Abstract
The minimal adverse-effect profile and positive clinical response of low-dose oral minoxidil (LDOM) have recently caused the drug to gain popularity for the treatment of hair disorders in adults. However, in the pediatric population, hesitancy still surrounds the use of oral minoxidil given the wide profile of potential side effects the drug offers. This review aims to characterize the safety and use of oral minoxidil in children for the treatment of all disorders to equip physicians with ample knowledge when prescribing oral minoxidil in the pediatric population. A total of 41 studies (19 case reports, 10 cohort studies, 7 retrospective chart reviews, and 5 case series) that reported data on 442 pediatric patients for whom oral minoxidil was used for treatment were included. Conditions for which treatment with minoxidil was described were hair disorders (83.9%, 371/442) and hypertension (11.3%, 50/442); accidental usage (4.8%, 21/442) was also noted in the literature and included in this review. This review is broken down by dosage and describes the safety and efficacy of oral minoxidil in pediatric patients aged 0 to 18 years old for the treatment of hair disorders. This review found that LDOM may represent a safe option for the treatment of hair disorders in children. This study also suggests moderate and high doses of oral minoxidil may not be safe for use in children. Additional studies are needed to further understand this drug’s efficacy and safety in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oral minoxidil is utilized off-label for the treatment of hair disorders in adults, but hesitancy surrounds its use in children. |
This review aims to characterize the safety and use of oral minoxidil in children for the treatment of all disorders. |
This review contained 41 studies that reported data on 442 pediatric patients for whom oral minoxidil was used for treatment. |
This review found that low-dose oral minoxidil may represent a safe option for the treatment of hair disorders in children. |
This study suggests moderate and high doses of oral minoxidil may not be safe for use in children. |
Introduction
Minoxidil was initially developed in the 1970s as a potent peripheral vasodilator agent for the treatment of severe refractory hypertension. Minoxidil exerts its antihypertensive effect by opening adenosine triphosphate (ATP)-sensitive potassium channels, thereby reducing peripheral resistance and lowering blood pressure levels in patients. Given the unexpected side effect of hair growth in patients using minoxidil for hypertension, in 1987, a topical formulation of minoxidil was developed to treat androgenetic alopecia. Although topical minoxidil is an effective treatment option for hair loss, many patients are poorly compliant because of the necessity to apply the medication twice a day, undesirable hair texture, and scalp irritation. As a result of these adverse effects, oral minoxidil has more recently been explored for off-label use in hair disorders [1]. The minimal adverse-effect profile and positive clinical response of low-dose oral minoxidil (LDOM) have caused the drug to gain popularity for the treatment of hair disorders in adults [2]. However, in the pediatric population, hesitancy still surrounds the use of oral minoxidil given the wide profile of potential side effects the drug offers.
This review aims to characterize the safety and use of oral minoxidil in children for the treatment of all disorders to equip physicians with ample knowledge when prescribing oral minoxidil in the pediatric population.
Methods
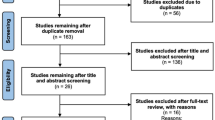
On March 22, 2024, a systematic search of PubMed, Embase, and Scopus was performed using the search terms “minoxidil” with “children”, “pediatric”, “adolescent”, “child”, “infant”, and “childhood” respectively, identifying 2163 articles. A total of 41 studies with 442 participants were included. Screening and review of articles were completed according to Preferred Reporting Items for Systematic and Meta-Analysis guidelines (Fig. 1). Two researchers (K.W. and C.O.) independently reviewed titles and abstracts to remove duplicate, review, nonhuman, and abstract-only articles, yielding 127 records that were accessed for eligibility. Only articles that described patients aged 0 to 18 years old and received oral or sublingual minoxidil were included. Full-text reviews were conducted on articles in which eligibility was uncertain, and discrepancies were resolved by discussion and mutual agreement between authors. Data extraction was performed independently by K.W. and C.O. through a full-text review of eligible articles. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Of the 127 articles, 41 studies (19 case reports, 10 cohort studies, 7 retrospective chart reviews, and 5 case series) that reported data on 442 pediatric patients for whom oral minoxidil was used for treatment were included. Conditions for which treatment with minoxidil was described were hair disorders (83.9%, 371/442) and hypertension (11.3%, 50/442); accidental usage (4.8%, 21/442) was also noted in the literature and included in this review.
Of the studies where gender was reported, the cohort consisted of 257 girls (60.3%) and 169 boys (39.7%). The population was divided by age group with infants (0–23 months), children (2–12 years), and adolescents (13–18 years) included in 7, 23, and 23 out of 41 studies, respectively.
Minoxidil dosage was separated by the mean dose of each study and studies were separated into their respective dose section when possible. Studies of minoxidil utilized for hypertension were reviewed for safety; however, evaluation of their efficacy will not be included in this review. A summary of the use of minoxidil broken down by dosage level is included in the “Discussion” section and Table 1.
Discussion
Low-Dose (≤ 2.5 mg)
We identified 17 studies (7 case reports, 7 retrospective chart reviews, 1 cohort study, and 2 case series) describing 373 patients where the mean dose of oral minoxidil utilized was 2.5 mg or less daily. For the purposes of this study this dose will be classified as LDOM. Of the 373 patients, oral minoxidil was utilized for hair in 99.5% of patients. The remaining two patients were treated for hypertension. The comorbidities in this group include two patients with eczema, one patient with Klinefelter syndrome, one patient with idiopathic short stature, and two patients with refractory hypertension secondary to underlying renal disease [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19].
Safety
Of the 373 patients, the duration of use spanned from 3 months to 5 years. The most reported adverse effect was hypertrichosis in 12.1% of patients. The severity of hypertrichosis documented progressed from involvement of only the legs, back, or face to involvement of all three [14, 16,17,18]. In John and Sinclair [18], a dose to severity relationship was noted between minoxidil and the hypertrichosis with patients taking larger doses of minoxidil experiencing an increase in unwanted hair growth. Interestingly, in Olamiju and Craiglow [13] no patients experienced hypertrichosis at a dose of 2.5 mg/day for 5 to 13 months. The authors hypothesized this to be due to the concomitant use of spironolactone, providing anti-androgen properties; this was a unique perspective as some consider minoxidil-induced hypertrichosis to be androgen independent [20].
Hypotension was the next most reported adverse effect for this group occurring in 5.6% of patients [16,17,18], followed by headaches (2.1%) [17, 18], elevated LFTs (1.9%) [18], nausea (1.6%) [18], palpitations/tachycardia (1.3%) [18], and anemia (1.1%) [18]. Other adverse side effects resulting in less than 1.0% of patients include change in hair color [11, 14], fluid retention [18], mood disturbance [18], menstrual irregularities [18], shedding [16], acne [18], hypercholesterolemia [18], abdominal pain [18], weight gain [18], and lethargy [18]. Of the 373 patients there were no reports of discontinuing minoxidil due to adverse events suggesting the side effects were mild.
Two studies explored the safety of LDOM in children through retrospective chart reviews [17, 18]. The largest prevalence of side effects (33.9%) was found in John and Sinclair [18], a study which retrospectively reviewed 192 patients aged 13 to 18 years old treated with LDOM or sublingual minoxidil for a minimum of 3 months. At a mean dose of 1.0 mg/day, 33.9% of patients experienced side effects. However, the symptoms were noted to be mild and, in all cases, did not lead to discontinuation of the drug. Of note, this study reported no difference in adverse event frequency between LDOM and sublingual minoxidil at equivalent dosages [18]. The second chart review was also conducted by John and Sinclair [17], however, in 63 children aged 0 to 12 years treated with LDOM or sublingual minoxidil for a minimum of 3 months. At a mean dose of 12.5 μg/kg and a maximum dose of 1.8 mg/day. Similar to the adolescents, LDOM and SLM were found to be well tolerated with facial/back hypertrichosis being the most reported adverse effect in 20.6% of patients followed by hypotension (6.3%), and headaches (3.2%). In all cases, symptoms were mild and did not lead to discontinuation of the drug.
Efficacy
Of the 373 patients, the hair disorders treated include loose anagen syndrome, androgenetic alopecia (AGA), alopecia areata, telogen effluvium, short anagen syndrome, trichotillomania, wooly hair hypotrichosis, diffuse congenital hereditary generalized hypotrichosis, kerion alopecia, premature graying, and hypotrichosis simplex–corneodesmosin (CDSN) deficiency [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. The majority of patients utilizing LDOM for hair noted improvement in hair density and a decrease in shedding [6,7,8,9,10,11,12,13,14,15,16, 19]. A minority of patients experienced only stabilization of their condition [15, 16], and one patient experienced a relapse [4].
We identified three studies (one cohort study and two case reports) utilizing low doses of oral minoxidil for the treatment of solely AGA [5, 10, 13]. In Olamiju and Craiglow [13] 2.5 mg/day of oral minoxidil was combined with varying doses of spironolactone in six girls aged 13 to 18 years old for 5 to 13 months. The result was objective improvement in five out of six patients. Two case reports described the use of oral minoxidil at doses of 0.6 mg and 2.5 mg daily in two patients with Klinefelter syndrome and idiopathic short stature, respectively, with success in both patients. Concomitant drugs utilized were finasteride and red light laser therapy respectively [5, 10].
We identified five studies (three retrospective chart reviews and two case reports) utilizing LDOM for the treatment of only alopecia areata [4, 8, 9, 15, 19]. One chart review analyzed the use of systemic minoxidil as maintenance monotherapy in the treatment of four adolescents with alopecia areata in remission. Two adolescents utilized oral minoxidil at a dose of 0.3 mg/day and two adolescents utilized sublingual minoxidil at a dose of 0.2 mg/day and 0.6 mg/day. The authors concluded low-dose systemic minoxidil appeared to be well tolerated and beneficial when used as long-term maintenance therapy following AA remission with three out of four patients not experiencing a relapse of the disease [4]. Two chart reviews explored the use of LDOM dosed at a mean of 0.7 mg/day and 0.5 mg/day in conjunction with baricitinib. While significant hair regrowth was noted in majority of these patients, the authors attributed this to the use of baricitinib rather than LDOM [15, 19]. Two case reports were also described in the literature treating alopecia areata and alopecia universalis utilizing LDOM in conjunction with simvastatin/ezetimibe and upadacitinib. Both patients noted improvement of their conditions with the 6-year-old girl and 14-year-old boy receiving a maximum dose of 1.0 mg/day for 5 years and 1.3 mg/day for 5 months, respectively [8, 9].
Two studies (one retrospective chart review and one case report) utilized LDOM for the treatment of only loose anagen syndrome in a total of eight patients. A mean dose of 0.2 mg/day was utilized in the chart review for a duration spanning 7 to 26 months and a dose of 0.5 mg/day was utilized in the case report. Eight out of nine patients noted an improvement in hair length and density, with all patients reporting decreased shedding. Of note, both studies reported darkening of hair color in a total of three patients. However, this can be a finding consistent with the improvement of loose anagen syndrome and less likely due to the LDOM [11, 14].
Three studies (two case reports and one case series) utilized LDOM in the treatment of hypotrichosis in a total of three patients. Doses of 0.3, 1.8, and 2.0 mg/day were used with improvement of hair density and thickness noted in all three cases [6, 7, 12].
Three chart reviews explored the effects of LDOM on a variety of hair conditions [16,17,18]. Only one of these studies reported outcomes of treatment in a total of 45 pediatric patients treated for AGA and telogen effluvium [16]. The mean dose prescribed was 1.2 mg/day spanning 3 to 24 months with a mean duration of 9.8 months. The outcomes in this group were clinical improvement in hair density for 80.0% of patients with stabilization of hair loss in the remaining 20.0%.
Moderate-Dose (2.6–15 mg)
We identified 14 studies (7 case reports, 5 cohort studies, and 2 case series) describing 42 patients utilizing an oral minoxidil dose of 3.0 to 15.0 mg daily [21,22,23,24,25,26,27,28,29,30,31,32,33,34]. For the purposes of this study, this dose will be classified as moderate-dose oral minoxidil (MDOM). Of the 42 patients, oral minoxidil was used for the treatment of hypertension in 22 (52.3%) [21,22,23,24,25,26,27,28,29,30, 32,33,34]. Twenty (47.7%) patients experienced a systemic accidental overdose when minoxidil was mistakenly replaced with omeprazole pills prior to dissemination [31]. All patients utilizing MDOM had comorbidities and required multiple therapies. This included diseases which are associated with secondary hypertension in children such as renovascular disease, pheochromocytoma, hemolytic uremic syndrome, transfusion, and aortic syndromes [21,22,23,24,25,26,27,28,29,30,31,32,33,34].
Safety
Of the patients utilizing MDOM, hirsutism and hypertrichosis affected the majority of patients (66.7%) [22, 23, 25, 32,33,34]. In Sanchez-Diaz et al. [31] the initial zone of appearance was the face (mostly forehead and temples) and the back and limbs. During evolution, hypertrichosis eventually developed in the facial area in all patients. Upon follow-up, all patients with hypertrichosis developed generalized hypertrichosis.
Pericardial effusion was the next most reported symptom in this group at 9.5% [23, 34]. The most severe patient was a 2-month-old girl with malignant renovascular hypertension who experienced minoxidil toxicity on a maximum dose of 5.9 mg/day of minoxidil. Antihypertensives were unable to improve her pressures and the introduction of minoxidil resulted in pericardial effusion with cardiac tamponade and left pleural effusion. The patient ultimately required endovascular correction of her underlying cause to correct the hypertension. The authors suggested prior to moderate dosing with minoxidil, vascular surgery should first be considered to prevent serious adverse events [23].
Reflex tachycardia and peripheral fluid retention occurred in three patients each (7.1%) [24, 34]. Abnormal kidney function while on minoxidil was noted during this review; 4.8% of patients experienced continued creatinine elevation despite improvement in blood pressure. However, this finding occurred in children with chronic hypertension and the role of minoxidil could not be distinguished from the effects of hypertensive nephropathy [25, 28].
Other reported side effects include congestive heart failure (4.7%) [34], rebound hypertension (4.7%) [21, 22], diarrhea (2.4%) [31], anxiety (2.4%) [31], headaches (2.4%) [31], severe asthenia (2.4%) [31], and facial edema (2.4%) [31]. Only one child experienced anorexia associated with the initiation of minoxidil. On a dose of 0.2 mg/kg, the 2-month-old infant’s oral intake decreased from 210.0 to 63.0 ml/kg/day and she failed to gain weight over 1 week, requiring a feeding tube. The minoxidil was utilized for 18 days [21]. Of note, there were two patients (4.7%) who did not experience any side effects [27, 29].
Interestingly, in an instance where oral minoxidil was mistakenly replaced with omeprazole pills prior to dissemination, the parents of 20 children were surveyed regarding systemic effects. The authors found there were no discernible variances in the daily dosage, total accumulated dosage, or adjusted daily dosage among patients with and without hypertrichosis with the average dose in this population being 13.2 mg/day. Additionally, neither sex nor age exhibited any significant correlation with hypertrichosis occurrence; this study was conducted in children aged 0 to 14 years. However, a noteworthy association was observed between treatment duration and the onset of hypertrichosis demonstrating patients with hypertrichosis had longer treatment times. Also interestingly, two patients noted a change in their hypertrichotic hair color, one experiencing lightening and the other darkening [31].
High-Dose (15.1–135 mg)
We identified 13 studies (6 cohort studies, 5 case reports, 2 case series) describing 27 patients utilizing a mean oral minoxidil dose of 15.1 to 135.0 mg daily [25, 32,33,34,35,36,37,38,39,40,41,42,43]. For the purposes of this study this dose will be classified as high-dose oral minoxidil (HDOM). Of the 27 patients, oral minoxidil was used for the treatment of hypertension in 26 (96.3%) [25, 32,33,34,35,36,37,38,39,40,41,42]. The remaining one patient experienced an accidental overdose [43]. All patients utilizing oral minoxidil for hypertension at the high-dose level had comorbidities including systemic lupus erythematosus, congestive heart failure, hypertensive encephalopathy, and a wide array of renal disease [25, 32,33,34,35,36,37,38,39,40,41,42,43].
Safety
Of the 26 hypertensive patients, the duration of use spanned 6 weeks to 24 months with the remaining overdose patient utilizing minoxidil for 1 day [25, 32,33,34,35,36,37,38,39,40,41,42]. In all HDOM patients, hirsutism and hypertrichosis were the most reported side effects, affecting 77.8% of patients (21/27) [25, 32, 34, 36,37,38, 42]. Compared to other groups, the patients treated with HDOM experienced the most severe forms of hypertrichosis. The most impressive case presented with hair growth over the forehead, eyebrows, eyelashes, face, and extremities after utilizing minoxidil at a dose of 40 mg/day for 9 months in a 10-year-old girl [25]. Within all HDOM studies, the hypertrichosis occurred at earliest within 2 weeks of beginning minoxidil therapy [36]. It is important to note that in each case where minoxidil therapy was discontinued, the hair growth remitted within 2 to 3 months [32, 36]. In one cohort study, Sinaiko and Mirkin [36] noted increasing intensity of hypertrichosis throughout the 6-week course of minoxidil therapy with no apparent relationship to the dosage administered.
Pericardial effusions are a rare side effect considered to be associated with minoxidil use [44]. In this review, 14.8% of patients utilizing HDOM experienced this adverse effect, with the specific doses in these patients ranging from 21.0 to 83.0 mg/day [34, 39,40,41]. Four cases were identified of pericardial effusions associated with minoxidil use in children. In every case, the patient had refractory hypertension secondary to an underlying disorder [34, 39,40,41]. Of the three patients with pericardial effusion, two also had signs of cardiac tamponade [39, 40]. In the most severe case, a 17-year-old boy was prescribed a maximum dose of 21.0 mg/day before experiencing a pericardial effusion which led to tamponade necessitating surgery. It is important to note that this patient was on dialysis and the authors suspect he had underlying uremic or lupus pericarditis. The patient worsened to eventually die of fulminant lupus [39].
Edema was the next most reported adverse effect for this group occurring in 11.1% of patients with the specific doses in these patients ranging from 20.0 to 50.0 mg/day. All cases of fluid retention reported in the literature occurred in patients suffering from secondary hypertension due to renal disease of varying degrees. While all cases managed the fluid retention with the addition of furosemide to the patient’s regimen, one study noted success in treatment of the edema with 120.0 mg/day of furosemide [25, 33, 38]. Another case discontinued use because of the severity of the edema and concurrent shortness of breath; this patient was on a dose of 50.0 mg/day for a duration of 24 months [33].
Other side effects noted in the literature include hypertensive encephalopathy (11.1%) [25]. Adverse effects occurring in only one patient each were minoxidil withdrawal with rebound hypertension [42], shortness of breath without a documented effusion [33], headaches [42], seizures [42], reflex tachycardia [43], and drug resistance [25].
Notably, one case report described a healthy 2-year-old boy who accidentally ingested 20 of his father’s 5.0 mg minoxidil tablets in 1 h totaling 100.0 mg of minoxidil. It was reported this patient experienced reflex tachycardia with a pulse of 160/min on presentation and 120/min 40 h later at discharge. The patient was normotensive on presentation and did not develop hypotension at any point throughout the hospitalization [43].
Conclusion
Developed in the 1970s, oral minoxidil has recently gained popularity for the treatment of adult hair disorders. While used off-label in the pediatric population for hair disorders, hesitancy still surrounds its use.
This review found that LDOM has promising results as an effective and safe option for treatment in the pediatric population for hair disorders including androgenetic alopecia, alopecia areata, loose anagen syndrome, telogen effluvium, and hypotrichosis. With the most common adverse effect being hypertrichosis in only 12.1% of patients and zero patients discontinuing use due to adverse effects, this review suggests oral minoxidil at a dose ranging from 0.0 to 2.5 mg/day should be considered safe for use in infants, adolescents, and children for the treatment of hair disorders. Given the low power of patients utilizing LDOM for the treatment of hypertension, more studies may be necessary to ascertain use in this condition.
At moderate doses of oral minoxidil the drug was primarily used for the treatment of hypertension. However, a large study also noted the accidental use of minoxidil in place of omeprazole. Patients taking MDOM were noted to have higher rates of hypertrichosis at 66.7% compared to 12.1% in the LDOM group. Notably, 9.5% of MDOM patients experienced pericardial effusions.
In this review, HDOM was found to have a similar adverse effect profile to MDOM but with slightly higher rates of hypertrichosis (77.8%). Interestingly, throughout this review as the dose of minoxidil increased, the prevalence of hypertrichosis and degree of hypertrichosis also increased suggesting a dose to severity relationship as previously postulated by John and Sinclair [18]. At high doses, oral minoxidil was associated with several severe cardiac effects including pericardial effusions noted in 14.8% of patients, a slightly higher prevalence than in MDOM. Fluid retention and tamponade were among other side effects noted in the HDOM group.
In conclusion, LDOM may represent a safe option for the treatment of hair disorders in children. While it is important to keep in mind the majority of patients in the MDOM and HDOM groups had severe underlying disease, this study suggests moderate and high doses of oral minoxidil may not be safe for use in children. Additional studies are needed to further understand this drug’s efficacy and safety in children.
References
Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls. Treasure Island (FL): StatPearls; 2024. http://www.ncbi.nlm.nih.gov/books/NBK482378/. Accessed 2024 Apr 8.
Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737–46.
Halling SE, Asling-Monemi K, Herthelius M, et al. Minoxidil therapy in children and young adult patients with renal disease and refractory hypertension: value when multidrug regimens have failed to achieve blood pressure control. J Hum Hypertens. 2010;24:552–4.
Moussa A, Bokhari L, Sinclair RD. Systemic minoxidil as maintenance treatment in alopecia areata: a retrospective case series of 24 patients. Clin Exp Dermatol. 2022;47:753–5.
Perper M, Herskovitz I, Tosti A. Aromatase inhibitor-induced hair loss in two adolescents. Pediatr Dermatol. 2020;37:1125–7.
López-Balboa P, Martos-Cabrera L, Ramírez-Lluch M, et al. Hypotrichosis simplex of the scalp and peeling skin disease, two sides of the same coin. J Eur Acad Dermatol Venereol. 2022;36:e789–90.
Willems A, Sinclair R. Diffuse congenital hypotrichosis simplex with associated hair shaft fragility. Australas J Dermatol. 2022;63:e96–7.
Bourkas AN, Sibbald C. Upadacitinib for the treatment of alopecia areata and severe atopic dermatitis in a paediatric patient: a case report. SAGE Open Med Case Re. 2022;10:2050313X221138452.
Ismail FF, Sinclair R. Alopecia universalis treated with simvastatin/ezetimibe, minoxidil, and prednisolone in a 6-year-old girl. Int J Dermatol. 2020;59:e103–5.
Alsalhi W, Tosti A. Androgenetic alopecia in a patient with Klinefelter syndrome: case report and literature review. Skin Appendage Disord. 2021;7:135–8.
Cranwell WC, Sinclair R. Loose anagen hair syndrome: treatment with systemic minoxidil characterised by marked hair colour change. Australas J Dermatol. 2018. https://doi.org/10.1111/ajd.12812.
Vastarella M, Martora F, Ocampo-Garza S, et al. Treatment of hereditary hypotrichosis simplex of the scalp with oral minoxidil and growth factors. Dermatol Ther. 2022. https://doi.org/10.1111/dth.15671.
Olamiju B, Craiglow BG. Combination oral minoxidil and spironolactone for the treatment of androgenetic alopecia in adolescent girls. J Am Acad Dermatol. 2021;84:1689–91.
Jerjen R, Koh W-L, Sinclair R, Bhoyrul B. Low-dose oral minoxidil improves global hair density and length in children with loose anagen hair syndrome. Br J Dermatol. 2021;184:977–8.
Asfour L, Bokhari L, Bhoyrul B, et al. Treatment of moderate-to-severe alopecia areata in pre-adolescent children with baricitinib. Br J Dermatol. 2023;189:248–50.
De Nicolas-Ruanes B, Moreno-Arrones OM, Saceda-Corralo D, et al. Low-dose oral minoxidil for treatment of androgenetic alopecia and telogen effluvium in a pediatric population: a descriptive study. J Am Acad Dermatol. 2022;87:700–2.
John JM, Sinclair RD. Systemic minoxidil for hair disorders in pediatric patients: a safety and tolerability review. Int J Dermatol. 2023;62:257–9.
John JM, Sinclair R. Safety and tolerability of low-dose oral minoxidil in adolescents: a retrospective review. J Am Acad Dermatol. 2023;88:502–4.
Moussa A, Eisman S, Kazmi A, et al. Treatment of moderate-to-severe alopecia areata in adolescents with baricitinib: a retrospective review of 29 patients. J Am Acad Dermatol. 2023;88:1194–6.
Comment on “Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: a retrospective review of 35 patients” - ClinicalKey. https://www-clinicalkey-com.access.library.miami.edu/#!/content/playContent/1-s2.0-S0190962222004364?returnurl=null&referrer=null. Accessed 2024 Apr 8.
Vesoulis ZA, Attarian SJ, Zeller B, Cole FS. Minoxidil-associated anorexia in an infant with refractory hypertension. Pharmacotherapy. 2014;34:e341-344.
Joekes AM, Thompson FD, O’Regan PF. Clinical use of minoxidil (Loniten). J R Soc Med. 1981;74:278–82.
Maroni A, Savary L, Deho A, et al. Malignant arterial hypertension in a 2-month-old girl: etiological diagnosis and treatment. Arch Pediatr. 2022;29:537–9.
Oka M, Mäkelä M. Minoxidil in severe hypertension. Acta Med Scand. 1978;203:43–7.
Makker SP. Minoxidil in refractory hypertension. J Pediatr. 1975;86:621–3.
Hack H. Use of the esophageal Doppler machine to help guide the intraoperative management of two children with pheochromocytoma. Paediatr Anaesth. 2006;16:867–76.
Felts JH, Charles J. Minoxidil in refractory hypertension. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S114–122.
Green TP, Nevins TE, Houser MT, Sibley R, Fish AJ, Sinaiko AR. Renal failure as a complication of acute antihypertensive therapy. Pediatrics. 1981;67:850–4.
Wood BC, Sharma JN, Crouch TT. Oral minoxidil in the treatment of hypertensive crisis. JAMA. 1979;241:163.
Miwa LJ, Shaefer MS, Stratta RJ, Wood RP, Langnas AM, Shaw BW. Drug-induced hypertrichosis: case report and review of the literature. DICP Ann Pharmacother. 1990;24:365–8.
Sánchez-Díaz M, López-Delgado D, Montero-Vílchez T, et al. Systemic minoxidil accidental exposure in a paediatric population: a case series study of cutaneous and systemic side effects. J Clin Med. 2021;10:4257.
Sinaiko AR, O’Dea RF, Mirkin BL. Clinical response of hypertensive children to long-term minoxidil therapy. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S181–188.
Colavita RD, Gaudio KM, Siegel NJ. Reversible reduction in renal function during treatment with captopril. Pediatrics. 1983;71:839–40.
Pennisi AJ, Takahashi M, Bernstein BH, et al. Minoxidil therapy in children with severe hypertension. J Pediatr. 1977;90:813–9.
Camel GH, Carmody SE, Perry HM. Use of minoxidil in the azotemic patient. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S173–180.
Sinaiko AR, Mirkin BL. Management of severe childhood hypertension with minoxidil: a controlled clinical study. J Pediatr. 1977;91:138–42.
Rosenthal T, Swartz J, Teicher A, Boichis H. Minoxidil in the treatment of refractory hypertension. Angiology. 1980;31:176–84.
Dumas R, Baldet P, Rolin B, Jean R. Hypertension and segmental renal hypoplasia causing a syndrome of haemolysis and uraemia. Arch Dis Child. 1981;56:403–4.
Bennett WM, Golper TA, Muther RS, McCarron DA. Efficacy of minoxidil in the treatment of severe hypertension in systemic disorders. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S142–148.
Bennett WM. Pericardial effusions associated with minoxidil. Lancet. 1977;2:1356.
Griswold W, McNeal R, O’Connor D, Reznik V, Mendoza S. Oral converting enzyme inhibitor in malignant hypertension. Arch Dis Child. 1982;57:235–7.
Makker SP, Moorthy B. Rebound hypertension following minoxidil withdrawal. J Pediatr. 1980;96:762–6.
Isles C, Mackay A, Barton PJ, Mitchell I. Accidental overdosage of minoxidil in a child. Lancet. 1981;1:97.
Lew MJ, Amato J. Minoxidil-associated pericardial effusion and impending tamponade. JAAPA. 2023;36:21–3.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kimberly Williams and Chrislene Olukoga. The first draft of the manuscript was written by Kimberly Williams, Chrislene Olukoga, and Dr. Antonella Tosti. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Antonella Tosti is a consultant for DS Laboratories, Monat Global, Almirall, Thirty Madison, Eli Lilly, Bristol Myers Squibb, P&G, Pfizer, and Myovant. Dr. Antonella Tosti is an Editorial Board member of Dermatology and Therapy. Dr. Antonella Tosti was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Kimberly Williams and Chrislene Olukoga have no competing interests.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, K.N., Olukoga, C.T.Y. & Tosti, A. Evaluation of the Safety and Effectiveness of Oral Minoxidil in Children: A Systematic Review. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01197-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01197-x