopigmentation are common sequelae or associated findings in psoriasis patients with skin of color [2, 5].
Historically, patients with skin of color have been underrepresented in clinical trials and underdiagnosed compared to White patients [1, 9,10,11]. In a recent study examining clinical trial representation in new drugs and biologics, clinical trial representation levels for Hispanic, Asian, and Other groups were below the US population estimates [12]. In an analysis of clinical trials at GlaxoSmithKline and Pfizer when combining all clinical trials regardless of therapeutic area, study populations were similar to those reported in the US census, but individual trials did not always meet census data [13, 14].
In one study analyzing several dermatology studies from 2010 to 2015, only 59.8% reported on racial or ethnic demographics. In psoriasis studies that did report race, 84.3% of their patients were White [15]. In another analysis of dermatology studies in the US from 2017 to 2021, Black patients were the most underrepresented with only 21.9% of studies including Black patients at or above census levels [16]. Hispanic patients were only included at or above census levels in 28.9% of studies, and only 36.6% of studies included minorities at or above the census level [16].
In an effort to more directly address efficacy and safety across races and ethnicities, we assess the efficacy and safety of risankizumab, a humanized monoclonal antibody that binds to the p19 subunit of interleukin 23, in the treatment of adults with moderate-to-severe plaque psoriasis in LIMMitless open label extension trial.
Methods
Study Design
This is a retrospective analysis of patients selected from LIMMitless (NCT03047395), a completed phase 3, single-arm, global, multicenter, open-label extension trial. A detailed description of LIMMitless has been previously reported [17]. Patients included in this analysis were initially randomized to receive 150 mg subcutaneous risankizumab at week 0, 4, and every 12 weeks thereafter in one of the five phase 2 or 3 studies (NCT02684370, NCT02684357, NCT03000075, NCT02694523, or IMMvent) and had received at least 100 weeks of continuous risankizumab treatment. Data reported here are from the November 2021 data cut and include a minimum of 256 weeks of ongoing treatment for safety monitoring. Patients self-reported race as White, Black or African American, Asian, or Other (including American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or Multi-race). Patients also self-reported ethnicity as Hispanic or Latino or non-Hispanic or Latino.
The LIMMitless study is being conducted in accordance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonisation, the Declaration of Helsinki, and/or all applicable federal and local regulations, and all patients provided written informed consent. All protocols were approved by an institutional review board (Quorom Review IRB, Seattle, WA, USA).
Assessments and Statistical Analysis
Efficacy was evaluated using Psoriasis Area and Severity Index (PASI) and the static Physician’s Global Assessment (sPGA). PASI 90 and 100 represent at least 90% and 100% reduction of PASI score from baseline, respectively. Erythema was generated using the following equation where E = intensity of erythema, A = area affected, and h, u, t, and l corresponded with the regions of the body (head and neck, upper limbs, trunk, and lower body, respectively).
Patient-reported health-related quality of life (HRQoL) was assessed using the Dermatology Life Quality Index (DLQI). The minimal clinically important difference (MCID) in DLQI is defined as a reduction of ≥ 4.
Categorical endpoints are reported by modified non-responder imputation (mNRI) in which a non-response is imputed only for treatment failures, defined as patients who have a worsening of psoriasis. Continuous endpoints are reported using last observation carried forward (LOCF). As this is a descriptive analysis, no p values were generated for these data.
Results
Patient Population
A total of 897 patients were included in this analysis (Fig. 1). Of these patients, 73.8% (n = 662) self-identified as White, 2.8% (n = 25) as Black or African American, 21.9% (n = 196) as Asian, and 1.6% (n = 14) as Other (Fig. 1).
Baseline Demographics and Disease Characteristics
Baseline demographics and disease characteristics varied among racial and ethnic groups (Table 1). When comparing across races, women comprised between 17.9% (35/196, Asian) to 56.0% (14/25, Black or African American) of the population, and the mean age ranged from 46.6 years (Other) to 52.4 years (Black or African American). White, Black or African American, and Other races all had the highest proportion of patients with a BMI ≥ 30, while most Asian patients had a BMI < 25 (Table 1). The mean PASI score ranged from 19.3 (Other) to 23.5 (Asian), and mean BSA ranged from 24.8 (White) to 33.3 (Asian) (Table 1). Mean DLQI ranged from 13.6 (White) to 16.5 (Other) (Table 1).
Efficacy in the Skin
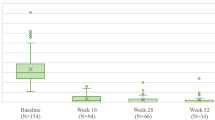
At week 100, patients, regardless of race or ethnicity, demonstrated at least a 94.1% mean improvement in PASI scores from baseline (Fig. 2). By race, patients had a 95.5%, 99.3%, 94.6%, and 97.3% mean reduction from baseline PASI for White, Black or African American, Asian, and Other patients, respectively (Fig. 2). Hispanic or Latino patients had a 94.1% mean reduction, and non-Hispanic or Latino patients had a 95.6% mean reduction in PASI scores from baseline (Fig. 2). The median reduction from baseline PASI was 100% (IQR 95.0%, 100.0%), 100% (100.0%, 100.0%) for Black or African American, 98.4% (92.3%, 100%) for Asian, and 100% (100%, 100%) for Other patients (data not shown). Hispanic or Latino patients had a median reduction of 100% (96.0%, 100.0%), and non-Hispanic or Latino patients had a median of 100% reduction (94.2%, 100%) of PASI from baseline (data not shown). PASI 90 achievement rates at week 100 ranged from 81.1 to 100.0% across races (Fig. 2). Of Hispanic or Latino patients, 85.7% and 85.0% of non-Hispanic or Latino patients achieved PASI 90 at week 100 (Fig. 2). At week 100, PASI 100 achievement rates ranged from 44.9% (Asian) to 84.0% (Black or African American) and 60.2% for Hispanic or Latino and 59.1% for non-Hispanic or Latino patients (Fig. 2).
At week 100, the mean absolute PASI score was 0.88 for White, 0.12 for Black or African American, 1.28 for Asian, 0.61 for Other, 1.43 for Hispanic or Latino, and 0.88 for non-Hispanic or Latino patients (data not shown). A PASI ≤ 3 at week 100 was achieved by 91.4% of White, 100.0% of Black or African American, 85.7% of Asian, 92.9% of Other, 87.8% of Hispanic or Latino, and 90.7% of non-Hispanic or Latino patients (data not shown).
We also independently assessed erythema, a component of the PASI score. A mean reduction in erythema ranging from 94.1 to 99.3% was observed across all racial and ethnic groups from baseline to week 100 (data not shown). Skin clearance was also assessed using sPGA with an sPGA score of 0 or 1 being considered clear or almost clear skin. At week 100, most patients achieved clear or almost clear skin with 85.8% of White, 100.0% of Black or African American, 83.2% of Asian, and 85.7% of Other patients meeting this mark (Fig. 2). Clear or almost clear skin was achieved for 83.7% of Hispanic or Latino patients and 85.9% of non-Hispanic or Latino patients (Fig. 2).
DLQI
Patients reported improved HRQoL as assessed by DLQI. After 100 weeks of treatment, the mean percent improvement from baseline DLQI score was 92.4% for White, 87.1% for Black or African American, 87.1% for Asian, and 92.5% for Other patients (Fig. 2). Hispanic or Latino patients reported a 93.7% and non-Hispanic or Latino patients reported a 90.8% mean reduction in baseline DLQI scores (Fig. 2). The median reduction in DLQI from baseline was 100% (92.9%, 100.0%) for White, 100.0% (100.0%, 100.0%) for Black or African American, 95.2% (82.8%, 100.0%) for Asian, and 100.0% (88.9%, 100.0%) for Other patients (data not shown). The median reduction in DLQI from baseline for Hispanic or Latino patients was 100.0% (95.8%, 100.0%) and 100.0% (90.0%, 100.0%) for non-Hispanic or Latino patients (data not shown). Most patients achieved a DLQI score of 0 or 1 at week 100, ranging from 73.5% (Asian) to 82.6% (White), and from 80.0% (non-Hispanic or Latino) to 83.7% (Hispanic or Latino) of patients (Fig. 3). The mean DLQI score at 100 weeks was 1.0 for White, 1.8 for Black or African American, 1.7 for Asian, 1.5 for Other, 1.3 for Hispanic or Latino, and 1.1 for non-Hispanic or Latino patients (data not shown). MCID in DLQI was achieved by 97.7% of White, 87.5% of Black or African American, 97.3% of Asian, 100% of Other, 98.9% of Hispanic or Latino, and 97.2% of non-Hispanic or Latino patients (data not shown).
Safety
Safety is reported through a minimum of 256 weeks of ongoing treatment. Patient-years of exposure varied across races and ethnicities with 3571.2 PYs for White, 133.3 PYs for Black, 1082.4 PYs for Asian, 73.8 PYs for Other, 531.3 PYs for Hispanic or Latino, and 4329.4 PYs for non-Hispanic or Latino patients (Table 2). Serious adverse events (reported as E/100PYs) were 7.4 for White, 6.0 for Black or African American, 5.7 for Asian, 2.7 for Other, 2.4 for Hispanic or Latino, and 7.5 for non-Hispanic or Latino patients (Table 2). MACE, serious infections, malignancy, and serious hypersensitivity occurrences were low across all racial and ethnic groups studied (Table 2). While there are numerical differences in the rates of certain adverse events, the variability in sample size and patient-years of exposure may be a contributing factor.
Discussion
Across races (White, Black or African American, Asian, or Other) and ethnicities (Hispanic or Latino or non-Hispanic or Latino), patients receiving risankizumab had reduced signs and symptoms of psoriasis and improved quality of life. Additionally, these improvements were often similar among racial/ethnic groups, and no new safety signals were detected. Overall, mean PASI improvements from baseline ranged from 94.1 to 99.3%. The percentage of patients achieving PASI 90 ranged from 81.1 to 100.0%, and 44.9–84.0% of patients achieved PASI 100 at week 100. Between 83.2 and 100.0% of patients achieved sPGA 0/1, and 73.5–83.7% of patients achieved DLQI 0/1 at week 100. Patients reported a mean percent improvement from baseline DLQI between 87.1 and 93.7%. Previous studies have reported comparable efficacy for psoriasis across racial and ethnic groups [18, 19] as well as other skin diseases [20].
An area of future development includes focusing on improving diversity in clinical trials, as evidenced within this article. Diversity in clinical trials can help build trust in medical research and institutions, promote fairness for participants and their communities, and help generate biomedical knowledge [21]. Additionally, studies focusing directly on psoriasis (and other dermatological conditions) in diverse patient populations are needed to further our understanding of the clinical, therapeutic, and quality of life impact of psoriasis in patients with skin of color.
Health care differences have also been reported for patients of different races and ethnicities, and another area of future development to further serve patients with skin of color is an improvement in medical resources. Many of these resources lack skin of color representation [22]. In one study, 47% of dermatologists felt their training was inadequate to diagnose skin disease in patients with skin of color, with most reporting they had never received lectures or clinical rotations involving experience in working with patients with skin of color [15]. From 1996 to 2006, only 2% of teaching events sponsored by the American Academy of Dermatology focused on skin of color, and < 5% of major pre-clinical anatomy textbooks had images with darker skin tones [15].
Additionally, patients with skin of color reported that they are less likely to seek a dermatologist for treatment citing lack of cultural competency and costs, though they reported similar willingness to seek medical care as White patients [7, 15, 23, 24]. Asian patients have been reported to receive less face-to-face time with their providers compared to patients of other races despite often presenting with more severe psoriasis [25]. Despite similar results, biologics are often prescribed at different rates across racial/ethnic groups in the US [26, 27]. Differences in care can be associated with the cost or cultural views of clinical care, differences in clinical presentation, underrepresentation in clinical trials, or health care utilization [28]. While our data support similar efficacy across races and ethnicities, non-White patients had lower achievement of DLQI 0/1 at week 100, suggesting that there is still an unmet need in order to improve the HRQoL for these patients.
Our analysis suggests that treatment with risankizumab resulted in robust reduction of erythema across all racial and ethnic groups. However, because psoriasis often presents differently in various skin tones, assessment of erythema, or normalization of natural skin tone, may be difficult to accurately assess. Additional education and training are likely to improve the accuracy with which skin erythema and/or discoloration is assessed in various skin tones.
This analysis has limitations in that this is a retrospective study with small subpopulations, specifically the Black or African American population was only 25 patients. As such, it is not powered to detect statistical differences, and scientific conclusions should not be drawn. The populations were also not balanced to have similar severity levels across races and ethnicities. For example, the Asian population had numerically higher PASI and BSA compared to White, Black or African American, and Other patients. Despite this, patients that identified as Other reported the highest DLQI at baseline. Additionally, these studies did not collect skin phenotype or objective measures of skin pigmentation (e.g., by colorimetry). Enrollment in these studies began before the increased awareness of lack of diversity in clinical trials and serves to emphasize the importance of enrolling patients with skin of color.
Conclusion
In conclusion, this analysis reports that across the range of racial and ethnic groups studied, patients receiving risankizumab often experienced similar reductions of signs and symptoms of psoriasis and improved quality of life. In addition, no new safety signals were detected.
Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select "Home".
References
Gelfand JM, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52(1):23–6.
Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7(11):16–24.
Taylor SC. Epidemiology of skin diseases in people of color. Cutis. 2003;71(4):271–5.
Klopot A, et al. Transcriptome analysis reveals intrinsic proinflammatory signaling in healthy African American Skin. J Investig Dermatol. 2022;142(5):1360-1371.e15.
Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-White Racial/Ethnic Groups. Am J Clin Dermatol. 2018;19(3):405–23.
Yan D, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018. https://doi.org/10.5070/D3247040909.
McKenzie S, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color. Part II: differences in clinical presentation and disparities in cutaneous disorders in skin of color. J Am Acad Dermatol. 2022;87(6):1261–70.
Takeshita J, et al. Health-Related QOL Differs by Race/Ethnicity in North American Patients with Psoriasis: results from PSOLAR. J Investig Dermatol. 2022;142(9):2528-2531.e3.
Ha MV, Wong C. Racial representation in clinical trials for dermatological new molecular entities. Clin Exp Dermatol. 2022;47(2):386–8.
Ricardo JW, Lipner SR. Under-representation of racial and ethnic minorities in nail psoriasis randomized clinical trials: a call to action. J Am Acad Dermatol. 2022;86(6):e267–8.
Ricardo JW, Qiu Y, Lipner SR. Racial, ethnic, and sex disparities in nail psoriasis clinical trials: a systematic review. Skin Appendage Disord. 2022;8(3):171–8.
Lolic M, et al. Racial and ethnic representation in US clinical trials of new drugs and biologics, 2015–2019. JAMA. 2021;326(21):2201–3.
Reid MM, et al. Demographic diversity of US-based participants in GSK-sponsored interventional clinical trials. Clin Trials. 2023;20(2):133–44.
Rottas M, et al. Demographic diversity of participants in Pfizer sponsored clinical trials in the United States. Contemp Clin Trials. 2021;106: 106421.
Narla S, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315(5):1215–23.
Mineroff J, Nguyen JK, Jagdeo J. Racial and ethnic underrepresentation in dermatology clinical trials. J Am Acad Dermatol. 2023;89(2):293–300.
Papp KA, et al. Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up. Br J Dermatol. 2021;185(6):1135–45.
Enos CW, et al. Similar response to biologic therapy across racial and ethnic groups among patients with psoriasis enrolled in the CorEvitas Psoriasis Registry. J Am Acad Dermatol. 2022;87(5):1087–9.
Ferguson JE, et al. Racial/ethnic differences in treatment efficacy and safety for moderate-to-severe plaque psoriasis: a systematic review. Arch Dermatol Res. 2023;315(1):41–50.
Alexis AF, et al. Efficacy of dupilumab in different racial subgroups of adults with moderate-to-severe atopic dermatitis in three randomized, placebo-controlled phase 3 trials. J Drugs Dermatol. 2019;18(8):804–13.
Schwartz AL, et al. Why diverse clinical trial participation matters. N Engl J Med. 2023;388(14):1252–4.
Perlman KL, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7(2):195–6.
Fischer AH, et al. Health care utilization for psoriasis in the United States differs by race: an analysis of the 2001–2013 medical expenditure panel surveys. J Am Acad Dermatol. 2018;78(1):200–3.
Tripathi R, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154(11):1286–91.
Wu KK, Armstrong AW. Differences in face-to-face time spent with a dermatologist among patients with psoriasis based on race and ethnicity. JAMA Dermatol. 2022;158(10):1210–2.
Hodges WT, et al. Biologics utilization for psoriasis is lower in black compared with white patients. Br J Dermatol. 2021;185(1):207–9.
Bell MA, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112(6):650–3.
Yadav G, et al. Unmet need in people with psoriasis and skin of color in Canada and the United States. Dermatol Ther. 2022;12(11):2401–13.
Acknowledgements
AbbVie and the authors thank the participants, study sights, and investigators who participated in the clinical trial
Funding
AbbVie Inc., participated in the study design; study research; collection, analysis and interpretation of data; and writing, reviewing, and approving of this manuscript for submission. All publication fees are funded by AbbVie Inc. All authors had access to the data; participated in the development, review, and approval of the manuscript and agreed to submit this manuscript to the American Journal of Clinical Dermatology for consideration.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection were performed by Ramon Espaillat, Tianshuang Wu, Linyu Shi, Mark I Kaldas, and Deanne Dilley. Analysis was performed by all authors. The first draft of the manuscript was written by Trisha Rettig and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A Alexis has received grants from Leo, Amgen, Galderma, Arcutis, Dermavant, AbbVie, and Castle, has served on advisory boards or as an consultant for Leo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Beiersdorf, Ortho, L’Oreal, BMS, Bausch health, UCB, Vyne, Arcutis, Janssen, Allergan, Almirall, AbbVie, Amgen, VisualDx, Eli Lilly, Swiss American, Cutera, Cara, EPI, Incyte, Castle, Apogee, Canfield, and Alphyn, has received speaker fees from Regeneron, SANOFI-Genzyme, BMS, L’Oreal, Janssen, and J&J, has received royalties from Springer, Wiley-Blackwell, and Wolters Kluwer Health and has received an equipment loan from Aerolase. M Gooderham has received speaker or consulting fees and/or research grants from: AbbVie, Amgen, Akros, Aslan, AnaptysBio, Aristea, Arcutis, Bausch Health, BMS, Boehringer Ingelheim, Cara Therapeutics, Celgene, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Merck, Mooklake, Meiji, Nimbus, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi Genzyme, Sun Pharma, UCB. S G Kwatra has received speaker or consulting fees and/or research grants AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Kiniksa Pharmaceuticals, Pfizer Inc., and Sanofi. A Amin has received speaker or consulting fees from AbbVie, Eli Lilly, Janssen, Dermavant, Leo Pharma, Regeneron, Sanofi/Genzyme, UCB, Amgen, BMS, and Pfizer. S Taylor has served on advisory boards for AbbVie, Avita Medical, Beiersdorf, Eli Lilly, EPI Health, Evolus, Galderma, Hugel, Incyte, Johnson & Johnson, L’Oreal, Medscape, Pfizer, Scientis, UCB and Vichy Laboratories, has served as an investigator for Allergan, Concert Pharamceuticals, Croma-Pharma GmbH Austria, Eli Lilly, Pfizer, and Sun Pharma, has served as a constulta form Armis Biopharma, Beiersdof, Bristol-Meyers Squibb, Cara Therapeutics, Dior, GloGetter, Piction Health, and Sanofi, has received speaker fees for Beiersdort, Catalyst Medical Education, Medscape, and MJH Life Sciences, and serves on the board of directors and has received employee fees from Mercer Strategies. R Espaillat, T Rettig, M Kaldas, T Wu, L Shi, DM Dilley, R Sinvhal, and C Nduaka are full-time salaried employees of AbbVie and may own stock/options. B Lockshin has received speaker and/or consulting fees and/or research grants from AbbVie, Novartis, Eli Lilly, Sanofi, Regeneron, Incyte, Leo, UCB, DermTech, Strata Skin Sciences, Corrona Registry, Boehringer Ingelheim, Pfizer, Franklin Bioscience, Trevi Therapeutics, Inc., Vanda, Celgene, Galderma, Amgen, and Dermira.
Ethical approval
The LIMMitless study is being conducted in accordance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonisation, the Declaration of Helsinki and/or all applicable federal and local regulations, and all patients provided written informed consent. All protocols were approved by an institutional review board (Quorom Review IRB, Seattle, WA, USA).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alexis, A.F., Gooderham, M., Kwatra, S.G. et al. A Descriptive, Post Hoc Analysis of Efficacy and Safety of Risankizumab in Diverse Racial and Ethnic Patient Populations With Moderate-to-Severe Psoriasis. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01268-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01268-z