Abstract
Pemphigus vulgaris (PV) is a rare autoimmune bullous dermatosis (AIBD) characterized by painful blistering of the skin and mucosa caused by autoantibodies that lead to loss of adhesion in the epidermis. Standard therapy for PV is corticosteroids, either alone or in combination with steroid-sparing immunosuppressants or infusions with rituximab. According to the published European guideline, high-dose intravenous immunoglobulin (IVIg) therapy with a dosage of 2 g per kg body weight distributed over 2–5 days every 4 weeks is a promising treatment option, especially for severe or refractory disease. This report describes a 73-year-old female patient with severe and recurrent disease who achieved stabilization with IVIg treatment. However, the patient experienced side effects such as headaches, nausea, and vomiting, which affected daily life. Hence, she was transitioned to a new IVIg preparation with a new manufacturing process, resulting in fewer side effects and an improved quality of life. Further follow-up is necessary to fully evaluate the effectiveness and tolerability of this new IVIg product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intravenous immunoglobulin (IVIg) therapy is a promising treatment option for severe pemphigus vulgaris (PV), particularly in the case of failure on standard treatment with steroids and additional immunosuppressants. |
Overall tolerability of IVIg is very good and severe side effects are rare, but may occur as a result of aggregation, complement activation, and protein impurities. |
Our case presents a patient with successful disease control under long-term IVIg therapy. Her quality of life improved owing to a switch of IVIg preparation—a subject that has not yet been sufficiently addressed in the literature. |
The new 10% IVIg preparation with a novel production process is a highly purified and thus well-tolerated product. |
Introduction
Autoimmune bullous dermatoses (AIBDs) are a diverse group of autoimmune diseases presenting with bullae and erosions on the skin and mucous membranes. Pemphigus vulgaris (PV) is one of the most common and severe AIBDs. PV is characterized by autoantibodies directed against desmosomal adhesion proteins of the epidermis—the so-called desmogleins (Dsg)—resulting in loss of epidermal integrity and acantholysis. This mechanism causes clinically apparent blistering and erosions, which can affect all lined mucosal surfaces and the skin. Histopathology typically reveals intraepidermal clefting. Direct immunofluorescence usually shows IgG and C3c reticular in the epidermis. Dsg1 mAb and Dsg3 mAb can be detected serologically [1, 2]. Untreated, the disease is frequently fatal, particularly due to infections, sepsis, or malnutrition [3]. Standard treatment consisting of corticosteroids, either alone or in combination with steroid-sparing immunosuppressants or rituximab, may effectively control the disease. However, some patients do not respond sufficiently or experience serious adverse events and side effects associated with the long-term use of corticosteroids, making treatment of PV still challenging [4].
Another therapeutic option, considered in the case of severe/refractory PV or in patients with contraindications or intolerability against standard therapy, is the use of intravenous immunoglobulins (IVIg). IVIg consist of pooled human IgGs from numerous healthy blood and plasma donors. The preparations contain antibodies against microbial antigens, self-antigens, and anti-idiotypic antibodies that recognize other antibodies [5]. IVIg modulate the immune system, acting on both innate and adaptive immunity. Modes of action involved include neutralization of circulating autoantibodies, reduction of their half-life and inhibition of autoantibody production. IVIg also modulate cytokines as well as dendritic cell properties. In addition, inhibition of complement-mediated tissue destruction can be achieved. Moreover, IVIg functionally block Fc receptors on phagocytes, resulting in reduced cellular destruction. By binding to the death receptor Fas (CD95) cell death can be influenced. Expanded T cell repertoire diversity and increased steroid sensitivity are among the effects being discussed [6, 7]. In summary, effective blockade of the dysregulated immune response is supposed to lead to long-term sustained, clinical, serological, and immunopathological remission. New data, presenting a 20-year follow-up, indicate that IVIg is effective in restoring immune regulation in patients with PV [8].
The half-life of IVIg in the blood is similar to that of endogenous immunoglobulin and therefore a monthly therapy with a standard dosage of 2 g per kg body weight over 2–5 days is recommended [6].
There are numerous different IVIg preparations which meet the high standards of safety and quality prescribed by the regulatory authorities [9]. Production commences with plasma collection following a sophisticated safety net that includes measures such as donor screening, blood tests, donor referral, plasma quarantine, and other investigations [9]. The production process itself is very complex and comprises techniques that ensure the maintenance of the structural and functional integrity of IgG antibodies with a purity of at least 95% and also includes highly effective and validated virus reduction steps. While efficacy of the preparations is almost equivalent, side effect profiles are slightly different; literature on this subject is lacking.
Here, we report on a patient with well-controlled PV under long-term IVIg therapy, who underwent a change of IVIg preparation as a result of headache and nausea.
Case Report/Case Presentation
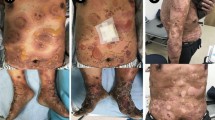
We present the case of a 73-year-old female patient, who first developed painful lesions of the oral mucosa in January 2012. After unsuccessful treatment with antiviral and antibiotic substances, the diagnosis of PV was confirmed by histology and immunosuppressive treatment with azathioprine 100 mg/day and prednisolone was initiated. As a result of significant worsening of disease activity under this treatment with lesions developing on pharynx and larynx accompanied by hoarseness and severe pain, the patient was admitted to our dermatology department in July 2012 (Fig. 1). Once the diagnosis was confirmed by histology and serology, high-dose immunosuppressive therapy with prednisolone 150 mg/day and mycophenolate mofetil 3 g/day was initiated. As a result of insufficient treatment response, dapsone 100 mg/day was added. Symptoms of epiglottitis and dyspnea associated with swelling of the airways rapidly improved under the aforementioned regimen and the immunosuppressive therapy could be gradually reduced. In March 2016, while maintaining dapsone 100 mg/day and prednisolone 5 mg/day, a fulminant relapse with multiple erosions and painful aphthae occurred, leading to a dramatic worsening of the general condition with difficulty swallowing, shortness of breath, and complete loss of voice as well as the inability to eat. As a result of a delayed response to high-dose immunosuppressive therapy and severity of the relapse, intravenous immunoglobulin (IVIg, Intratect® 100 g/l Biotest AG, 2 g per kg body weight distributed over 3 days every 4 weeks) therapy was started in July 2016. As a result of cardiac disease in the medical history, administration over 3 days was initiated. This ultimately led to a sustained treatment response, so that concomitant immunosuppression could carefully and slowly be reduced. Treatment with IVIg was interrupted from October to December 2020 because of personal reasons, whereupon another fulminant relapse occurred in January 2021, which again necessitated high-dose immunosuppressive therapy with prednisolone 150 mg/day and mycophenolate mofetil 3 g/day. Therapy with rituximab was not preferred at that time because of the ongoing Covid-19 virus pandemic and severe lung disease reported in the context of immunosuppression with rituximab [10, 11]. Additionally, disease had been controlled during IVIg therapy the years before and the relapse had occurred in the context of IVIg therapy being paused. Further on, IVIg therapy (Intratect® 100 g/l Biotest AG, 2 g per kg body weight distributed over 3 days every 4 weeks) was reinitiated and a long-lasting stabilization of skin lesions achieved. The concomitant immunosuppression was gradually reduced and will be completely discontinued in the future. However, the patient reported experiencing headaches and severe nausea following IVIg administration, which are common side effects of the therapy. Despite premedication with intravenously administered Tavegil 1 mg and orally administered paracetamol 1 g, a well as a reduced infusion rate of 200 ml/h, these symptoms persisted. Hence, in September 2023, therapy was switched to a new IVIg preparation (Yimmugo® 100 g/l Biotest AG, 2 g per kg body weight distributed over 3 days every 4 weeks). The patient continued to report headaches after infusions, but an improvement in nausea was observed. Episodes of vomiting that had previously accompanied nausea have no longer occurred.
Discussion
Nowadays, IVIg are used for the treatment of a wide range of diseases, and numerous clinical studies have shown their efficacy and tolerability. Indications include primary and secondary immunodeficiencies, neuromuscular diseases, primary immune thrombocytopenia, and Kawasaki disease [12]. In dermatology IVIg are used to treat autoimmune diseases such as pemphigus vulgaris and foliaceus, bullous pemphigoid, mucous membrane pemphigoid, epidermolysis bullosa acquisita, dermatomyositis, ANCA-associated vasculitis, and systemic lupus erythematosus [6]. For treatment of PV, IVIg may be used as a secondary or tertiary therapeutical option in patients with particularly severe and treatment-refractory disease, as the patient presented above. Besides numerous positive case reports and case series, efficacy of IVIg for PV has also been shown in a randomized controlled trial, providing a high level of evidence [13].
Although overall tolerability is very good, adverse effects do occur. Severe side effects such as aseptic meningitis, renal insufficiency, thrombosis, and hemolytic anemia are rare. Mild symptoms like headaches and nausea which had also been reported by our patient as well as fever, tiredness, and lethargy are more common [14]. With reports ranging from 2.5% to 87.5%, the incidence of adverse reactions associated with IVIg varies widely between studies and side effects are only rarely discussed in the literature [15]. Strategies for managing side effects include reducing the infusion rate or premedication with antihistamines or corticosteroids. In most cases, this only reduces side effects, without eliminating them altogether. However, since IVIg therapy is used in chronic diseases and is administered at regular intervals over a long period of time, tolerability and management of side effects are important factors with regard to the patient’s quality of life.
Side effects are associated with specific immunoglobulin preparations, yet do vary between individuals. Preparations contain variable amounts of other ingredients and excipients such as IgA, IgM, sugars, salts, traces of solvents, detergents and buffers, as well as different stabilizing agents, such as sucrose, glucose, maltose, d-sorbitol, l-proline, or glycine. Therefore, pre-existing medical conditions should be considered, e.g., sugar-free preparations are recommended for patients with renal insufficiency [16, 17].
IVIg preparation finally used in our patient is a new sugar-free 10% IVIg preparation with a novel manufacturing process including innovative features. As a result of the use of gentle vibromixing, the shear stress on the proteins can be reduced, resulting in extremely low aggregate levels for this preparation. Additionally, the gamma globulin properdin, which is an important complement activator, was largely eliminated by an additional chromatography step, resulting in reduced levels of anticomplementary activity. Overall, the preparation is highly purified [18]. For our patient, changing the preparation resulted in an improvement in her quality of life. The new preparation was equally effective but better tolerated, with no more vomiting after infusions and fewer limitations in daily life. In a clinical trial in patients with primary ITP, administration was also associated with a low rate of headaches, nausea, and vomiting in comparison with other studies that followed the revised European Medicines Agency (EMA) guidelines [19]. Since side effects of IVIg are patient-specific, further evidence from real-life experience with this new IVIg preparation is needed to fully evaluate the effectiveness and tolerability and to make favorable long-term management decisions.
Conclusion
High-dose intravenous immunoglobulins represent an excellent treatment option for severe pemphigus vulgaris failing standard therapy. A long treatment period of the chronic disease is to be expected, which is why side effects and tolerability of the therapy are quite relevant for patients. Particularly for patients who benefit from IVIg therapy but continue to suffer from infusion-related side effects, a change of preparation may be appropriate. The new high-quality immunoglobulin preparation has a good side effect profile and is therefore a promising treatment option.
Data Availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16(9):1077–91.
Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
Murrell DF, Peña S, Joly P, et al. Diagnosis and management of pemphigus: recommendations of an international panel of experts. J Am Acad Dermatol. 2020;82(3):575–85.e1.
Hsu DY, Brieva J, Sinha AA, Langan SM, Silverberg JI. Comorbidities and inpatient mortality for pemphigus in the U.S.A. Br J Dermatol. 2016;174(6):1290–8.
Durandy A, Kaveri SV, Kuijpers TW, et al. Intravenous immunoglobulins–understanding properties and mechanisms. Clin Exp Immunol. 2009;158 Suppl 1(Suppl 1):2–13.
Hoffmann JHO, Enk AH. High-dose intravenous immunoglobulin in skin autoimmune disease. Front Immunol. 2019;10:1090.
BarahonaAfonso AF, João CM. The production processes and biological effects of intravenous immunoglobulin. Biomolecules. 2016;6(1):15.
Ahmed AR, Kalesinskas M, Kaveri SV. Restoring immune tolerance in pemphigus vulgaris. Proc Natl Acad Sci U S A. 2024;121(5):e2317762121.
Brinkman N, McCann K, Gooch B. The purification of plasma proteins for therapeutic use. In: Rossi’s principles of transfusion medicine. Wiley; 2022. p. 216–35.
Grabbe S, Beissert S, Enk A. Systemic immunosuppression in times of COVID-19: do we need to rethink our standards? J Dtsch Dermatol Ges. 2020;18(8):810–3.
Elmas ÖF, Demirbaş A, Türsen Ü, Atasoy M, Lotti T. Pemphigus and COVID-19: critical overview of management with a focus on treatment choice. Dermatol Ther. 2020;33(6): e14265.
European Medicines Agency. Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) - Rev. 6 (2021). Reference Number: EMA/CHMP/BPWP/94038/2007 Rev. 6 Corr. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-core-smpc-human-normal-immunoglobulin-intravenous-administration-ivig-rev-6_en.pdf. Accessed 19 April 2024.
Amagai M, Ikeda S, Shimizu H, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60(4):595–603.
Cherin P, Marie I, Michallet M, et al. Management of adverse events in the treatment of patients with immunoglobulin therapy: a review of evidence. Autoimmun Rev. 2016;15(1):71–81.
Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299.
Prins C, Gelfand EW, French LE. Intravenous immunoglobulin: properties, mode of action and practical use in dermatology. Acta Derm Venereol. 2007;87(3):206–18.
Chérin P, Cabane J. Relevant criteria for selecting an intravenous immunoglobulin preparation for clinical use. BioDrugs. 2010;24(4):211–23.
Duellberg C, Hannappel A, Kistner S, Maneg O. Biochemical characterization of a new 10% IVIG preparation [IgG next generation (BT595)/Yimmugo®] obtained from a manufacturing process preserving IgA/IgM potential of human plasma. Drugs R D. 2023;23(3):245–55.
Demeter J, Hamed A, László S, et al. Efficacy and safety of BT595 (10% human intravenous immunoglobulin) in adult patients with chronic immune thrombocytopenia. Transfus Med. 2023;33(2):165–73.
Acknowledgements
We thank the patient reported on for the permission to publish this case report.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
All authors had full access to patient data and take responsibility for the integrity and accuracy of the manuscript. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Alexander H. Enk received advisory board honoraria, consultancy fees and support for this publication from Biotest AG. Julia K. Winkler received travel expanse and honoraria from Biotest AG. Nadine Wiedenmayer and Anastasia S. Vollmer declare to have no competing interests.
Ethical Approval
Ethical approval was not required for this case report, in accordance with local and national guidelines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wiedenmayer, N., Vollmer, A.S., Winkler, J.K. et al. Successful Treatment of Severe Pemphigus Vulgaris with Reduced Side Effects Using a Novel IVIg Preparation. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01191-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01191-3