Abstract
Introduction
Early prediction of abrocitinib efficacy in atopic dermatitis (AD) could help identify candidates for an early dose increase. A predictive model determined week 12 efficacy based on week 4 responses in patients receiving abrocitinib 100 mg/day and assessed the effect of an abrocitinib dose increase on platelet counts.
Methods
Analysis included the phase 3 trials JADE MONO-1 (NCT03349060), MONO-2 (NCT03575871), COMPARE (NCT03720470), and TEEN (NCT03796676). For platelet counts and simulations, a phase 2 psoriasis trial (NCT02201524) and phase 2b (NCT02780167) and phase 3 (MONO-1, MONO-2, and REGIMEN (NCT03627767)) abrocitinib trials were pooled. A training-and-validation framework assessed potential predictors of response at week 4: score and score change from baseline in the Eczema Area and Severity Index (EASI), Investigator's Global Assessment (IGA), and Peak Pruritus Numerical Rating Scale (PP-NRS), and percentage change from baseline in EASI. The dependent variables at week 12 were ≥ 75% improvement in EASI (EASI-75) and IGA score of 0 (clear) or 1 (almost clear) and ≥ 2-point improvement from baseline. The probability of each variable to predict week 12 EASI-75 and IGA responses was calculated.
Results
In the training cohort (n = 453), 72% of the ≥ 50% improvement in EASI (EASI-50) at week 4 responders and 16% of the nonresponders with abrocitinib 100 mg achieved EASI-75 at week 12; 48% and 6% of the week 4 EASI-50 responders and nonresponders, respectively, achieved week 12 IGA response. Similar results occurred with week 4 IGA = 2, ≥ 4-point improvement from baseline in PP-NRS, or EASI = 8 responders/nonresponders. Platelet counts after an abrocitinib dose increase from 100 to 200 mg were similar to those seen with continuous dosing with abrocitinib 100 mg or 200 mg.
Conclusion
Achieving week 4 clinical responses with abrocitinib 100 mg may be useful in predicting week 12 responses. Week 4 nonresponders may benefit from a dose increase to abrocitinib 200 mg, and those that receive this dose increase are likely to achieve treatment success at week 12, with no significant impact on platelet count recovery.
Video abstract available for this article.
Clinical Trial Registration
NCT03349060, NCT03575871, NCT03720470, NCT03796676, NCT02201524, NCT02780167 and NCT03627767.
Plain Language Summary
Abrocitinib is an approved treatment for people with moderate or severe atopic dermatitis. Abrocitinib tablets are available in two doses (100 and 200 mg) and are taken by mouth once daily. Some people with atopic dermatitis who are taking abrocitinib 100 mg may need to increase the dose to 200 mg to get adequate symptom relief. We studied whether people with atopic dermatitis who did or did not experience clear skin or itch relief after taking abrocitinib 100 mg for 4 weeks are likely or not likely to experience relief after 12 weeks of treatment. We also defined the level of response after 4 weeks of treatment that best differentiates people who did or did not experience symptom relief, and we identified who might benefit from increasing the abrocitinib dose from 100 to 200 mg. We found that people with atopic dermatitis who had symptom relief after 4 weeks of abrocitinib 100 mg treatment were much more likely to have greater relief after 12 weeks, and people who did not achieve symptom relief after 4 weeks may benefit from a dose increase at week 4. Some people who receive abrocitinib 200 mg may have a temporary decrease in the number of certain blood cells called platelets at week 4, but platelets return to near-normal levels by week 12. This analysis showed that increasing the abrocitinib dose from 100 to 200 mg at week 4 did not seem to affect the platelet numbers after week 4.
Video abstract (MP4 174529 KB)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Abrocitinib is an oral, once-daily, Janus kinase 1-selective inhibitor approved for the treatment of adults and adolescents with moderate-to-severe atopic dermatitis (AD) and is available in doses of 100 mg and 200 mg. |
In randomized clinical trials with abrocitinib, most patients who achieved clinically meaningful improvements in skin lesions and AD severity at week 12 after treatment with abrocitinib 100 mg had already achieved these improvements by week 4, an early time point in treatment. |
Identifying clinical responses at early time points that can reliably predict treatment success in the long term will enable the optimization of AD management, including decisions on continuing, stopping, or modifying therapy. |
What was learned from the study? |
Achieving clinical response as measured by ≥ 50% improvement in the Eczema Area and Severity Index (EASI-50) after 4 weeks of treatment with abrocitinib 100 mg may be useful in predicting week 12 response. |
Other suitable alternative predictors of week 12 response are an Investigator’s Global Assessment score of 2, a ≥ 4-point improvement from baseline on the Peak Pruritus Numerical Rating Scale, or an EASI score of 8 after 4 weeks of treatment with abrocitinib 100 mg. |
Patients who do not reach these responses at week 4 may benefit from a dose increase to abrocitinib 200 mg to improve the likelihood of treatment success at week 12 without incurring negative effects on platelet counts. |
Digital Features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25772895.
Introduction
Abrocitinib is an oral, once-daily, Janus kinase (JAK) 1-selective inhibitor approved for the treatment of adults [1,2,3,4,5] and adolescents [1,2,3] with moderate-to-severe atopic dermatitis (AD) and is available in doses of 100 mg and 200 mg. Dose selection at treatment initiation is dependent on several variables, including local prescribing and reimbursement criteria as well as shared decision-making between the patient and their physician. Depending on the local prescribing information, abrocitinib may be started at 100 mg orally once daily, and if an adequate response is not achieved after 12 weeks, patients may be eligible to receive 200 mg orally once daily [5].
Clinical trial data show that 24–42% of patients receiving abrocitinib 100 mg/day achieved Investigator’s Global Assessment (IGA) response, defined as an IGA score of 0 (clear) or 1 (almost clear) (IGA 0/1) and a ≥ 2-point improvement from baseline, and 40–68% achieved a ≥ 75% improvement from baseline in the Eczema Area and Severity Index (EASI-75) at week 12; approximately 50–75% of these patients had already achieved IGA 0/1 and EASI-75 responses by week 4 [6,7,8,9]. Being able to predict a patient’s response at week 12 based on their week 4 assessments could optimize the management of AD and inform the most favorable dosing strategy for individual patients. If clinically appropriate, patients who are anticipated not to achieve an adequate response at week 12 may benefit from an earlier dose increase to improve patient outcomes and prevent suboptimal dosing over a prolonged period. Abrocitinib is known to be associated with a dose-dependent increased risk of thrombocytopenia, lymphopenia, and hyperlipidemia in clinical trials [5,6,7, 9, 10]. Per local prescribing information, patients should be assessed for a complete blood count and lipid profile 4 weeks after treatment initiation [1,2,3,4,5].
Here we developed an approach to identify patients who would benefit from an abrocitinib dose increase from 100 mg/day to 200 mg/day after 4 weeks of treatment instead of waiting until week 12 to make that decision. We also studied whether an earlier dose increase would have a negative impact on platelet count in patients receiving treatment with abrocitinib for moderate-to-severe AD.
Methods
Study Design and Patients
Efficacy data were pooled from four phase 3 trials in which patients with moderate-to-severe AD received abrocitinib once daily as monotherapy (JADE MONO-1 (NCT03349060) [6]; JADE MONO-2 (NCT03575871) [7]) or in combination with background topical therapy (JADE COMPARE (NCT03720470) [8]; JADE TEEN (NCT03796676) [9]). Only patients initially randomly assigned to abrocitinib 100 mg were included in the analysis of predictor variables. Patients were aged ≥ 18 years (JADE COMPARE) [8], ≥ 12 years (JADE MONO-1 [6] and JADE MONO-2 [7]), or 12 to < 18 years (JADE TEEN [9]). Study design details for these trials were reported previously [6,7,8,9].
The observed platelet count and simulations used pooled efficacy, safety, dosing, and pharmacokinetic data from patients receiving JAK1 inhibitors in a phase 2 trial for psoriasis (NCT02201524) [11] and in phase 2b (NCT02780167) [12] and phase 3 (JADE MONO-1 [6], JADE MONO-2 [7], and JADE REGIMEN (NCT03627767) [13]) trials for AD. The phase 2 trial for psoriasis [11] was not included in the assessment of the relationship between percentage change in EASI and absolute platelet count at week 4. Patients who received abrocitinib 100 mg and abrocitinib 200 mg were included in the platelet count and simulation analyses.
All study documents and procedures were approved by the appropriate institutional review boards/ethics committees at each study site. The studies were conducted in compliance with the ethical principles from the Declaration of Helsinki and all International Council for Harmonisation Good Clinical Practice Guidelines. All local regulatory requirements were followed. Informed consent was obtained from all individual participants included in the study.
Data Analysis
Predictors of efficacy were assessed using a training-and-validation framework. Using a logistic regression model, training was conducted to identify predictors of response in a randomly selected cohort stratified by study ID, which comprised 70% of the pooled population (i.e., the training cohort). In the validation step, the identified predictors were tested using the remaining 30% of patient-level data (i.e., the validation cohort).
The full analysis set was defined as all randomized individuals who received at least one dose of study medication. Baseline was defined as the last measurement before the first dosing (day 1). The dependent variables at week 12 were EASI-75 and IGA response. Predictor variables included week 4 scores for the EASI, IGA, and Peak Pruritus Numerical Rating Scale (PP-NRS; used with permission from Regeneron Pharmaceuticals, Inc., and Sanofi); changes from baseline to week 4 in EASI, IGA, and PP-NRS; and percentage change from baseline to week 4 in EASI.
For each value of the predictor variable, the probability to predict EASI-75 and IGA responses at week 12 was evaluated in the context of sensitivity, defined as the proportion of patients with an early response among those who had a response at week 12, i.e., \(\left( {\frac{{{\text{correctly predicted week 12 }}{\text{responder}}}}{{{\text{all week 12 responders}}}}} \right)\)\(.\) Specificity was defined as the proportion of patients without an early response among those who had no response at week 12, i.e., \(\left( {\frac{{{\text{correctly predicted week 12}}\,{\text{nonresponder}}}}{{{\text{all week 12 nonresponders}}}}} \right)\). Receiver operating characteristic (ROC) curves were plotted as sensitivity versus 1 − specificity; ROC areas under the curve (AUCs) were used to assess the predictive value of each variable in the training cohort (see the Supplementary Information). The Youden index, a common summary measure of the ROC curve, was calculated as (sensitivity + specificity – 1). Week 4 predictor cutoff values were defined as those that corresponded to the maximum Youden index. Only patients with observed week 4 predictor values were included in this analysis. The negative predictive value (NPV), \(\frac{{{\text{correct prediction}} \left( {\text{i.e., true negative}} \right)}}{{{\text{correct prediction}} \left( {\text{i.e., true negative}} \right) + {\text{wrong prediction}} \left( {\text{i.e., false negative}} \right)}},\) was calculated for the following Week 4 or Week 8 predictor variables: ≥ 50% improvement from baseline in EASI [EASI-50], EASI-75, ≥ 2-point improvement from baseline in PP-NRS [PP-NRS2], or ≥ 1-point improvement from baseline in IGA to predict Week 12 dependent variables (EASI-75, PP-NRS4, or IGA response) in patients pooled from the JADE MONO-1, JADE MONO-2, and JADE COMPARE studies only (Supplementary information). Responders were defined as those who achieved EASI-75 or IGA response at week 12, and nonresponders were defined as those who did not achieve these endpoints at week 12 [6,7,8,9]. Patients who withdrew from the study prior to week 12 were also considered nonresponders.
Week 4 absolute platelet counts were assessed against EASI percentage change from baseline at week 4 and analyzed using linear regression. Platelet simulations were performed using the same population pharmacokinetic-pharmacodynamic modeling reported in a recent analysis [14]. Simulations were generated from week 2 through week 12 based on patients with baseline platelet counts of 170/nL or 270/nL treated with continuous dosage of abrocitinib 200 mg/day or 100 mg/day or based on those who received a dosage increase from 100 mg/day to 200 mg/day between week 2 and week 8.
Results
Patient Demographics and Baseline Disease Characteristics
A total of 647 patients were included in this post hoc analysis. Of these, 453 patients were part of the training cohort and 194 comprised the validation cohort. Baseline demographics and clinical characteristics were comparable across the training and validation cohorts (Table 1).
Values of Week 4 Predictor Variables
In the training cohort, ROC AUCs for logistic regression of EASI-75 and IGA responses at week 12 suggested a reasonable discriminative power of EASI, IGA, and PP-NRS scores at week 4, EASI, IGA, and PP-NRS score changes from baseline to week 4, and percentage change in EASI from baseline to week 4 (Supplementary Information).
EASI-75 Response at Week 12: Performance of Predictor Variables
Analyses of EASI-75 response at week 12 as a function of week 4 score (EASI, IGA, PP-NRS), score change from baseline to week 4 (EASI, IGA, PP-NRS), and percentage change from baseline to week 4 (EASI) in the training cohort suggested that a 63% improvement in EASI at week 4 would be a good predictor of week 12 response with abrocitinib 100 mg/day, based on a reasonably high sensitivity (0.77), specificity (0.78), and an acceptable Youden index (0.56) (Table 2).
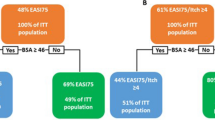
Based on the identification of a 63% improvement in EASI at week 4 as a good predictor of achieving EASI-75 at week 12, we assessed performance of EASI-50, a widely accepted measure of clinical improvement (sensitivity, 0.90; specificity, 0.60; Youden index, 0.51). In the training cohort, 72% of patients who were EASI-50 responders at week 4 achieved EASI-75 response at week 12, compared with 16% in the EASI-50 nonresponder subgroup; in the validation cohort, those values were 69% and 23%, respectively (Fig. 1). Among patients who were considered responders at week 4 in the training cohort based on IGA = 2, a ≥ 4-point improvement from baseline in PP-NRS (PP-NRS4), or EASI = 8, 73%, 73%, and 83%, respectively, attained EASI-75 response at week 12; those values were 29%, 46%, and 31% for IGA = 2, PP-NRS4, and EASI = 8 nonresponders at week 4. Similar results were seen for these subgroups in the validation cohort (Fig. 1).
Proportions of week 4 responders and nonresponders based on EASI-50, IGA = 2, PP-NRS4, or EASI = 8 who achieved EASI-75 response at week 12. EASI Eczema Area and Severity Index, EASI-50 ≥ 50% improvement from baseline in EASI, EASI-75 ≥ 75% improvement from baseline in EASI, IGA Investigator’s Global Assessment, PP-NRS4 ≥ 4-point improvement from baseline on the Peak Pruritus Numerical Rating Scale, CI confidence interval
IGA Response at Week 12: Performance of Predictor Variables
In the training cohort, week 4 EASI = 8 and a 58% improvement from baseline in EASI were considered the best predictors of week 12 IGA response, based on their Youden index values (Table 3). Attainment of EASI-50 at week 4 would be an inferior predictor of IGA response at week 12 (sensitivity, 0.94; specificity, 0.48; Youden index, 0.42) compared with the prediction of EASI-75.
In the training cohort, 48% of the EASI-50 responders at week 4 achieved IGA response at week 12, compared with 6% of EASI-50 nonresponders; in the validation cohort, those values were 41% and 12%, respectively (Fig. 2). Among patients who were considered responders at week 4 in the training cohort based on IGA = 2, PP-NRS4, or EASI = 8, 53%, 50%, and 60%, respectively, achieved IGA response at week 12; those values were 10%, 26%, and 14% for IGA = 2, PP-NRS4, and EASI = 8 nonresponders at week 4. Similar results were seen for these subgroups in the validation cohort (Fig. 2).
Proportions of week 4 responders and nonresponders based on EASI-50, IGA = 2, PP-NRS4, or EASI = 8 who achieved IGA responsea at week 12. aIGA response was defined as a score of 0 (clear) or 1 (almost clear) and a ≥ 2-point improvement from baseline. EASI Eczema Area and Severity Index, EASI-50 ≥ 50% improvement in EASI, IGA Investigator’s Global Assessment, PP-NRS4 ≥ 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale, CI confidence interval
Negative Predictive Values for IGA, EASI, and PP-NRS Responses at Week 12
Using efficacy measures at week 4, not achieving EASI-50, EASI-75, and a ≥ 1-point improvement from baseline in IGA at week 4 were reliable predictors of patients not achieving IGA response at week 12 (NPV: EASI-50, 0.93; EASI-75, 0.84; ≥ 1-point improvement from baseline in IGA, 0.90) (Supplementary Information). Similarly, not achieving EASI-50 or PP-NRS2 responses at week 4 were reliable predictors that patients would not achieve EASI-75 or PP-NRS4, respectively, at week 12 (NPV: EASI-50, 0.86; PP-NRS2, 0.86). High NPVs were also observed for the outcomes EASI-50, EASI-75, a ≥ 1-point improvement from baseline in IGA, and PP-NRS2 at week 8 as predictors of week 12 EASI, IGA, or PP-NRS response.
Platelet Counts and Platelet Simulations
Around week 4, a transient nadir occurred with abrocitinib 200 mg/day and 100 mg/day in the observed platelet count in a dose-dependent manner. After that timepoint, values returned towards baseline levels (Fig. 3). There was a weak association between absolute platelet count and EASI response at week 4 (Supplementary Information). Simulation data of patients with baseline platelet counts of 170/nL and 270/nL suggest that the platelet count dynamics with an abrocitinib dosage increase from 100 mg/day to 200 mg/day would be similar to the dynamics of continuous dosing with abrocitinib 100 mg or abrocitinib 200 mg. According to these simulations, nadir in platelet count could be expected about 4 weeks after the initiation of abrocitinib treatment, followed by a period of recovery (Fig. 4). A higher platelet count at baseline would increase the probability of keeping the platelet count values above 150/nL (Fig. 4).
Observed platelet-count time course stratified by treatment group. Blue circles represent the observed platelet counts. A solid black line indicates the loess smooth of the platelet time course. Horizontal lines represent platelet counts of 150/nL (dashed black), 100/nL (dashed red), 50/nL (dotted red), and 25/nL (solid red). QD, once daily
Typical platelet-count time coursesa with different dosage regimens stratified by baseline platelet count. The typical patient was White, male, 30 years old, and 70 kg. For the scenarios with a dosage increase, the patient was assumed to have started on abrocitinib 100 mg/day. aThe typical platelet-count time course did not include between-patient variability or residual variability
Discussion
Our findings, based on a predictor analysis conducted using common statistical techniques, suggest that patients with moderate-to-severe AD who start abrocitinib treatment with 100 mg/day are more likely to achieve EASI-75 response at week 12 if they reach EASI-50 by week 4. Other suitable alternative predictors are week 4 IGA = 2, PP-NRS4, or EASI = 8. These week 4 responses, though suitable, were less-reliable predictors of IGA response at week 12. Thus, if clinically appropriate and permitted by local product information, patients who do not achieve one of the predictors of response (i.e., EASI-50, IGA = 2, PP-NRS4, or EASI = 8) by week 4 may benefit from increasing the abrocitinib dosage to 200 mg once daily at week 4 instead of waiting until week 12 to change the dosing strategy. A similar analysis, performed to identify clinical predictors of response in patients with psoriasis treated with tofacitinib [15], suggested that patients who achieved a ≥ 50% improvement in the Psoriasis Area and Severity Index (PASI) at week 8 of treatment were more likely to achieve a ≥ 75% improvement in PASI at week 16 [15]. In an analysis of a clinical trial with another JAK inhibitor, baricitinib, investigators found that in patients with AD with a baseline affected body surface area (BSA) of 10–50% who had an inadequate response or intolerance to topical corticosteroids, either a ≥ 50% improvement in BSA or a ≥ 3-point improvement in Itch NRS at week 4 or 8 was associated with EASI-75, IGA 0/1, and PP-NRS4 response at week 16 [16]. In another study of baricitinib, composite predictors of response (EASI-50 or a ≥ 3-point improvement in Itch NRS and a validated Investigator Global Assessment for AD score ≤ 2 or a ≥ 3-point improvement in Itch NRS) at week 4 or 8 were associated with EASI-75, a ≥ 4-point improvement in Itch NRS, or a combination of both, and they showed higher predictive accuracy compared with single parameters [17].
In the overall global clinical trial population, 40–69% of patients achieved EASI-75 response with abrocitinib 100 mg at week 12 [6,7,8,9, 12]. In this analysis, we show that EASI-50 response with abrocitinib 100 mg early in the treatment regimen (i.e., at week 4) is likely to yield EASI-75 response in ~ 70% of patients later, at week 12. These efficacy responses were observed in conjunction with high NPV, suggesting that patients without an early EASI-50 response are unlikely to have a later response and highlighting its utility as an early predictor to help guide decisions regarding stopping, switching, or increasing the treatment dose. Our findings are in agreement with the treat-to-target framework, which recommends the achievement of EASI-50 after 3 months or EASI-75 after 6 months of treatment as acceptable targets for decision-making on treatment management for patients with AD [18].
JAK inhibitors, including abrocitinib, have been shown to affect multiple laboratory parameters, including platelet, lymphocyte, or neutrophil counts, serum creatine phosphokinase (CPK), and high- and low-density lipoprotein cholesterol (HDL-C and LDL-C) [10, 19, 20]. The time-course effect of abrocitinib on platelet dynamics in patients treated with continuous abrocitinib 100 mg or 200 mg is well understood and was previously established using pharmacokinetic/pharmacodynamic modeling [14]. Abrocitinib reduced platelet counts in a dose-dependent manner, and this decrease was transient, reaching nadir at week 4, followed by a period of recovery [10, 14]. The current analysis assessed predictors of week 12 efficacy response at week 4, which coincides with the nadir observed for platelet counts. To understand the impact of increasing the abrocitinib dose from 100 mg/day to 200 mg/day at week 4, changes in platelet counts were assessed using the same model. Simulation of platelet dynamics suggests that an abrocitinib dose increase from 100 mg/day to 200 mg/day between week 4 and week 8 is unlikely to have any further negative effect on platelet count recovery. The mechanism involved in the platelet count reduction up to week 4 and subsequent recovery at later time points is currently unclear, but the inhibition of thrombopoietin production via JAK1 inhibition and the subsequent compensatory responses to thrombopoietin inhibition may play a role [21,22,23]. Thus, it is likely that a dynamic platelet recovery phase may be less influenced by increasing abrocitinib dosage.
Further studies are needed to elucidate other safety implications of an abrocitinib dose increase before week 12; however, the safety profile of abrocitinib in this scenario is likely to be consistent with that seen with the continuous dose of abrocitinib 200 mg, especially given the lower dose received during induction.
Limitations of this analysis include its post hoc nature and the small sample size of the cohorts, especially the validation cohort, which may limit the interpretation of results. In addition, common clinical study assessments, such as EASI and PP-NRS, are not always performed in routine real-world clinical practices, which limits the practical applicability of our findings.
Conclusions
Patients with moderate-to-severe AD who start abrocitinib treatment with 100 mg/day are more likely to achieve EASI-75 response at week 12 if they reach EASI-50 by week 4. Other suitable predictors are week 4 IGA = 2, PP-NRS4, or EASI = 8. Patients who failed to reach these responses at week 4 with abrocitinib 100 mg/day may be good candidates for an early dose increase to 200 mg, provided they satisfy safety criteria and the dose increase is done in a manner consistent with local product information.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Pfizer Limited. Cibinqo 100 mg film-coated tablets. Summary of product characteristics. Tadworth: Pfizer Limited; 2021.
Pfizer Japan Inc. Cibinqo™ (abrocitinib) tablets for oral use (50 mg, 100 mg, 200 mg). Tokyo: Pfizer Japan Inc.; 2021.
Pfizer Canada ULC. Cibinqo (abrocitinib). Product monograph. Kirkland: Pfizer Canada ULC; 2022.
European Medicines Agency. Cibinqo® (abrocitinib). Summary of product characteristics (SmPC). Pfizer Europe MA EEIG. Belgium: European Medicines Agency; 2024.
Pfizer Inc. Cibinqo (abrocitinib) tablets, for oral use. Prescribing information. New York: Pfizer Inc.; 2023.
Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–66.
Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–73.
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–12.
Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and Safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165–73.
Simpson EL, Silverberg JI, Nosbaum A, Winthrop KL, Guttman-Yassky E, Hoffmeister KM, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693–707.
Schmieder GJ, Draelos ZD, Pariser DM, Banfield C, Cox L, Hodge M, et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br J Dermatol. 2018;179(1):54–62.
Gooderham MJ, Forman SB, Bissonnette R, Beebe JS, Zhang W, Banfield C, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–9.
Blauvelt A, Silverberg JI, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104–12.
Wojciechowski J, Malhotra BK, Wang X, Fostvedt L, Valdez H, Nicholas T. Population pharmacokinetic-pharmacodynamic modelling of platelet time-courses following administration of abrocitinib. Br J Clin Pharmacol. 2022;88(8):3856–71.
Tan H, Valdez H, Griffins CE, Mrowietz U, Tallman A, Wolk R, et al. Early clinical response to tofacitinib treatment as a predictor of subsequent efficacy: results from two phase 3 studies of patients with moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2017;28(1):3–7.
Silverberg JI, Boguniewicz M, Waibel J, Weisman J, Strowd L, Sun L, et al. Clinical tailoring of baricitinib 2 mg in atopic dermatitis: baseline body surface area and rapid onset of action identifies response at week 16. Dermatol Ther (Heidelb). 2022;12(1):137–48.
Bieber T, Thyssen JP, Irvine AD, Tsunemi Y, Chen YF, Sun L, et al. Early improvements in signs and symptoms predict clinical response to baricitinib in patients with moderate-to-severe atopic dermatitis. Clin Exp Dermatol. 2023;48(8):881–8.
De Bruin-Weller M, Biedermann T, Bissonnette R, Deleuran M, Foley P, Girolomoni G, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402.
Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–68.
Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: Clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729–49.
Grozovsky R, Giannini S, Falet H, Hoffmeister KM. Novel mechanisms of platelet clearance and thrombopoietin regulation. Curr Opin Hematol. 2015;22(5):445–51.
Stivala S, Codilupi T, Brkic S, Baerenwaldt A, Ghosh N, Hao-Shen H, et al. Targeting compensatory MEK/ERK activation increases JAK inhibitor efficacy in myeloproliferative neoplasms. J Clin Invest. 2019;129(4):1596–611.
Williams DA. Pairing JAK with MEK for improved therapeutic efficiency in myeloproliferative disorders. J Clin Invest. 2019;129(4):1519–21.
Acknowledgements
We thank the participants in these studies. The authors would also like to thank Pinaki Biswas for his assistance with the additional data analyses for this manuscript.
Medical Writing/Editorial Assistance
Editorial/medical writing support under the guidance of the authors was provided by Renata Cunha, PharmD, and Vojislav Pejovic, PhD, and support for the development of the video abstract was provided by Amanda Mabhula, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175(9):1298–1304; https://doi.org/10.7326/M22-1460).
Funding
Sponsorship for this study and the Rapid Service Fee were funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Claire Feeney, Mark Levenberg, and Gary Chan contributed to the study concept and design. Fan Zhang and Luke Fostvedt conducted the statistical analysis. April W. Armstrong, Andrew F. Alexis, Andrew Blauvelt, Jonathan I. Silverberg, Claire Feeney, Mark Levenberg, Gary Chan, Fan Zhang, and Luke Fostvedt interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
April W. Armstrong has served as a research investigator and/or scientific advisor for Pfizer Inc., AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, EPI Health, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Regeneron Pharmaceuticals, Sanofi Genzyme, Sun Pharma, and UCB. Andrew F. Alexis has received grants made to the institution from AbbVie, Almirall, Amgen, Arcutis, Bristol Myers Squibb, Cara Therapeutics, Castle, Dermavant, Galderma, LEO Pharma, Novartis, Valeant (Bausch Health), and Vyne; is an advisory board member or consultant for Pfizer Inc., AbbVie, Allergan, Almirall, Alphyn Biologics, Amgen, Apogee Pharmaceuticals, Arcutis, Bausch Health, Beiersdorf, Bristol Myers Squibb, Canfield, Cara Therapeutics, Castle, Cutera, Dermavant, Eli Lilly and Company, EPI Health, Galderma, Incyte, Janssen, LEO Pharma, L’Oréal, Ortho Pharmaceutical, Regeneron Pharmaceuticals, Sanofi Genzyme, Swiss American, VisualDx, Vyne, and UCB; has served as a speaker for Pfizer Inc., Bristol Myers Squibb, Regeneron Pharmaceuticals, and Sanofi Genzyme; and has received royalties from Springer, Wiley-Blackwell, and Wolters Kluwer Health. Andrew Blauvelt has served as a speaker (received honoraria) for Pfizer Inc., AbbVie, Eli Lilly and Company, and UCB; served as a scientific advisor (received honoraria) for Pfizer Inc., AbbVie, Abcentra, Aclaris Therapeutics, Affibody Medical, ALIGOS Therapeutics, Almirall, Alumis Inc., Amgen, AnaptysBio, Apogee Pharmaceuticals, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient Pharmaceuticals, Evelo Biosciences, Evommune Inc., Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, LEO Pharma, Lipidio, Microbion Biosciences, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Q32 Bio Inc., Rani, Rapt, Regeneron Pharmaceuticals, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB, Union, Ventyx, Vibliome, and Xencor; and has acted as a clinical study investigator (the institution has received clinical study funds) for Pfizer Inc., AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo Biosciences, Evommune Inc., Galderma, Incyte, Janssen, LEO Pharma, Merck, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Sun Pharma, UCB, and Ventyx. Jonathan I. Silverberg served as an investigator for Celgene, Eli Lilly and Company, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, Regeneron Pharmaceuticals, and Sanofi Genzyme; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly and Company, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron Pharmaceuticals, and Sanofi Genzyme; and as a speaker for Regeneron Pharmaceuticals and Sanofi Genzyme. Fan Zhang was a former employee and shareholder of Pfizer Inc. and is currently an employee of Google LLC. Claire Feeney, Mark Levenberg, Gary Chan, and Luke Fostvedt are employees and shareholders of Pfizer Inc.
Ethical Approval
All study documents and procedures were approved by the appropriate institutional review boards/ethics committees at each study site. The studies were conducted in compliance with the ethical principles from the Declaration of Helsinki and all International Council for Harmonisation Good Clinical Practice Guidelines. All local regulatory requirements were followed. Informed consent was obtained from all individual participants included in the study.
Additional information
The affiliation given for Fan Zhang was their affiliation at the time the study was conducted.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Armstrong, A.W., Alexis, A.F., Blauvelt, A. et al. Predicting Abrocitinib Efficacy at Week 12 Based on Clinical Response at Week 4: A Post Hoc Analysis of Four Randomized Studies in Moderate-to-Severe Atopic Dermatitis. Dermatol Ther (Heidelb) 14, 1849–1861 (2024). https://doi.org/10.1007/s13555-024-01183-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01183-3