Abstract
Introduction
Acne vulgaris, a chronic inflammatory condition, is associated with significant physical and psychosocial burden. Since 2019, three new topical agents for acne vulgaris have been approved in the USA and Canada. We performed a systematic review and meta-analysis to compare the efficacy between twice-daily clascoterone cream 1%, once-daily trifarotene 0.005% cream, and once-daily tazarotene 0.045% lotion for acne treatment.
Methods
Randomized controlled trials (RCTs) comparing clascoterone, trifarotene, or tazarotene with vehicle in patients with moderate-to-severe acne were identified from a systematic literature review and included in a meta-analysis. Primary outcomes were percentage reduction in inflammatory and noninflammatory lesion count (ILC and NILC, respectively) and treatment success rate (≥ 2-grade improvement in Investigator’s Global Assessment or Evaluator’s Global Severity Score and a rating of clear or almost clear) at week 12. DerSimonian and Laird random-effects models with the inverse variance method were used to calculate the mean difference (MD) for percentage reduction in ILC and NILC, and odds ratios (ORs) for the rate of treatment success.
Results
Six Phase 3 RCTs were included in the meta-analysis. The analyses showed robust differences favoring the interventions for ILC (MD: − 11.5; 95% confidence interval [CI]: − 14.39, − 8.62), NILC (MD: − 12.25; 95% CI: − 15.21, − 9.29), and treatment success rate (OR: 2.14; 95% CI: 1.81, 2.53). No differences were observed between clascoterone, trifarotene, and tazarotene for ILC (MD: − 12.8, − 11.2, and − 10.1, respectively), NILC (MD: − 11.6, − 13.9, and − 12.8, respectively), or treatment success rate (OR: 2.9, 1.9, and 2.1, respectively (all P > 0.05).
Conclusion
No significant differences in efficacy were observed between clascoterone, trifarotene, and tazarotene after 12 weeks of treatment in patients with moderate-to-severe acne. Differences in application frequency and safety profile should also be taken into consideration when making treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Three new topical agents have been approved for acne vulgaris treatment in the USA and Canada: the retinoids trifarotene 0.005% cream and tazarotene 0.045% lotion, and the androgen receptor inhibitor clascoterone cream 1%. |
A meta-analysis comparing the efficacy of these agents as compared with vehicle treatments revealed robust differences for all endpoints favoring the intervention versus vehicle groups. |
No significant differences were found between clascoterone, trifarotene, and tazarotene for reductions in lesion counts or for the rate of treatment success following 12 weeks of treatment. |
The findings of this analysis suggest that clascoterone, trifarotene, and tazarotene exhibit similar efficacy in the treatment of patients with moderate-to-severe acne. |
Differences in application frequency and safety profile are important to take into consideration when making decisions regarding the best treatment options for patients. |
Introduction
Acne vulgaris is a prevalent, chronic, inflammatory condition characterized by the appearance of inflammatory (i.e., papules, pustules, and nodules) and noninflammatory (i.e., open and closed comedones) lesions on the face and/or trunk [1]. The disorder most commonly occurs in adolescents and young adults, but it can affect individuals at any age [2,3,4]. Globally, acne has a prevalence of 9.4% [5] and is ranked second highest among all skin conditions in disability-adjusted life years [6]. Acne causes significant physical and psychosocial burden, including residual scarring, erythema, postinflammatory hyperpigmentation, social avoidance, and higher prevalence of anxiety and depression [1, 7,8,9], and is associated with negative impacts on quality of life and decreased school and work productivity [7]. Collectively, these underscore the significant disease burden experienced by individuals with acne and the importance of safe and effective treatment to mitigate its long-term sequelae.
The pathophysiology of acne vulgaris is multifactorial and involves follicular hyperkeratinization, increased sebum production, colonization by Cutibacterium acnes, and inflammation [1]. Sex steroid hormones, particularly androgens, also play a prominent role in the development of acne lesions by inducing the production of sebum and proinflammatory cytokines in sebocytes [10, 11]. As outlined by the American Academy of Dermatology, topical therapy is the foundation of initial treatment for acne vulgaris, and the use of multiple topical agents that affect different aspects of acne pathophysiology is recommended [1]. Current treatment guidelines outline the use of benzoyl peroxide or a topical retinoid (e.g., tretinoin, adapalene) as first-line agents for cases of mild acne, with combination topical therapy recommended for moderate or severe cases [1]. However, these agents may cause side effects such as irritation, dryness, and erythema, which can limit their tolerability and use in clinical practice [1].
Since 2019, three new topical agents for acne vulgaris have been approved in the USA and Canada: the retinoids trifarotene 0.005% cream and tazarotene 0.045% lotion (previously available as 0.1% cream) [12,13,14,15,16], and the androgen receptor inhibitor clascoterone cream 1% [17, 18]. Both trifarotene and tazarotene exhibit selective agonistic activity at retinoic acid receptors present within keratinocytes, thereby modulating cellular differentiation, keratinization, and inflammation [12, 13]. In contrast, clascoterone is thought to exert its effects by competing with dihydrotestosterone for binding to androgen receptors to prevent androgen-stimulated sebum production [11, 17]. Treatment by all three agents elicited significantly greater reductions in inflammatory and noninflammatory lesion counts versus vehicle in phase 3 clinical trials [19,20,21].
The objective of this systematic literature review and meta-analysis of outcomes from phase 3 clinical trials was to compare the efficacy between clascoterone, trifarotene, and tazarotene for the treatment of acne vulgaris to guide the clinical management of acne vulgaris treatment.
Methods
Ethics
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Selection Criteria
Only randomized controlled trials (RCTs) evaluating the efficacy of clascoterone cream 1%, trifarotene 0.005% cream, or tazarotene 0.045% lotion for the treatment of acne vulgaris were included. For inclusion in the meta-analysis, studies must have reported the following outcomes: (1) mean percentage change in inflammatory lesion count (ILC) at week 12; (2) mean percentage change in noninflammatory lesion count (NILC) at week 12; and (3) percentage of patients achieving treatment success, defined as a ≥ 2-grade improvement in the Investigator’s Global Assessment (IGA) scale or Evaluator's Global Severity Score (EGSS). Studies were excluded if they did not report on all the primary outcomes. Only studies published in English were included. Retrospective studies, systematic reviews, meta-analyses, research letters, case reports, and abstracts were excluded.
Literature Search
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Embase and MEDLINE (Ovid) databases were searched from inception to October 2023 using specific keywords (Electronic Supplementary Material [ESM] Table S1). Reference lists of the included articles were also searched for studies not captured by the literature search.
Selection Process and Data Extraction
Two researchers (MS and MUA) independently assessed the trials according to the study selection criteria. Trial design, trial size, information about the intervention (dose, frequency, and treatment duration), participant inclusion and exclusion criteria, follow-up period, and outcome data for each endpoint were extracted.
Key Endpoints
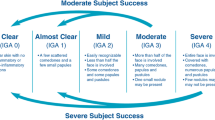
The primary outcomes were mean percentage reduction in ILC at week 12, mean percentage reduction in NILC at week 12, and percentage of patients achieving treatment success, defined as a ≥ 2-grade improvement in IGA or EGSS and a rating of clear or almost clear at week 12. The IGA is a 5-point scale used to assess the severity of acne numbered from 0 to 4, defined as 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe). Similarly, the EGSS is an ordinal scale for evaluating acne severity ranging from 0 to 4, defined as clear (0), almost clear (1), mild (2), moderate (3), and severe (4).
Risk of Bias Assessment
The methodological quality of the included studies was assessed using the revised Cochrane risk of bias tool for randomized trials. Risk of bias was assessed across various domains, including the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results (ESM Table S2).
Statistical Analysis
All data analyses were planned a priori. A meta-analysis was performed to compare the efficacy of the three topical acne therapies (clascoterone, trifarotene, and tazarotene) as compared with vehicle treatments using the published data from included studies. For continuous outcomes (i.e., ILC and NILC), the mean ± standard deviation (SD) percentage change in lesion counts from baseline to the immediate posttreatment follow-up data at week 12 was utilized for both intervention and control groups. In studies in which confidence intervals or standard errors were reported as a measure of variance, Cochrane-recommended methods were used to convert to the SD [22]. For dichotomous outcomes (i.e., treatment success rate), the number of events or proportion data at the immediate posttreatment follow-up at week 12 were utilized for both intervention and control groups. DerSimonian and Laird random-effects models with the inverse variance method were used to calculate the mean difference (MD) for continuous outcomes and odds ratios (ORs) for dichotomous outcomes [23]. Cochran’s Q (α = 0.05) was employed to detect statistical heterogeneity, and the I2 statistic was applied to quantify the magnitude of statistical heterogeneity between studies; I2 > 50% represents moderate and I2 > 75% represents substantial heterogeneity across studies [24]. Primary subgrouping in each meta-analysis was based on the medication type reported in each study. All analyses were performed using R software (metafor package) [25, 26].
Results
Search Results
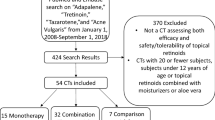
The search yielded 277 articles from MEDLINE (Ovid). Following the removal of 77 duplicate articles, the remaining 200 articles were screened, among which six were assessed for eligibility. Of these six articles, three publications [19,20,21] pertaining to six unique phase 3 RCTs—two RCTs each for clascoterone, trifarotene, and tazarotene—were eligible for inclusion (Fig. 1). The details of the included studies pertaining to treatment dose and duration, inclusion criteria, and number of enrolled patients are shown in Table 1.
Study Characteristics
A total of 5474 patients were enrolled across all included trials, of which 2735 were randomized to active treatment (clascoterone, n = 722; trifarotene, n = 1214; tazarotene, n = 799), and 2739 were randomized to vehicle treatment. The median age was 18 (range 9–58) years in the studies of clascoterone, 19.6 (range 9–58) years in the studies of trifarotene, and 20.5 (range 10–65) years in the studies of tazarotene. The mean facial inflammatory and noninflammatory baseline lesion counts were 42.4 and 61.5 for clascoterone, 35.7 and 52.2 for trifarotene, and 28.1 and 41.1 for tazarotene, respectively. At baseline, 83.4% of patients enrolled in the clinical trials of clascoterone, 100% of patients enrolled in the clinical trials of trifarotene, and 90.9% of patients enrolled in the clinical trials of tazarotene had acne of moderate severity (defined as an IGA or EGSS score of 3). All trials were double blinded and used a 1:1 randomization pattern. Consistent with the post-approval product labeling of these three agents [12, 13, 17], treatment was administered once daily for both tazarotene and trifarotene and twice daily for clascoterone, for a total duration of 12 weeks.
Comparisons of Efficacy
For comparisons of the mean percentage reduction in ILC and NILC at week 12, the MDs of clascoterone, trifarotene, and tazarotene as compared with vehicle were ranked using forest plots (Fig. 2). The analyses showed robust differences favoring interventions for ILC (MD: − 11.5; 95% CI: − 14.4, − 8.6; Fig. 2a) and NILC (MD: − 12.3; 95% CI: − 15.2, − 9.3; Fig. 2b) at week 12. However, tests for subgroup differences did not identify significant differences between clascoterone, trifarotene, and tazarotene for ILC (MD: − 12.8, − 11.2, and − 10.1, respectively; P = 0.82; Fig. 2a) or NILC (MD: − 11.6, − 13.9, and − 12.8, respectively; P = 0.81; Fig. 2b).
For comparisons of the rate of treatment success at week 12, the ORs of clascoterone, trifarotene, and tazarotene as compared with vehicle were ranked using forest plots (Fig. 3). The OR for the rate of treatment success similarly indicated favorable treatment efficacy for the interventions at week 12 (OR: 2.1; 95% CI: 1.8, 2.5). However, no significant differences were observed between clascoterone, trifarotene, and tazarotene (OR: 2.9, 1.9, and 2.1, respectively; P = 0.16; Fig. 3).
Heterogeneity and Risk of Bias
A low overall heterogeneity of the studies existed in the analysis. The I2 statistic was 22.8% for the percentage change in ILCs, 0% for the percentage change in NILCs, and 28.9% for the rate of treatment success. However, for certain outcomes, there was moderate-to-high heterogeneity across trials for the same treatment. The I2 statistic was 78.1% for the percentage change in ILC across the two clascoterone trials and 44.2% for the percentage change in NILC across the two trials for tazarotene. Differences in population, baseline characteristics, and treatment setting may have contributed to the observed intertrial heterogeneity. No trials were identified with a high risk of bias.
Discussion
This meta-analysis provides an indirect evaluation of the comparative efficacy of three new topical agents for the reduction of inflammatory and noninflammatory lesions and the rate of treatment success in patients with acne vulgaris. The analyses revealed robust differences for all efficacy endpoints favoring the interventions versus vehicle groups, corroborating the established efficacy of these agents in acne. However, no significant differences were found between the three agents for reductions in lesion counts or for the rate of treatment success after 12 weeks of treatment. Based on this analysis, clascoterone, trifarotene, and tazarotene exhibit similar efficacy in the treatment of patients with moderate-to-severe acne.
The efficacy and safety of clascoterone, trifarotene, and tazarotene were previously established in separate 12-week, double-blinded, vehicle-controlled, phase 3 studies [19,20,21]. The rate of treatment success (evaluated based on IGA or EGSS) and the absolute change in inflammatory and noninflammatory lesion counts were coprimary endpoints across all studies [19,20,21], which facilitates indirectly comparing these outcomes between products. However, there are some differentiating factors that could impact their relative efficacy, safety, tolerability and, therefore, treatment selection in the clinical setting. For example, trifarotene and tazarotene are both applied once daily, whereas clascoterone is applied twice daily [19,20,21]. In clinical trials, application-site reactions such as pain, dryness, and irritation were the most common adverse events (AEs) reported in patients who received treatment with trifarotene or tazarotene [20, 21], whereas application-site reactions were not frequently reported in patients who received clascoterone [19, 27]. Furthermore, the mechanism of action of clascoterone, an androgen receptor inhibitor, differs from that of the two retinoids, and so it targets a different aspect of acne pathophysiology. These factors are important and must be taken into consideration when making decisions regarding the best treatment options for patients.
A previous meta-analysis of both pharmacological and nonpharmacological treatments for acne reported that combination treatment consisting of a topical retinoid and benzoyl peroxide is the most effective for reducing the number of inflammatory and noninflammatory lesions [28]. However, comparative efficacy was only presented for treatments grouped across categories (e.g., topical retinoid); therefore, inferences cannot be made regarding differences in efficacy between specific treatments. A different meta-analysis similarly reported that combination treatment with adapalene and benzoyl peroxide ranked the most effective for both reductions in total lesion counts and IGA success, but this analysis did not include newer agents approved for acne treatment such as clascoterone and trifarotene [29].
A more recent network meta-analysis of the comparative efficacy of pharmacological treatments for acne included 221 RCTs evaluating 37 interventions, making it the largest study to date [30]. Following oral isotretinoin, combination therapies consisting of an oral or topical antibiotic, topical retinoid, and benzoyl peroxide were found to be the most effective. Among topical retinoids, tazarotene was ranked the second most effective therapy following topical isotretinoin for reductions in total lesions and was the most effective for reductions in noninflammatory lesions. For IGA treatment success, clascoterone was ranked the most effective among topical monotherapies, followed by tazarotene. Trifarotene was generally ranked lower across outcomes compared with tazarotene and clascoterone. The results of this analysis are consistent with those of the current three-treatment comparison. A primary distinction is that the previous analysis focused on comparisons between single-agent and combination therapies (both topical and oral) to oral isotretinoin, whereas the current analysis focused on a comparison of three topical monotherapies. Topical therapies are preferred as initial first-line therapies for acne, and comparisons between these may have wider applicability than comparisons to oral isotretinoin, which is typically reserved for severe, recalcitrant, nodular acne [1].
In the current analysis, the primary focus was on efficacy outcomes, and differences in safety between treatments were not assessed, which is a limitation of the study. It is notably more difficult to compare safety directly due to differences in the outcome measures used across studies. In the meta-analysis by Huang et al. [30], trifarotene and tazarotene had the highest ORs for discontinuation due to AEs, whereas clascoterone had the lowest OR for discontinuation. However, discontinuation rates were generally low across all three treatments; in clinical trials, AEs leading to discontinuation only occurred in 1.2% to 1.9% of patients who received trifarotene [20] and in 0.5% to 0.8% of patients who received clascoterone [19]. Additional, more detailed comparisons of safety outcomes between these agents will be necessary to determine whether significant differences exist.
Conclusion
There were no significant differences in efficacy between clascoterone, trifarotene, and tazarotene for either reductions in lesion counts or treatment success in the current study. Differences in dosing schedule, mechanism of action, and safety profile should also be taken into consideration when making treatment decisions in the clinical setting.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
References
Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90(5):1006.e1–e30.
Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–9.
Chen H, Zhang TC, Yin XL, Man JY, Yang XR, Lu M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: an analysis from the Global Burden of Disease Study 2019. Br J Dermatol. 2022;186(4):673–83.
Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):5754.
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96.
Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153(5):406–12.
Hazarika N, Archana M. The psychosocial impact of acne vulgaris. Indian J Dermatol. 2016;61(5):515–20.
Samuels DV, Rosenthal R, Lin R, Chaudhari S, Natsuaki MN. Acne vulgaris and risk of depression and anxiety: a meta-analytic review. J Am Acad Dermatol. 2020;83(2):532–41.
Layton AM, Thiboutot D, Tan J. Reviewing the global burden of acne: how could we improve care to reduce the burden? Br J Dermatol. 2021;184(2):219–25.
Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35(1):2298878.
Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone 17alpha-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412–8.
Bausch Health, Canada Inc. ARAZLO™ (tazarotene 0.045%) lotion. Product monograph. Laval: Bausch Health, Canada Inc.; 2021.
Galderma Canada Inc. AKLIEF™ (trifarotene 50 mcg/g) cream. Product monograph. Thornhill: Galderma Canada Inc.; 2019.
Allergan Inc. TAZORAC® (tazarotene cream 0.1%). Product monograph. Markham: Allergan Inc.; 1997.
Galderma Laboratories. AKLIEF® (trifarotene) cream, for topical use. Prescribing information. Fort Worth: Galderma Laboratories, LP; 2019.
Bausch Health US, LLC. ARAZLO™ (tazarotene) lotion, for topical use. Prescribing information. Bridgewater: Bausch Health US, LLC; 2020.
Sun Pharma Canada Inc. WINLEVI® (clascoterone cream 1%). Product monograph. Brampton: Sun Pharma Canada Inc.; 2023.
Sun Pharmaceutical Industries, Inc. WINLEVI® (clascoterone cream 1%). Prescribing information. Cranbury: Sun Pharmaceutical Industries, Inc.; 2023.
Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(6):621–30.
Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 µg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–9.
Tanghetti EA, Werschler WP, Lain T, Guenin E, Martin G, Pillai R. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19(1):70–7.
Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). 2023. Accessed 9 Feb 2024. www.training.cochrane.org/handbook.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). 2023. Accessed 9 Feb 2024. www.training.cochrane.org/handbook.
Veichtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48.
Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R package for the guide 'doing meta-analysis in R.' R package version 0.1.0. 2019. Accessed 9 Feb 2024. http://dmetar.protectlab.org/.
Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83(2):477–85.
Shi Q, Tan L, Chen Z, et al. Comparative efficacy of pharmacological and nonpharmacological interventions for acne vulgaris: a network meta-analysis. Front Pharmacol. 2020;11:592075.
Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild-to-moderate acne vulgaris: systematic review and network meta-analysis. Br J Dermatol. 2021;185(3):512–25.
Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21(4):358–69.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Dana Lengel, PhD, of AlphaBioCom, a Red Nucleus company, and funded by Sun Pharma.
Funding
No funding was received for this study. Medical writing support and the Rapid Service Fee were funded by Sun Pharma.
Author information
Authors and Affiliations
Contributions
Mahek Shergill, Muhammad Usman Ali, and Mohannad Abu-Hilal contributed to the study conception and design. Data collection and analysis were performed by Mahek Shergill and Mohammad Usman Ali. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Mahek Shergill and Mohammad Usman Ali report nothing to disclose. Mohannad Abu-Hilal received advisory board honoraria and consulting fees from AbbVie, Eli Lilly, Galderma, Hikma Pharmaceuticals, Janssen, LEO Pharma, L’Oréal, Medexus Pharma, Novartis, Pfizer, Sanofi, and Sun Pharma.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shergill, M., Ali, M.U. & Abu-Hilal, M. Comparison of the Efficacy of Clascoterone, Trifarotene, and Tazarotene for the Treatment of Acne: A Systematic Literature Review and Meta-Analysis. Dermatol Ther (Heidelb) 14, 1093–1102 (2024). https://doi.org/10.1007/s13555-024-01175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01175-3