Abstract
Introduction
The aim of this study was to assess the efficacy and safety of 15% azelaic acid (AzA) gel in treating acne-induced post-inflammatory erythema (PIE) and post-inflammatory hyperpigmentation (PIH). The effects of 15% AzA gel on acne, skin barrier function, and quality of life were also evaluated.
Methods
A total of 72 patients with mild to moderate acne were enrolled in a randomized, double-blind, placebo-controlled trial. Patients were divided into two groups: patients in the AzA group applied 15% AzA gel twice daily for 12 weeks, and those in the placebo group applied AzA-free gel. Clinical evaluations using non-invasive skin detection technologies, including VISIA skin analysis, dermoscopy, and skin physiological function tests, were performed at 0, 4, 8, and 12 weeks. Main outcome measures included the post-acne hyperpigmentation index (PAHPI), melanin, hemoglobin, individual typology angle, water content, transepidermal water loss, and sebum. Investigator Global Assessment) and Dermatology Life Quality Index (DLQI) assessments were conducted at weeks 0 and 12. Adverse reactions were recorded.
Results
Of the 72 patients at study initiation, 60 completed the trial. At 8 and 12 weeks, patients in the AzA group showed significantly reduced PAHPI for PIE lesions compared to baseline and patients receiving placebo (P < 0.05). Patients in both groups exhibited reduced PIH lesions at weeks 8 and 12 that differed significantly from baseline (P < 0.05). Hemoglobin content decreased significantly in AzA-treated PIE lesions compared to those treated with placebo at week 12 (P < 0.05). Melanin content decreased significantly in AzA-treated PIH lesions at week 12 (P < 0.05). The AzA group showed higher improvement in DLQI (P < 0.05), and greater overall satisfaction (P < 0.05) compared to placebo.
Conclusion
The results indicate that 15% AzA gel effectively improved acne-induced PIE and PIH with minimal adverse reactions, making it a viable clinical application. In the study population, it had no adverse effects on skin barrier function and contributed positively to acne improvement and patient quality of life.
Trial Registration
This study was registered with the Chinese Clinical Trial Registry (ChiCTR.org.cn) under the identifier ChiCTR2300076959. The registration date was 25 October 2023, retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Acne-induced post-inflammatory erythema (PIE) and post-inflammatory hyperpigmentation (PIH) pose notable challenges in dermatology. |
This study aimed to evaluate the efficacy and safety of 15% azelaic acid (AzA) gel in treating acne-induced PIE and PIH. |
What was learned from this study? |
The study demonstrated that 15% AzA gel significantly decreased the intensity, size, and number of PIE and PIH lesions, along with improving overall acne severity. |
Importantly, 15% AzA gel treatment did not negatively impact skin barrier function, indicating its safety profile. |
The findings suggest that 15% AzA gel could be a valuable treatment option for acne-induced PIE and PIH, with potential implications for future research and treatment strategies. |
Introduction
Acne vulgaris, an inflammatory skin disorder, poses a significant challenge in dermatology, affecting approximately 9% of the global population and up to 85% of adolescents [1]. Its pathogenesis is complex, involving genetic predisposition, hormonal fluctuations, hyperkeratosis of pilosebaceous duct epithelium, colonization by Cutibacterium acnes, and dietary factors. Inflammation plays a central role in acne progression, leading to persistent vasodilation, capillary hyperplasia, and subsequent melanin synthesis abnormalities, culminating in post-inflammatory erythema (PIE) and post-inflammatory hyperpigmentation (PIH). These pigmentary complications affect a wide range of individuals, particularly those with Fitzpatrick skin types III through VI, with rates ranging from 45.5% to 87.2% [2]. PIE predominantly affects individuals with lighter skin tones, while PIH is more prevalent in those with darker complexions.
These pigmentary changes often coexist and primarily manifest on the cheeks, mandibular, and forehead regions. While inflammation serves as the primary etiological factor, the precise mechanisms remain incompletely understood. Although these lesions may regress spontaneously, their resolution can be protracted, lasting months to years or persisting indefinitely. In Asian and Middle Eastern populations, a substantial proportion of acne sufferers endure PIH for extended periods, signifying the need for effective management strategies.
Current management approaches for PIE include topical agents like tranexamic acid, timolol, vitamin C, and tacrolimus, alongside photoelectric therapies such as pulsed dye laser and intense pulsed light treatments. Likewise, depigmenting agents like hydroquinone and kojic acid, as well as chemical peels and laser interventions, are employed to address PIH. However, these treatments are often expensive and require multiple sessions, limiting their accessibility and efficacy, especially among patients with darker skin tones or active disease. While PIE and PIH may not directly impact physical health, their visible effects can significantly affect patients’ self-esteem and overall well-being, potentially leading to psychological issues like anxiety, depression, and even suicidal thoughts [3].
Despite the impact of these pigmentary changes on patients, research on clinical management remains limited. Azelaic acid (AzA), known for its anti-inflammatory, antibacterial, and anti-keratinizing properties, has emerged as a promising therapeutic option [4,5,6]. Given its multifaceted pharmacological effects, we hypothesized its efficacy in treating acne-induced PIE and PIH. Thus, our study aimed to evaluate the effectiveness and safety of 15% AzA gel in treating these conditions, as well as its impact on acne, skin barrier function, and quality of life following treatment.
Methods
Ethical Approval
This study received ethical approval from the Bioethics Committee of West China Hospital, Sichuan University (Protocol 2022-1439). All participants were recruited and included in the study following informed consent procedures in accordance with the principles outlined in the Declaration of Helsinki. Additionally, written informed consent was obtained from all participants for the publication of their photographs.
Study Design
The study was conducted at the Department of Dermatology and Venereology, West China Hospital, Sichuan University. Patients with mild to moderate acne vulgaris who met the predefined inclusion criteria were enrolled and randomly assigned to either the AzA group or the placebo group (Fig. 1). In the AzA group, participants received 15% AzA gel (Kelun Pharmaceutical, Chengdu, China) applied twice daily to the cleansed facial area for 12 weeks. Conversely, the placebo group received an AzA-free gel with identical ingredients (Kelun Pharmaceutical) applied twice daily for the same duration. Concurrent use of alternative acne treatments or functional skincare products was prohibited, and strict adherence to physical sun protection measures was mandated.
Patients
A total of 72 patients were enrolled in the study, among whom 12 patients were ultimately lost to follow-up (4 in the AzA group, 8 in the placebo group) and eight patients missed the visit at the 8th week. Missing visit data at week 8 were imputed using the last observation carried forward (LOCF) method. Ultimately, 30 participants were included in each group, for a total of 60 participants (25 men, 35 women), with an average (± standard deviation [SD]) age of 22.68 ± 3.60 (range 18–33) years. According to Fitzpatrick skin typing (58 patients type III, 2 patients type IV), there were no statistically significant differences in gender (Chi-square [χ2] = 0.069), age (t-test = 0.969), or skin type between the two groups before treatment (P > 0.05) (Table 1).
The duration of PIE and PIH in the enrolled patients was 6–12 months in 49 cases (81.6%) and exceeded 12 months in 11 cases (18.3%). Analysis of medical history using χ2 tests for categorical variables and t-tests for continuous variables showed no statistically significant differences between the two groups before treatment (P > 0.05), indicating comparability (Table 2).
Cutaneous symptom scores for PIE and PIH, and for the post-acne hyperpigmentation index (PAHPI), exhibited a non-normal distribution. Therefore, non-parametric Mann–Whitney U-tests were conducted, which revealed no statistically significant differences between the two groups (P > 0.05) (Table 3).
Prior to treatment, the Dermatology Life Quality Index (DLQI) and physiological parameters of acne, PIE, PIH, skin melanin, hemoglobin, individual typology angle (ITA) value, water content of the stratum corneum, transepidermal water loss (TEWL), and sebum levels were measured in both groups. All data were normally distributed, and independent sample t-tests indicated no statistically significant differences between the groups (P > 0.05) (Table 4).
Inclusion and Exclusion Criteria
The diagnosis of PIE and PIH relied on clinical assessment, given the absence of universally established diagnostic criteria. In this study, we defined diagnostic criteria for acne-induced PIE and PIH as follows: (1) prior clinical diagnosis of facial acne vulgaris; (2) development of smooth pigmentary changes following the resolution of inflammatory acne lesions like papules and pustules; and (3) possible concurrent presence of a limited number of active acne lesions or scars.
Inclusion criteria included: (1) age between 18 and 35 years; (2) a diagnosis of acne-induced PIE or PIH; (3) mild to moderate acne severity, indicated by Investigator Global Assessment (IGA) score 1–3; (4) provision of informed consent and willingness to adhere to the prescribed skincare regimen; and (5) avoidance of strenuous physical activity within 72 h preceding each scheduled visit.
Exclusion criteria comprised: (1) facial pigmentary skin disorders that might interfere with the accurate interpretation of test outcomes; (2) documented allergy or sensitivity to AzA; (3) pregnancy or lactation; (4) evidence of impaired liver or kidney function; (5) tretinoin use within 3 months; (6) facial plastic surgery within 3 months; (7) oral acne treatments within 4 weeks; (8) chemical peels or photoelectric therapy within 2 weeks; and (9) a history of immunodeficiency disorders.
Clinical Evaluations
The primary assessment parameter was the change in PAHPI within PIE and PIH lesions before and after AzA treatment. This index encompasses an evaluation of the intensity, size, and number of skin lesions [7], resulting in a cumulative score ranging from 6 to 22 points, with higher scores correlating with greater degrees of pigmentation severity (Tables 5, 6, 7). To facilitate accurate PAHPI scoring, the VISIA Skin Analysis system (Canfield Scientific, Parsippany, NJ, USA) and dermatoscopy (JEDA, Nanjing, China) were deployed in accordance with the respective manufacturers’ instructions. In a complementary manner, the IGA scale served as a secondary evaluation parameter.
Skin Physiology Assessments
Skin physiology assessments were conducted non-invasively using equipment from Courage + Khazaka electronic GmbH (Köln, Germany) to evaluate changes in melanin, hemoglobin, and ITA values within PIE and PIH lesions, as described in following text. Typical PIE and PIH lesions on the face, identified through dermoscopy, were selected for analysis. Three consecutive measurements were taken at each of these marked locations, with a 3-s interval between each reading, and the average value was recorded. Consistency was maintained by ensuring measurements were taken at the same marked locations during each visit.
Melanin and hemoglobin content were assessed using the Mexameter MX18 probe, following the manufacturer’s instructions. These measurements aided in determining the Fitzpatrick skin type, with average melanin content serving as an indicator (1–150 for Type II skin, 150–250 for Type III skin, and 250–350 for Type IV skin). Erythema values were also determined, ranging from no erythema (0–170) to maximum erythema (> 570).
Brightness changes within PIE and PIH skin lesions were analyzed using the ITA value, assessed with the Skin-Colorimeter CL400 probe in accordance with the manufacturer’s instructions. Outcomes were categorized based on ITA values as very bright (ITA > 55°), bright (55° > ITA > 41°), moderate (41° > ITA > 28°), and tan or dull (28° > ITA > 10°) complexions.
Skin Barrier Function Evaluations
Skin barrier function was evaluated through the assessment of water content, TEWL, and sebum levels within the stratum corneum, utilizing equipment sourced from Courage + Khazaka electronic GmbH, as described in following text.
Water content was quantified using the Corneometer CM825 probe. Triple consecutive measurements were performed at each assessment point, including the forehead and both cheeks, with the mean value calculated for accuracy. Interpretation referenced healthy skin on the inner forearm, categorized from very dry (< 30) to extremely moist (> 40).
TEWL was determined using the Tewameter TM300 probe. Measurements, lasting 30 s each, were conducted on the forehead and both cheeks. The average value for each region was computed from three consecutive measurements. Interpretation compared TEWL values to those of healthy skin (2.26 ± 1.36 g/m2/h) [8], with higher values indicating compromised skin barrier function.
Sebum levels were measured using the Sebumeter SM815 probe. Assessment points included the forehead and both cheeks. Elevated sebum values were associated with heightened sebum secretion. For sebum level assessment, a thin special extinction tape was utilized to absorb sebum on the skin, and then the transparency of the tape was measured using a photodetector tube. Following calculation, the sebum content appeared on the computer. This method exclusively assesses skin sebum, mitigating interference from moisture levels.
Patient Outcome Evaluations
The DLQI was evaluated during the initial and final visits. Participants were instructed to document any adverse effects encountered during the study period.
Statistical Analysis
For the statistical analysis, SPSS version 27 software (IBM SPSS, Armonk, NY, USA) was utilized. Normally distributed data were presented as the mean ± standard deviation (x ± s) and analyzed using t-tests for paired data or repeated measures analysis of variance for multiple groups. Non-normally distributed data were expressed using the median and 25th/75th percentiles (P25, P75) and analyzed using non-parametric tests such as the Wilcoxon rank-sum test for related samples. Multigroup analysis was conducted using generalized estimating equations. Categorical and ranked data were assessed using the Chi-square test and rank-sum test, respectively. Statistical significance was considered at P < 0.05. Graphs were generated using GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA).
Results
Decreased Intensity, Size, Number, and PAHPI in Acne-Induced PIE and PIH, and Decreased Overall IGA
The data for skin lesion scores and PAHPI before and after treatment in the AzA and placebo groups exhibited non-normal distribution, necessitating the use of generalized estimating equations for analysis. A reduction in the intensity, size, number, and PAHPI in acne-induced PIE and PIH, along with a decrease in overall IGA, was observed. Both groups demonstrated a decrease in PIE and PIH following treatment compared to baseline.
For PIE, statistically significant differences in intensity between the groups were noted at weeks 8 (Wald χ2 = 5.190, P = 0.023) and 12 (Wald χ2 = 32.347, P < 0.001), with the AzA group showing superior improvement over time (Table 8; Fig. 2a). Similarly, for size, statistically significant differences between the groups were observed at week 12 (Wald χ2 = 3.888, P = 0.049), favoring the AzA group (Table 9; Fig. 2b). Regarding the number, statistically significant differences between the groups were also seen at week 12 (Wald χ2 = 8.303, P = 0.004), with the AzA group exhibiting better improvement (Table 10; Fig. 2c). PAHPI results showed significant differences between the groups at weeks 8 (Wald χ2 = 3.99, P = 0.046) and 12 (Wald χ2 = 20.89, P < 0.001), with the AzA group demonstrating superior improvement over time (Table 11; Fig. 2d).
Effect of AzA on acne-induced PIE and PIH. a–d Graphical representation illustrating the reduction in intensity, size, and number of acne-induced PIE lesions. e–h Graphical representation illustrating the reduction in intensity, size, and number of acne-induced PIH lesions. AzA significantly decreased the overall PAHPI scores and IGA ratings. Asterisks indicate significant differences between groups at *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001. AzA azelaic acid, IGA Investigator Global Assessment, ns not significant, PAHPI post-acne hyperpigmentation index PIE post-inflammatory erythema, PIH post-inflammatory hyperpigmentation
Results for PIH exhibited a similar trend to that of PIE. Statistically significant differences between the AzA and placebo groups, as well as significant improvement, were observed at week 12 for intensity (Wald χ2 = 19.470, P < 0.001), size (Wald χ2 = 19.470, P = 0.003), and number (Wald χ2 = 12.199, P < 0.001) (Tables 12, 13, 14; Fig. 2e–g). PAHPI also showed statistically significant differences between the groups at weeks 8 (Wald χ2 = 4.51, P = 0.034) and 12 (Wald χ2 = 22.51, P < 0.001), with the AzA group displaying better improvement over time (Table 15; Fig. 2h).
The changes observed in the IGA scores before and after treatment were consistent with the PAHPI results. Based on IGA scores, in the AzA group prior to treatment, 83.3% of cases were assessed as mild and 16.66% as moderate; after treatment, there was a shift to 70% mild cases, 3.33% moderate cases, with fully recovery in 26.66% of cases. Conversely, in the placebo group, prior to treatment, 86.66% of cases were assessed as mild and 13.33% as moderate; after treatment, this changed to 87% mild cases, 10% moderate cases, and 3.33% severe cases (Fig. 3). A statistical analysis conducted at week 12 (Z = − 3.386, P < 0.001) revealed a significant difference between the groups, indicating notable improvement in the AzA group compared to the placebo group (Table 16).
Decreased Melanin and Hemoglobin, and Increased ITA in Acne-Induced PIE, and Decreased Melanin and Increased ITA in Acne-Induced PIH
Melanin, hemoglobin, and ITA values before and after treatment in the AzA and placebo groups exhibited normal distribution, allowing for repeated measures analysis of variance. A decrease in melanin and hemoglobin, along with an increase in ITA, was observed in acne-induced PIE and PIH.
For PIE, melanin levels decreased significantly in the AzA group compared to the placebo group at week 12 (F = 4.335, P = 0.042) (Table 17; Fig. 4a). Hemoglobin levels and ITA values also showed statistically significant improvement in the AzA group at week 12 (hemoglobin: F = 4.783, P = 0.033; ITA: F = 4.539, P = 0.037) (Tables 18, 19; Fig. 4b, c).
Effect of AzA on melanin, hemoglobin, and ITA in acne-induced PIE and PIH. a–c Reduction in melanin (a) and hemoglobin (b) levels and increase in ITA (c) within acne-induced PIE. d, f Reduction in melanin (d) and increase in ITA (f) within acne-induced PIH. e Hemoglobin in PIH; no significant difference between the two groups was observed. Asterisks indicate significant differences between groups at *P < 0.05, **P < 0.01, and ***P < 0.005. AzA Azelaic acid, ITA individual typology angle, ns not significant, PIE post-inflammatory erythema, PIH post-inflammatory hyperpigmentation
In terms of PIH, statistically significant improvements in melanin and ITA were observed in the AzA group at week 12 (melanin: F = 8.146, P = 0.006; ITA: F = 5.502, P = 0.022) (Tables 20, 21; Fig. 4d, f). No statistical difference was observed in hemoglobin levels between the two groups (Table 22; Fig. 4e).
No Influence on Water Content, Increased TEWL, and Decreased Sebum Level of Skin
Water content of the stratum corneum showed no significant changes compared to baseline after treatment in both groups. There was no statistical difference between the AzA and placebo groups (Table 23; Fig. 5a).
Effect of AzA on skin physiology. a Water content; there was no significant change with treatment. b, c there was an increase in TEWL (b) and decrease in sebum level (c) of skin after AzA treatment. Asterisks indicate significant differences between groups at **P < 0.01 and ***P < 0.005. AzA azelaic acid, ns not significant, TEWL transepidermal water loss
TEWL values increased in both groups after treatment, with no statistical difference between the AzA and placebo groups (Table 24; Fig. 5b).
Sebum levels decreased in both groups after treatment, with no statistical difference observed between the two groups (Table 25; Fig. 5c).
Increased Overall Subjective Improvement in Patients, and Decreased DLQI with Observation of Clinical Improvement and No Serious Adverse Effects
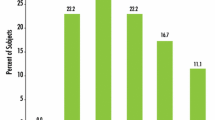
By week 12, patients assessed their satisfaction with the improvement in acne-induced PIE and PIH. In the AzA group, the effective rate, indicating improvement rates exceeding 60%, was 73.33%, contrasting with 13.33% in the placebo group. This discrepancy yielded a statistically significant difference between the two groups (P < 0.05), with a total effective rate of 100% in the AzA group compared to 56.66% in the placebo group. Notably, 10% of patients in the placebo group experienced worsening symptoms (Tables 26, 27; Fig. 6a).
DLQI scores decreased significantly in the AzA group compared to the placebo group at week 12 (Z = − 3.221, P = 0.001), indicating an improvement in the quality of life of patients with acne-induced PIE and PIH in the AzA group (Table 27; Fig. 6b).
Clinical evaluations using the VISIA Skin Analysis system and dermatoscopy demonstrated marked improvements in PIE and PIH within the AzA group compared to the placebo group. Specifically, analysis with VISIA revealed a reduction in the area of redness and brown spots, accompanied by darkening of color at the 12-week mark compared to baseline in the AzA group (Fig. 7a, b). In contrast, the placebo group exhibited no significant change in redness area, if not exacerbation, with color deepening (Fig. 7e, f). Dermoscopy examinations further indicated lightening of color and reduction in brown spot area and background erythema in the AzA group at week 12 (Fig. 7c, d), whereas no notable changes were observed in the placebo group (Fig. 7g, h).
Clinical evaluation of PIE and PIH using VISIA Skin Analysis system and dermatoscopy. Comparison between the AzA group and the placebo group at week 12 revealed significant improvements in PIE and PIH. Analysis with VISIA demonstrates a notable reduction in redness area and brown spots in the AzA group (a, b), while the placebo group shows minimal change or exacerbation (e, f). Dermoscopy examinations reveal lightening of color and reduction in brown spot area and background erythema in the AzA group (c, d), contrasting with the placebo group's lack of notable changes (g, h). PIE Post-inflammatory erythema, PIH post-inflammatory hyperpigmentation
In the AzA group, 20 patients (66.67%) reported experiencing mild facial erythema, tingling, dryness, itching, peeling, and other symptoms during the treatment process. Upon experiencing these symptoms, patients were advised to temporarily discontinue AzA usage for 3–5 days while intensifying facial moisturization and sun protection. Generally, these symptoms completely subsided within approximately 7 days, and patients developed tolerance by gradually extending the duration of AzA application. Notably, these adverse reactions did not persist into the 8th and 12th weeks of the trial. Conversely, no adverse reactions were observed in the placebo group throughout the entire study duration, and no serious adverse events were reported.
Discussion
Acne is known as a prevalent chronic inflammatory condition that particularly affects adolescents, presenting a spectrum of clinical manifestations and a multifaceted pathogenesis. Inflammation remains a hallmark of acne, often leading to the emergence of PIE and PIH during the healing phase of inflammatory lesions.
Notably, persistent telangiectasia and inflammatory mediators are frequently observed in acne PIE lesions, particularly among individuals with lighter skin tones [9]. The precise pathogenesis remains incompletely elucidated, with several key points meriting consideration: (1) inflammation triggers an increase in red blood cell count, leading to vascular dilation and hyperplasia; (2) sustained inflammation induces skin tissue edema and collagen degradation, resulting in epidermal thinning and heightened light reflection from expanded microvasculature [9]; (3) certain anti-acne agents, including topical or systemic retinoic acid and benzoyl peroxide, may serve as sources of local irritation [10]. Dermoscopic examination of PIE lesions often reveals telangiectasia and vascular patterns, predominantly in the form of punctate vessels, followed by glomeruli, linear vessels, serpentine structures, dendrites, and large vascular lakes. Studies utilizing optical coherence tomography-based microangiography have demonstrated rougher and less organized blood vessels in acne-affected areas compared to surrounding normal tissue, with an early surge in blood vessel density during acne inflammation followed by a gradual decline [11].
In comparison, acne PIH lesions typically present as brown to gray-brown patches with well-defined borders, intensifying following the resolution of PIE. Histopathologically, PIH can be categorized into epidermal and dermal types [12]: the former primarily results from increased melanin within keratinocytes, while the latter stems from heightened dermal melanophages, with potential melanin decrease in the epidermis due to lymphocyte infiltration. Dermoscopically, epidermal PIH appears as light brown to tan, while dermal PIH manifests as light blue, dark gray, or blue-black [13]. Melanin synthesis is intricately regulated, with tyrosinase playing a key role [14]. The pathogenesis of PIH involves local inflammation triggering the production of inflammatory mediators, such as arachidonic acid, prostaglandin, leukotriene, interleukin (IL), tumor necrosis factor (TNF), and thromboxane, thereby stimulating melanocyte proliferation and volume increase, ultimately leading to heightened melanin synthesis. Inflammation also targets and disrupts melanocytes and the dermal–epidermal junction, facilitating the migration of melanin granules from the epidermis to the dermis via dendrites. Additionally, inflammation diminishes sulfhydryl groups in the skin, hampering tyrosinase activity and augmenting melanin production. Cutibacterium acnes has been implicated in elevating messenger RNA (mRNA) expression of TNF-alpha, secretion levels of IL-6 and IL-8, mRNA levels of tyrosinase, and dopachrome tautomerase, consequently activating melanocytes and increasing melanin output [15].
A comparative analysis of soluble proteins in pathological sections of acne-induced PIE and PIH revealed elevated levels of dermal remodeling proteases and inhibitors in PIE lesions, indicative of dermal remodeling during excision [16]. Conversely, PIH lesions exhibited heightened levels of IL-1β and transforming growth factor-beta (TGF-β), suggesting macrophage infiltration and persistent inflammation during resection. Moreover, PIH lesions displayed a significant increase in Keap1 protein, implicating a role in repressing oxidative and electrophilic stress and leading to premature endothelial cell senescence [16]. The resolution time of skin lesions is influenced by factors such as patient skin color, inflammation severity, and basement membrane zone damage [17]. Early intervention and inflammation control are crucial for effective treatment.
AzA exerts anti-inflammatory effects by inhibiting the expression and secretion of inflammatory mediators, thereby attenuating inflammation. AzA has been shown to impede the antimicrobial peptide channel of serine protease kallikrein-5 (KlK-5), reduce serine protease synthesis, and subsequently diminish pro-inflammatory cytokine release [5, 18]. Moreover, AzA inhibits the CD36/NADPH oxidase pathway and upregulates peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression, thereby modulating inflammation [2, 6, 19]. Furthermore, AzA scavenges intracellular reactive oxygen species (ROS), including superoxide anions and hydroxyl radicals, thereby mitigating ROS release from neutrophils and inhibiting the degradation of aromatic compounds (including tyrosine acid) and arachidonic acid [20, 21]. AzA also impedes tyrosinase activity, exerting a direct inhibitory and cytotoxic effect on hyperfunctional melanocytes with structural anomalies, with minimal impact on normal cells, potentially due to enhanced permeability in aberrant cells. Additionally, AzA inhibits mitochondrial metabolism, DNA, and protein synthesis in melanocytes [22].
In our study, 15% AzA gel demonstrated efficacy in improving acne-induced PIE and PIH, augmenting acne management, and enhancing patient quality of life. Through non-invasive skin physiology assessments and light color space principles, we objectively assessed alterations in melanin and hemoglobin levels in acne-induced PIE and PIH lesions, together with ITA values. Our evaluation of skin barrier function revealed no significant impact following AzA treatment. However, some patients exhibited adverse reactions, likely influenced by prior retinoic acid use, potentially leading to compromised skin barrier integrity. Acne patients frequently exhibit elevated sebum levels and diminished total ceramide content in the stratum corneum, rendering them susceptible to barrier disruption. Moreover, Cutibacterium acnes colonization can further compromise skin barrier function. Despite these considerations, our findings demonstrate the efficacy of AzA in ameliorating acne-induced PIE and PIH, necessitating future studies with expanded sample sizes, consistent environmental conditions, and extended observation periods to corroborate our findings and elucidate the long-term effects of AzA on acne management and skin health.
Conclusion
In summary, our investigation into the efficacy and safety of 15% AzA gel for treating acne-induced PIE and PIH employed a rigorous randomized, double-blind, placebo-controlled methodology. Utilizing a comprehensive, multifaceted approach, including VISIA analysis, dermoscopy, and non-invasive physiological index detection technology, together with assessments like the PAHPI, acne IGA score, and DLQI, our study yielded compelling results. We found that 15% AzA gel significantly and safely ameliorated acne-induced PIE and PIH, accompanied by minimal adverse reactions, thus substantiating its clinical utility. Notably, our study revealed that AzA had no detrimental effect on the skin barrier function of patients with acne-induced PIE and PIH, while suggesting potential ancillary benefits in augmenting overall acne management and enhancing patients' quality of life. These findings emphasize the promising role of AzA in the therapeutic armamentarium for acne-associated cutaneous sequelae, advocating for its integration into clinical practice as a valuable treatment option.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–85.
Nazzaro-Porro M, Passi S. Identification of tyrosinase inhibitors in cultures of Pityrosporum. J Invest Dermatol. 1978;71(3):205–8.
Thiboutot D. Versatility of azelaic acid 15% gel in treatment of inflammatory acne vulgaris. J Drugs Dermatol. 2008;7(1):13–6.
Sieber MA, Hegel JK. Azelaic acid: properties and mode of action. Skin Pharmacol Physiol. 2014;27(Suppl 1):9–17.
Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10(6):586–90.
Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–7.
Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73(8):779–87.
Chandra F, Sandiono D, Sugiri U, Suwarsa O, Gunawan H. Cutaneous side effects and transepidermal water loss to gefitinib: a study of 11 patients. Dermatol Ther (Heidelb). 2017;7(1):133–41.
Bae-Harboe YS, Graber EM. Easy as PIE (postinflammatory erythema). J Clin Aesthet Dermatol. 2013;6(9):46–7.
Agamia N, Essawy M, Kassem A. Successful treatment of the face post acne erythema using a topically applied selective alpha 1-adrenergic receptor agonist, oxymetazoline 1.5%, a controlled left to right face comparative trial. J Dermatolog Treat. 2022;33(2):904–9.
Baran U, Li Y, Choi WJ, Kalkan G, Wang RK. High resolution imaging of acne lesion development and scarring in human facial skin using OCT-based microangiography. Lasers Surg Med. 2015;47(3):231–8.
Park JY, Park JH, Kim SJ, et al. Two histopathological patterns of postinflammatory hyperpigmentation: epidermal and dermal. J Cutan Pathol. 2017;44(2):118–24.
Jurairattanaporn N, Suchonwanit P, Rattananukrom T, Vachiramon V. A Comparative study of dermatoscopic features of acne-related postinflammatory hyperpigmentation in facial and nonfacial areas in Asian patients. J Clin Aesthet Dermatol. 2022;15(8):16–21.
Pillaiyar T, Manickam M, Jung SH. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017;40:99–115.
Yu Y, Shen Y, Zhang S, Wang N, Luo L, Zhu X, et al. Suppression of Cutibacterium acnes-mediated inflammatory reactions by fibroblast growth factor 21 in skin. Int J Mol Sci. 2022;23(7):3589.
Karaman-Jurukovska N, Kohli I, Nicholson C, et al. 633 Comparison of soluble proteins from skin sections of acne and TCA induced postinflammatory hyperpigmentation and erythema. J Invest Dermatol. 2022;142(8):S109.
Silpa-Archa N, Kohli I, Chaowattanapanit S, Lim HW, Hamzavi I. Postinflammatory hyperpigmentation: a comprehensive overview: epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J Am Acad Dermatol. 2017;77(4):591–605.
Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131(3):688–97.
Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Exp Dermatol. 2010;19(9):813–20.
Akamatsu H, Komura J, Asada Y, Miyachi Y, Niwa Y. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283(3):162–6.
Passi S, Picardo M, Zompetta C, De Luca C, Breathnach AS, Nazzaro-Porro M. The oxyradical-scavenging activity of azelaic acid in biological systems. Free Radic Res Commun. 1991;15(1):17–28.
Breathnach AS, Nazzaro-Porro M, Passi S. Azelaic acid. Br J Dermatol. 1984;111(1):115–20.
Acknowledgements
We extend our sincere gratitude to all the participants who generously contributed their time and cooperation to this trial.
Funding
The study was funded by the National Natural Science Foundation of China (Grant No. 81502719). The journal’s Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Huidi Shucheng: conceptualization, methodology, project administration, investigation, formal analysis, writing—original draft preparation, and writing—editing and revision. Xinyu Zhou: conceptualization, methodology, investigation, formal analysis, and writing-original draft preparation. Dan Du: investigation and formal analysis. Jiaqi Li: investigation and formal analysis. Chenyang Yu: investigation and writing—original draft preparation. Xian Jiang: project administration, writing—editing and revision, funding acquisition, and supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors (Huidi Shucheng, Xinyu Zhou, Dan Du, Jiaqi Li, Chenyang Yu, and Xian Jiang) declare no conflicts of interest.
Ethical Approval
This study received ethical approval from the Bioethics Committee of West China Hospital, Sichuan University (Protocol 2022-1439). All participants were recruited and included in the study following informed consent procedures in accordance with the principles outlined in the Declaration of Helsinki. Additionally, written informed consent was obtained from all participants for the publication of their photographs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shucheng, H., Zhou, X., Du, D. et al. Effects of 15% Azelaic Acid Gel in the Management of Post-Inflammatory Erythema and Post-Inflammatory Hyperpigmentation in Acne Vulgaris. Dermatol Ther (Heidelb) 14, 1293–1314 (2024). https://doi.org/10.1007/s13555-024-01176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01176-2