Abstract
Introduction
Standard therapy for patients with mild to moderate atopic dermatitis (AD) typically includes topical therapies; however, patients with more extensive AD and/or AD refractory to topical therapy may benefit from systemic treatment. Ruxolitinib cream monotherapy has demonstrated superior antipruritic and anti-inflammatory effects versus vehicle in patients with mild to moderate AD, and long-term disease control with as-needed use. Here, efficacy/safety of 1.5% ruxolitinib cream through 52 weeks was assessed in a subset of patients with moderate and/or more extensive disease.
Methods
This post hoc analysis of TRuE-AD1/TRuE-AD2 included patients who, at baseline, had Investigator’s Global Assessment (IGA) score of 3, Eczema Area and Severity Index (EASI) ≥ 16, and affected body surface area (BSA) ≥ 10% (higher severity of disease threshold subgroup). Disease control and safety were assessed.
Results
Of 1249 patients in the overall population, 78 (6.2%) met all higher severity of disease threshold criteria (continuous-use vehicle-controlled period: 1.5% ruxolitinib cream, n = 32; vehicle, n = 13); 28 and 4 of these patients, respectively, continued as-needed 1.5% ruxolitinib cream during the long-term safety (LTS) period. At week 8 (continuous-use), IGA-treatment success (IGA 0/1, with ≥ 2-grade improvement from baseline) was achieved by 19/32 (59.4%) patients applying 1.5% ruxolitinib cream versus no patients applying vehicle. In the LTS period, those achieving clear/almost clear skin increased from 19/28 patients (67.9%; continuous-use: week 8) to 18/23 patients (78.3%; as-needed use: week 52) in patients applying ruxolitinib cream from day 1. Ruxolitinib cream was well tolerated, with few application site reactions, regardless of disease severity threshold. Efficacy and safety results were similar to the overall study population.
Conclusion
Patients with AD who meet standard disease severity eligibility criteria for systemic therapy may achieve IGA-treatment success with clear/almost clear skin with continuous-use ruxolitinib cream, and maintain long term-disease control with as-needed ruxolitinib cream monotherapy.
Trial Registration Number
NCT03745638/NCT03745651.
Plain Language Summary
Atopic dermatitis (AD) is a skin condition that causes itchy, dry, and inflamed skin. For many people AD is controlled with medication that is applied to the skin. However, for some people medication that is taken orally or injected (i.e., systemic treatment) may be needed. Systemic treatment can sometimes be challenging. Doctors use a variety of tools to measure AD severity and apply standard criteria to help determine if a person should receive systemic treatment. In the TRuE-AD1/TRuE-AD2 clinical trials, itch and inflammation improved in people with mild to moderate AD after they applied ruxolitinib cream twice daily for 8 weeks. When people then applied ruxolitinib cream to areas of AD only when it was needed for another 44 weeks, ruxolitinib cream provided long-term control of their AD. The aim of this analysis was to assess disease control with ruxolitinib cream in people with AD severe enough to meet the standard criteria indicating a need for systemic treatment. In this group, the majority had clear or almost clear skin after applying ruxolitinib cream twice daily for 8 weeks. After 44 weeks of as-needed application of ruxolitinib cream, most people still had clear or almost clear skin. In this group of people who may have otherwise needed treatment with systemic therapy, ruxolitinib cream twice daily for 8 weeks and then as-needed was generally well tolerated. These results show that as-needed ruxolitinib cream may provide long-term control of AD in people who may otherwise have needed systemic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Atopic dermatitis (AD) is typically treated with topical therapies; however, patients with more extensive AD or AD refractory to topical therapy may require systemic treatment. |
Ruxolitinib cream demonstrated superior antipruritic and anti-inflammatory effects versus vehicle in patients with mild to moderate AD, and long-term disease control with as-needed use. |
The aim of this study was to assess the efficacy and safety of 1.5% ruxolitinib cream through 52 weeks in patients meeting standard disease severity eligibility criteria for systemic therapy. |
What was learned from the study? |
Patients with AD meeting standard disease severity eligibility criteria for systemic therapy may derive clinical benefit from continuous-use ruxolitinib cream monotherapy and maintain long term-disease control with as-needed use. |
Ruxolitinib cream represents a safe and effective, non-steroidal, topical treatment option for patients with moderate and/or extensive AD, potentially reducing the need for therapy escalation. |
Introduction
Atopic dermatitis (AD) is a highly pruritic inflammatory skin disease [1]. Topical therapies are the standard of care for most patients with AD and are the first treatment step in protocols recommended by an expert panel of the International Eczema Council (IEC), by the consensus-based European guidelines, and by the American Academy of Dermatology [2,3,4,5,6]. As per these protocol recommendations, patients with more extensive AD or those with AD refractory to topical therapy may be appropriate candidates for systemic treatment.
The IEC does not provide strict thresholds for identifying patients appropriate for systemic therapy. However, studies of systemic therapies have tended to use similar inclusion criteria that incorporate minimum disease severity thresholds from clinician-reported assessments, including affected body surface area (BSA) of at least 10%, an Eczema Area and Severity Index (EASI) of at least 16 (EASI ≥ 16), and an Investigator’s Global Assessment (IGA) of at least 3 (IGA ≥ 3) [7,8,9,10,11]. In clinical trial settings, these thresholds have been generally accepted to identify patients who may benefit from systemic therapy. Additionally, more subjective tools, such as the itch numerical rating scale (itch NRS), have also been used to assess AD severity [12, 13].
Ruxolitinib cream is a topical formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and JAK2 [14]. In two phase 3 studies of identical design (Topical Ruxolitinib Evaluation in Atopic Dermatitis [TRuE-AD] Study 1 [TRuE-AD1] and Study 2 [TRuE-AD2]), ruxolitinib cream monotherapy demonstrated superior antipruritic and anti-inflammatory effects versus vehicle after 8 weeks of continuous treatment in adults and adolescents with mild to moderate AD. Long-term disease control was observed for most patients through the 44-week as-needed treatment period, and application site reactions were infrequent throughout the entire 52-week period [15, 16]. The aim of this analysis was to evaluate the efficacy and safety of 1.5% ruxolitinib cream in a subset of patients who were enrolled in TRuE-AD1/2 and met standard disease severity eligibility criteria for systemic therapy.
Methods
Patients and Study Design
This was a post hoc analysis of the pivotal phase 3 trials TRuE-AD1 (NCT03745638) and TRuE-AD2 (NCT03745651) [15, 16]. Eligible patients in the TRuE-AD1/2 studies were aged ≥ 12 years with AD for ≥ 2 years and had an IGA score of 2 or 3 and 3–20% affected BSA (excluding scalp). In both studies, patients were randomized (2:2:1) to either of two ruxolitinib cream strength regimens (0.75% twice daily [BID], 1.5% BID) or vehicle cream BID for 8 weeks of double-blinded continuous treatment (vehicle-controlled [VC] period); patients were instructed to continue treating lesions regardless of lesion clearance. Patients on ruxolitinib cream subsequently continued treatment for 44 weeks (long-term safety [LTS] period); patients initially randomized to vehicle were rerandomized 1:1 (blinded) to either ruxolitinib cream strength. During the LTS period, patients were instructed to treat skin areas with active AD lesions only and to stop treatment 3 days after clearance of lesions; patients were instructed to restart treatment with ruxolitinib cream at the first sign of lesion recurrence. Rescue treatment was not permitted at any time. Additional details regarding study design have been previously reported [15, 16]. These studies were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrollment. The protocols were approved by the relevant institutional review board or ethics committee at each study center (Supplementary Materials Tables S1 and S2). Following standard approaches to identify patients with more severe disease, this post hoc analysis included patients who had all three of the following at baseline: IGA score of 3, EASI ≥ 16, and affected BSA ≥ 10% (higher severity of disease threshold subgroup). Other definitions were also used to identify patients meeting higher thresholds of AD disease severity including (1) affected BSA ≥ 10% only; (2) IGA score of 3 only; and (3) all four of the following: IGA score of 3, EASI ≥ 16, affected BSA ≥ 10%, and itch NRS score ≥ 4.
In contemporary clinical trials of biologic and oral therapies [8, 9, 17], failure of previous topical therapy would occur prior to the introduction of systemic therapy, though this was not included in the eligibility criteria of the TRuE-AD1/2 studies and, thus, was not considered in this analysis. However, in the TRuE-AD1/2 studies, 89.5% of patients had received prior AD treatments, including low/medium/high potency topical corticosteroids (49.6%/42.4%/32.7%), topical calcineurin inhibitors (21.5%), or systemic corticosteroids (17.5%), and had met the study enrollment criteria of mild to moderate AD [18]. In the subgroup of patients meeting higher thresholds of AD disease severity, the percentage of patients who had received prior AD treatment was even higher (96.2%; Table 1).
Assessments
Efficacy assessments during the VC period included the percentage of patients achieving IGA treatment success (IGA-TS; defined as an IGA score of 0 or 1 with a ≥ 2-grade improvement from baseline), the percentage of patients achieving ≥ 75% improvement in Eczema Area and Severity Index (EASI75) from baseline, and the percentage of patients achieving a ≥ 4-point reduction in itch numerical rating scale score (NRS4). Additional efficacy assessments included the percentage of patients achieving ≥ 50% improvement in EASI (EASI50) and ≥ 90% improvement in EASI (EASI90). Disease control during the LTS period was assessed by the percentage of patients who achieved no or minimal skin lesions (IGA score of 0 or 1 [clear or almost clear skin]) and the mean percentage of BSA affected by AD at each visit (every 4 weeks). Safety and tolerability assessments included the frequency of reported treatment-emergent adverse events (TEAEs), treatment-related adverse events (AEs), application site reactions, and AEs leading to treatment discontinuation.
Statistical Analysis
All subgroup analyses were conducted using pooled data from both studies. The disease control analysis during the LTS period included a subgroup of patients who remained on their initial ruxolitinib cream strength regimen from the VC periods through the LTS period and a subgroup who crossed over to ruxolitinib cream from vehicle; data are reported as observed. The safety analysis included patients who received ruxolitinib cream in any period (VC or LTS). Data from the VC period were analyzed by logistic regression and reported descriptively; data from the LTS were summarized using descriptive statistics. Results for patients applying 1.5% ruxolitinib cream are reported.
Results
Patients
Of 1249 patients in the pooled randomized population in the TRuE-AD1 and TRuE-AD2 studies, 937 (75.0%) patients had a baseline IGA score of 3, 84 (6.7%) an EASI ≥ 16, and 535 (42.8%) an affected BSA ≥ 10%. Overall, a total of 78 (6.2%) patients met all three of these thresholds for higher severity of disease at baseline. Of these 78 patients, 32 applied continuous-use 1.5% ruxolitinib cream BID during the VC period and 13 patients applied vehicle during the VC period; 69 (88.5%) patients continued from the VC to the LTS period and were evaluated for disease control. Of these 69 patients, 28 (40.6%) patients had applied 1.5% ruxolitinib cream from day 1, and 4 (5.8%) patients were rerandomized from vehicle to as-needed 1.5% ruxolitinib cream at the start of the LTS period; these patients comprised the higher severity of disease threshold subgroup in the LTS period (N = 32). In either period, VC or LTS, 36 patients who met higher disease severity thresholds applied 1.5% ruxolitinib cream at least once and were evaluated for safety. Demographics and baseline clinical characteristics in the higher severity of disease threshold subpopulation are shown in Table 1.
Efficacy During the VC Period for the Higher Severity of Disease Threshold Subgroup
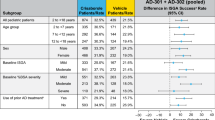
At week 8 of the VC period, 19/32 (59.4%) of those who applied continuous-use 1.5% ruxolitinib cream BID achieved IGA-TS, 23/32 (71.9%) achieved EASI75, and (for those with NRS ≥ 4 at baseline) 11/18 (61.1%) achieved NRS4 (Fig. 1). EASI50 and EASI90 were achieved by 25/32 (78.1%) and 15/32 (46.9%) patients applying continuous-use 1.5% ruxolitinib cream BID at week 8, respectively. No or few patients who applied vehicle achieved IGA-TS (0/13); EASI75 (1/13; 7.7%), or itch NRS4 (3/11; 27.3%) at week 8. Similarly, few patients who applied vehicle achieved EASI50 (5/13; 38.5%) or EASI90 (1/13; 7.7%).
Percentages of patients* achieving IGA-TS†, EASI75, or itch NRS4‡ at week 8 among the higher severity of disease threshold subgroup§and the overall TRuE-AD1/2 population. *Patients with missing postbaseline values were imputed as non-responders. †Defined as patients achieving an IGA score of 0 or 1 with an improvement of ≥ 2 points from baseline. ‡Patients in the analysis had an itch NRS score ≥ 4 at baseline. §Patients with IGA score of 3, EASI ≥ 16, and BSA ≥ 10% at baseline. BSA body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment
Ruxolitinib cream treatment effects were observed as early as the first postbaseline visit (week 2), with a higher percentage of responders among patients who applied 1.5% ruxolitinib cream (n = 32) than among those applying vehicle (n = 13) from week 2 (Fig. 2a–e).
Percentages of patients* achieving a IGA-TS, b EASI75, c itch NRS4, d EASI50, or e EASI90 with 0.75% or 1.5% ruxolitinib cream, or vehicle among higher severity of disease threshold subgroup†. *Patients with missing postbaseline values were imputed as non-responders at weeks 2, 4, and 8. †Patients with IGA score of 3, EASI ≥ 16, and BSA ≥ 10% at baseline. ‡Defined as patients achieving an IGA score of 0 or 1 with an improvement of ≥ 2 points from baseline. §Patients in the analysis had an itch NRS score ≥ 4 at baseline. BSA body surface area, EASI Eczema Area and Severity Index, HSDT higher severity of disease threshold, IGA Investigator’s Global Assessment
Disease Control During the LTS Period for the Higher Severity of Disease Threshold Subgroup
The percentage of patients achieving clear or almost clear skin (IGA 0/1) after 8 weeks of continuous-use 1.5% ruxolitinib cream BID was 67.9% (19/28) among patients who continued from the VC to the LTS period. Percentages of patients achieving clear or almost clear skin (IGA 0/1) with as-needed application of 1.5% ruxolitinib cream further increased during the LTS period (week 52: 78.3% [18/23]), similar to results observed in patients applying 1.5% ruxolitinib cream from day 1 in the overall population (Fig. 3a). Percentages of patients achieving clear or almost clear skin (IGA 0/1) were similar among patients who applied ruxolitinib cream from day 1 and those who crossed over from vehicle even when different definitions were used to define higher thresholds of AD disease severity (Fig. 3c shows results for patients who applied ruxolitinib cream from day 1).
Disease control* with 1.5% ruxolitinib cream: a percentages of patients with clear or almost clear skin (IGA of 0 or 1) and b mean percentages of BSA affected by AD among the higher severity of disease threshold subgroup† and the overall TRuE-AD1/2 population; and c percentages of patients with clear or almost clear skin (IGA of 0 or 1) and d mean percentages of BSA affected by AD among patients meeting various severity of disease thresholds. *Data are reported as observed. †Patients with IGA score of 3, EASI ≥ 16, and BSA ≥ 10% at baseline. BL baseline, BSA body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, LTS long-term safety, NRS numerical rating scale, VC vehicle-controlled
For the patients who applied 1.5% ruxolitinib cream from day 1, mean affected BSA decreased from 18.0% at baseline to 5.2% at week 8 (n = 28), and further decreased to 2.5% at week 52 (n = 23, as was observed with 1.5% ruxolitinib cream from day 1 in the overall population (Fig. 3b). Results were similar among patients who applied ruxolitinib cream from day 1 and those who crossed over from vehicle even when different definitions were used for higher thresholds of AD disease severity (Fig. 3d shows results for patients who applied ruxolitinib cream from day 1).
Safety for the Higher Severity of Disease Threshold Subgroup
In patients who applied 1.5% ruxolitinib cream from day 1, ruxolitinib cream was well tolerated and had a safety profile consistent with that observed in the overall study population (Table 2). No patients in this subgroup discontinued treatment as a result of a TEAE. Treatment-related AEs were reported for 6/36 (16.7%) patients in this subgroup and 39/545 (7.2%) patients in the overall population. The rate of application site reactions was low in this subgroup (n = 2/36 [5.6%]; application site irritation, n = 1; application site pruritus, n = 1) and in the overall population (n = 9/545 [1.7%]). One serious AE of chronic tonsilitis, which was not considered related to treatment, occurred in one patient in the 1.5% ruxolitinib cream subgroup. No notable infections, major cardiac events, malignancy, or thromboses were reported in this subgroup.
Discussion
Patients included in this post hoc, exploratory, subgroup analysis of the TRuE-AD1/2 studies had higher thresholds of disease severity at baseline, reflective of IGA, EASI, and BSA involvement inclusion criteria typically used for clinical trials of systemic treatments. Despite increased disease severity and/or extent as compared to the overall TRuE-AD1/2 study population, similar anti-inflammatory and antipruritic effects with 1.5% ruxolitinib cream were observed in this more severe subgroup, with similar safety and tolerability. These findings reinforce the efficacy of ruxolitinib cream in patients with mild to moderate AD and demonstrate benefits in a subset of these patients who present with moderate and/or more extensive disease.
Among patients with IGA ≥ 3, EASI ≥ 16, and affected BSA ≥ 10%, no patient assigned to vehicle achieved IGA-TS in the 8-week VC period, signaling the burden of disease while highlighting the efficacy of 1.5% ruxolitinib cream in this subgroup. Similar outcomes were observed when different definitions of disease severity were used to identify patients with moderate and/or extensive disease. In addition, these results align with those previously reported for 1.5% ruxolitinib cream under maximum-use conditions in adult and adolescent patients with mild to severe AD, and more extensive (range 25–90%) BSA involvement (week 8: IGA-TS, 56.8%; EASI75, 94.6%; NRS4, 90.5%) [19].
A similar percentage of patients in the higher severity of disease threshold subgroup achieved clear or almost clear skin at week 52 as in the overall TRuE-AD1/2 study population [16], thus suggesting that long-term maintenance of disease control may be independent of baseline disease severity.
The incidence and severity of TEAEs with continuous and as-needed application of ruxolitinib cream were similar in this higher severity of disease threshold subgroup compared to the overall TRuE-AD1/2 study population [15, 16, 19]. These safety results align with similar findings from the maximum-use study, which evaluated the pharmacokinetics, safety, and tolerability of 1.5% ruxolitinib cream in adult and adolescent patients in exaggerated conditions (e.g., application of 1.5% ruxolitinib cream to ≥ 25% BSA for an extended period of time) [19]. Over a period of 28 days, continuous-use 1.5% ruxolitinib cream BID showed mean plasma concentrations below the half-maximal inhibitory concentration for JAK-mediated myelosuppression and no evidence of accumulation of ruxolitinib in the plasma was observed [16]. Taken together, these data reinforce a lack of physiologically meaningful systemic JAK inhibition with ruxolitinib cream in patients with AD regardless of BSA involvement.
The management of AD is typically considered to be a stepwise approach, with topical treatment optimized before systemic treatments are considered [3, 4]. Topical treatment with corticosteroids and calcineurin inhibitors may be limited by tolerability issues, particularly with long-term use [5, 20], and this may hasten the escalation to systemic therapy. Treatment of AD must be individualized [3, 4]. In some cases, systemic therapy may be challenging, while in other cases systemic therapy may be preferred (e.g., in patients with such extensive disease that topical therapy is deemed impractical) or required (e.g., in patients with persistent disease after adequate trials of topical treatment) [3]. Ruxolitinib cream represents a safe and effective topical treatment option to consider for patients with mild to moderate disease, before escalation to systemic therapies.
Although approximately 90% of patients in the TRuE-AD1/2 studies and more than 95% in this post hoc analysis had received prior AD therapy (including topical corticosteroids, topical calcineurin inhibitors, and systemic agents), documented recent history of inadequate response to prior topical therapy was not a TRuE-AD1/2 inclusion criterion as is the case for studies of systemic therapy. Thus, comparisons to other trial populations can be challenging [18]. Further limitations of this post hoc subgroup analysis include the limited number of patients in some subgroups and the descriptive statistics. Finally, the results may not apply to patients of all disease severities.
Conclusion
Monotherapy with 1.5% ruxolitinib cream demonstrated similar efficacy, safety, and long-term disease control regardless of disease severity or extent in patients with mild to moderate AD enrolled in TRuE-AD1/2. The results of this subgroup analysis challenge thresholds of disease severity typical of contemporary systemic AD clinical trials as ruxolitinib cream presents an effective topical option for patients before escalation to systemic therapies.
Data Availability
Incyte Corporation (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g., US, EU, JPN). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
References
Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60.
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77(4):623–33.
Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–31.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–82.
Sidbury R, Alikhan A, Bercovitch L, et al. Executive summary: American Academy of Dermatology guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):128–9.
Guttman-Yassky E, Thaci D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–84.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–49.
Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156(4):411–20.
Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–12.
Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section II: tools for assessing the severity of atopic dermatitis. J Cutaneous Med Surg. 2018;22(suppl 1):10S–6S.
Vakharia PP, Chopra R, Sacotte R, et al. Severity strata for five patient-reported outcomes in adults with atopic dermatitis. Br J Dermatol. 2018;178(4):925–30.
Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–17.
Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from two phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–72.
Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88(5):1008–16.
Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–73.
Blauvelt A, Eichenfield LF, Kuligowski ME, et al. Efficacy of ruxolitinib cream among patients with atopic dermatitis based on previous medication history: pooled results from two phase 3 studies. Am Acad Dermatol Virt Meeting Exp. 2021;85:AB148.
Bissonnette R, Call RS, Raoof T, et al. A maximum-use trial of ruxolitinib cream in adolescents and adults with atopic dermatitis. Am J Clin Dermatol. 2022;23(3):355–64.
Muller SM, Tomaschett D, Euler S, Vogt DR, Herzog L, Itin P. Topical corticosteroid concerns in dermatological outpatients: a cross-sectional and interventional study. Dermatology. 2016;232(4):444–52.
Acknowledgements
The authors thank the patients and their families for their participation in the TRuE-AD1 and TRuE-AD2 studies.
Medical Writing/Editorial Assistance
Writing and editorial assistance were provided by Samantha Locke, PhD and Lee Hohaia, PharmD, employees of ICON (Blue Bell, PA, USA), and were funded by Incyte Corporation (Wilmington, DE, USA).
Funding
Sponsorship of this study and the journal’s Rapid Service Fee were funded by Incyte Corporation (Wilmington, DE, USA).
Author information
Authors and Affiliations
Contributions
Conceptualization: Eric L. Simpson, Howard Kallender, Daniel Sturm, and Lawrence F. Eichenfield; Methodology: Mingyue Wang, Howard Kallender, and Daniel Sturm; Formal analysis: Howard Kallender and Mingyue Wang; Investigation and interpretation of the data: all authors; Writing—review and editing: all authors; Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
Eric L. Simpson is an investigator for AbbVie, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, Merck, Pfizer, and Regeneron; and is a consultant with honorarium for AbbVie, Eli Lilly, Forte Bio, Galderma, Incyte, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, and Valeant. Leon Kircik has served as an investigator, consultant, or speaker for AbbVie, Amgen, Anaptys, Arcutis, Dermavant, Eli Lilly, Glenmark, Incyte, Kamedis, LEO Pharma, L’Oreal, Menlo Therapeutics, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and Taro. Andrew Blauvelt is a member and owner of Blauvelt Consulting and has served as a speaker (received honoraria) for AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Regeneron, and Sanofi, served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Merck, Novartis, Pfizer, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Vibliome, and Xencor, and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB Pharma. Howard Kallender and Daniel Sturm are employees and shareholders of Incyte. Mingyue Wang (currently at Boehringer Ingelheim) was an employee of Incyte at the time of the study and is a shareholder of Incyte. Lawrence F. Eichenfield has served as a consultant, speaker, advisory board member, or investigator for AbbVie, Amgen, Arcutis, Aslan, Bristol Myers Squibb, Castle Biosciences, Dermavant, Eli Lilly, Forte, Galderma, Incyte, Janssen, Johnson & Johnson, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi-Genzyme, Target RWE, and UCB.
Ethical Approval
These studies were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrollment. The protocols were approved by the relevant institutional review board or ethics committee at each study center (Supplementary Materials Tables S1 and S2).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Simpson, E.L., Kircik, L., Blauvelt, A. et al. Ruxolitinib Cream in Adolescents/Adults with Atopic Dermatitis Meeting Severity Thresholds for Systemic Therapy: Exploratory Analysis of Pooled Results from Two Phase 3 Studies. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01219-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01219-8