Abstract
Introduction
There are several treatment options for plaque psoriasis (PsO), but uncertainty remains around the optimal sequencing of treatments. The aims of this study were to investigate how adopting a best-treatment-first treatment sequence impacts patient outcomes and healthcare systems and to quantify the cost of treatment failure to the healthcare system.
Methods
A 3-year state-transition treatment-sequencing model which identifies all possible treatment sequences in PsO was adapted to the Italian healthcare setting. Treatments considered in the model are those with European Medicines Agency marketing authorization and reimbursement in Italy as of December 2022. Italian market share data (2019–2021) and list prices (2022) informed the current prescribed sequences; these sequences were compared against all possible sequences to determine opportunities for improvement. Both the national perspective in Italy as well as the local perspective from seven regions were considered. The cost of treatment failure was informed through a questionnaire circulated to Italian dermatologists.
Results
Overall, 1284 possible treatment sequences are possible when four lines of treatment are considered for patients with moderate-to-severe PsO in Italy. Within the estimated range of treatment failures across those sequences (0.97–2.56 per patient over 3 years), current prescribing behavior from the national perspective suggests patients will face 1.44 failures on average; this highlights the potential for improvement. For every treatment failure, the cost borne by the Italian National Healthcare Service (NHS) is €676.80. Overall, prescribing more optimized treatment sequences results in a 22.95% reduction in failures with a 2.27% increase in costs. The regional analyses found similar trends.
Conclusions
Results suggest that selecting the most effective treatment sequences for incident patients provides the greatest opportunity to reduce treatment failures and maximize patient outcomes with a modest impact on costs. While regional variations exist, there is room for improvement across the board, which could translate to more efficient local healthcare systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There are several treatment options to treat plaque psoriasis, which may be combined in several sequences, but the optimal approach for sequencing treatment is poorly characterized. |
Furthermore, in a budget-constrained environment, prescribing restrictions are often introduced that only consider the costs of therapeutics and not the impact on patient outcomes or the “knock-on” cost and resource impact on the healthcare system resulting from restricted prescribing (i.e., where the use of effective treatments is disallowed early in the care pathway). |
What did the study ask?/What was the hypothesis of the study? |
The study aimed to assess the change in patient outcomes and healthcare budgets when shifting treatment prescribing from a cheapest-treatment-first approach to a best-treatment-first approach for plaque psoriasis in Italy. |
What was learned from the study? |
Results suggest that selecting the most effective treatment sequences for incident psoriasis patients in Italy could reduce treatment failures by 22.95% with just a 2.27% increase in costs. |
If physicians were free to prescribe the most effective treatments first in moderate-to-severe psoriasis, despite their cost, important health gains would be obtained with relatively modest increases in spending. |
Introduction
Psoriasis is a chronic, relapsing, immune-mediated skin disease. In Europe, the prevalence of psoriasis ranges from 0.73% in Scotland to 2.9% in Italy [1], which is roughly equal to 1,500,000 Italians [2].
Plaque psoriasis (PsO) is the most common type of psoriasis and is characterized by erythematous, scaly plaques that can be localized or present over extensive areas of the body [2]. The quality of life of patients may be greatly affected, with up to 50% experiencing feelings of deep helplessness and loneliness, increasing their risk of depression and suicide [3].
Multiple new and effective treatments with highly favorable safety profiles have achieved marketing authorization through the European Medicines Agency (EMA) in the last decade. In particular, these include biologic therapeutics targeting specific pathways involved in the pathogenesis of the disease, such as tumor necrosis factor (TNF), interleukin (IL)-17, IL-12/23, and IL-23 [4]. However, uncertainty remains around the optimal sequence of treatments and, crucially, which treatment(s) should be used first. Despite the many effective and safe options available, patients are often required to start with less costly but less effective treatments due to national and/or local treatment guidelines, which are typically driven by budget constraints.
The Optimized Patient Treatment Initiative (OPT-In) model was developed to help inform decision-making regarding likely optimal treatment sequences for seven immune-mediated diseases (ankylosing spondylitis, Crohn’s disease, non-radiographic axial spondyloarthritis, psoriatic arthritis, plaque psoriasis, rheumatoid arthritis, and ulcerative colitis) [5]. For this manuscript, we focus on PsO within the Italian setting.
In Italy, there are 20 regions, and each has the authority to recommend regional treatment pathways to meet their respective needs in accordance with reimbursement decisions made by Agenzia Italiana del Farmaco (AIFA, the Italian Medicines Agency). In turn, this results in variability regarding access to treatments across regions. We therefore aim to investigate how shifting treatment prescribing from a cheapest-treatment-first approach to a best-treatment-first approach can impact patient outcomes and healthcare budgets nationally for Italy as well as for seven of its most populous regions: Apulia, Campania, Lazio, Liguria, Lombardy, Tuscany, and Veneto.
Methods
A 3-year state-transition treatment-sequencing model was developed using the R programming language to calculate potential effectiveness improvements and budget reallocation considerations associated with implementing optimal sequences for seven immunological diseases. The treatment sequences for each disease included three or fewer biological or disease-modifying treatments, followed by best supportive care. Disease-specific response measures were selected based on clinical relevance, data availability, and data quality. Efficacy and treatment persistency were differentiated between biologic-naïve and -experienced populations, where possible, using published network meta-analyses (NMAs) and real-world data, respectively. All possible treatment sequences, based on country-specific reimbursement decisions, were simulated.
The detailed methodology, including all efficacy inputs, assumptions, calculations, and scenario analyses due to variations in inputs and assumptions for the overall model, has been described in full elsewhere [5–7]. For ease of reference, the following sections summarize the model’s objective and a schematic as well as the overall data inputs and assumptions related to PsO. This is followed by details of specific data inputs that are utilized to adapt the model to Italy and the seven regions in focus. Finally, the methodology used to estimate the cost of treatment failure in Italy is described.
This study did not involve any human subject, and no ethical approval was needed due to the secondary nature of data analysis.
Overview of the OPT-In Model and PsO Data Inputs
The approach used to develop this model was to first estimate the number of sequences that could be prescribed (due to the variety of treatments available for a given immunological condition) and then estimate the associated range of efficacy that would be achievable across all the possible sequences; this range is referred to as the ‘efficacy variation.’ Once this range was established, the model considered the market share in a given country to understand where current prescribing practice is positioned within the range of possible sequences. This allowed for an understanding of whether current prescribing practice was already at an optimal level, leveraging the most effective treatments first, or whether there was room for improvement [6]. If room for improvement was identified, the model estimated the efficacy and cost associated with the current prescribing practice and compared it to the efficacy and cost associated with optimized prescribing sequences. The impact of optimized prescribing on patient outcomes was measured as the average number of treatment failures avoided, and the impact on treatment expenditure was measured based on the difference in costs (e.g., the budget for therapeutics) due to the change in prescribing practice (i.e., from cheapest treatment first to best treatment first) [7].
Each treatment sequence included up to three lines of treatments, followed by best supportive care. For PsO, due to the large number of available treatments with European Medicines Agency (EMA) marketing authorization, a ‘blended’ fourth line of treatment was implemented in the sequence. To model the blended line, the efficacy inputs of all treatments not previously used in the first, second, or third lines were averaged and applied.
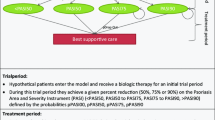
The transitions of patients through the model are summarized in Fig. 1. Patients were assumed to remain on treatment for as long as they responded to therapy, and response was defined as achieving a 90% improvement in Psoriasis Area and Severity Index score compared to baseline (PASI 90). In accordance with previous modeling in PsO, each treatment line is defined by two treatment phases: (i) induction as the starting period of treatment and (ii) a maintenance regimen from the end of the induction period until treatment failure. In alignment with clinical preference and systematic literature review findings, the PsO NMA was based on response data from the induction period; for the remaining time horizon of the model (the duration beyond the induction phase), published discontinuation and persistency data were used. The data are summarized in Table 1.
Model structure and data implementation. According to the model structure, patients remain on treatment for as long as they respond to therapy. Each treatment line is defined by two treatment phases: induction and the maintenance regimen from the end of the induction period until treatment failure. The NMA was based on response data from the induction period; for the remaining time horizon of the model (the duration beyond the induction phase), published discontinuation and persistency data were used. HTA health technology assessment, NMA network meta-analysis, RWE real-world evidence
We wished to investigate whether current prescribing practice in Italy was already at an optimal level, and, if current prescribing practice in Italy was not at an optimal level, we also wanted to determine the impact of optimized prescribing on patient outcomes and expenditure on therapeutics. To do this, Italian inputs were required. These are presented below.
Data Inputs for Italian Adaptation
To adapt this model to the Italian setting, five categories of Italian-specific data were required (Table 2).
These inputs are summarized in Tables 3, 4, and 5 and Fig. 2.
Cost of Treatment Failure in Italy
The cost associated with a patient failing PsO treatment (cost of switching) is not widely understood or well documented. Without this information, it is difficult to contextualize the value of avoiding treatment failures through improved prescribing patterns.
Therefore, to inform this key data input, seven experts were asked to complete an online questionnaire aimed at identifying the resources required to manage a treatment failure. The experts were Italian dermatologists specifically approached to represent each of the seven regions considered in the study. These data, together with the frequency of use and cost of each resource required, helped to inform an estimate of the cost associated with treatment failure. The questionnaire also gathered data on the average out-of-pocket expense the experts estimated was incurred by patients (e.g., to purchase the over-the-counter topical treatments needed to manage a failure). Table 6 summarizes the information gathered from the questionnaire.
Results
Efficacy Variation in PsO and Italian Prescribing Practice
Overall, 1284 possible PsO treatment sequences were identified after considering EMA approval and AIFA reimbursement status. Figure 3 shows the variation in terms of the average number of failures associated with each sequence, ranging from the lowest average number of treatment failures (green) to the highest average number of failures (red). The most ineffective sequence would result in a PsO patient facing more than two treatment failures (2.56) on average over a 3-year period, while the most effective treatment sequence would result in the patient facing less than one failure (0.97).
Number of treatment failures. Average number of treatment failures (triangles) from the most to the least effective sequence in Italy and all seven regions, based on a treatment target of PASI 90. Red indicates the least efficacious sequence, green the most efficacious sequence, and blue the current practice average
The current prescribing practice for Italy, as determined by market share data, is mapped onto the range of efficacy (as a blue triangle) to assess how current practice compares with the possible outcomes.
The analysis suggests that current prescribing practice results in more than one failure (1.44), on average, in Italy over 3 years. The difference (i.e., the gap) between the blue triangle and the bottom of the bar represents the opportunity for improvement; for Italy, this suggests there is considerable room for improvement from the perspective of patient outcomes.
Outcomes for current prescribing practice in the seven regions of Italy considered are presented along the vertical axis on the right in the figure and reflect the overall national pattern in Italy, with the current prescribing practice resulting in an average number of treatment failures over 3 years ranging from 1.39 to 1.52. Taken together, these findings indicate that improvements that would benefit patients can be made across the board.
Optimized Treatment Sequences
To better understand and consider options for improvement, the current prescribing practice relative to the 1284 possible treatment sequences was assessed in greater detail. Figure 4 plots each of the possible sequences (each identified by the treatment used as the first line) according to the efficacy they can achieve based on a treatment target of PASI 90 versus the average drug cost associated with each. The point at which the dotted lines intersect represents the current prescribing practice for Italy overall (the blue triangle in Fig. 3).
All possible treatment sequences plotted based on efficacy and cost. The 1284 possible sequences are represented as points with various shapes on the cost–effectiveness plane. The shape depends on the mechanism of action that is employed as the first line. The sequences are plotted according to the efficacy they can achieve based on a treatment target of PASI 90 versus the average drug cost associated with each. IL interleukin, MOA mechanism of action, PDE4 phosphodiesterase-4, TNF tumor necrosis factor
Figure 4 suggests there are a multitude of more efficacious treatment sequences to choose from compared to the current prescribing practice. Some options—those with similar levels of efficacy to the current practice—leverage TNF inhibitors as the first-line treatment within the sequence; however, more efficacious sequences leverage IL-17 inhibitors, and the most efficacious sequences leverage IL-23 inhibitors as first-line options.
Table 7 provides greater detail on the most efficacious treatment sequences by listing the treatment mechanisms of action (MOAs), by line of treatment, for the five most effective sequences and the corresponding average number of expected failures over a 3-year period. These results highlight that, in all cases, the MOAs used in the first-line treatments of the most efficacious sequences align with the MOA of the therapy with the best relative response rates from the literature [manuscript pending submission]. These results support the adoption of the most efficacious treatments early in the treatment pathway and therefore a ‘best-treatment-first’ approach in order to improve patient outcomes.
In light of these findings, it is important to consider the impact of a shift from the current prescribing practice to optimized prescribing on treatment expenditures. Figure 4 provides a top-line understanding of treatment costs associated with various sequences and showcases that more efficacious options with similar or increased costs are apparent.
An important additional consideration is the cost associated with the management of treatment failure. Improved efficacy through optimized prescribing may impact costs due to earlier use of more efficacious treatments compared to current prescribing; however, cost savings due to avoiding treatment failures must also be considered.
The information gathered from the questionnaire completed by expert dermatologists resulted in a greater understanding of the key cost components associated with managing each treatment failure:
-
Cost associated with visits to healthcare professionals: €166.17 per failure
-
Cost associated with additional laboratory tests: €89.24 per failure
-
Cost associated with additional treatments: €421.38 per failure.
Therefore, the total cost borne by the Italian NHS for every treatment failure faced by a PsO patient is estimated, at a minimum, to be €676.80 per failure.
Furthermore, the questionnaire highlighted an additional, often overlooked, cost: out-of-pocket costs incurred by patients. Based on output from the questionnaire, it was estimated PsO patients spend €203.90 out of pocket to purchase over-the-counter topical products per treatment failure, in addition to the resources provided by the Italian NHS. This brings the total cost, combining NHS and private spending, to at least €880.69 per failure.
Therefore, the adoption of optimized prescribing resulting in savings of €676.80 to €880.69 per treatment failure avoided should also be considered. Current prescribing practice patterns cost the Italian healthcare system €24,366,675 (3-year time horizon) through the management of treatment failures alone, considering the prevalent PsO population from 2021 and an average of 1.44 failures per patient over 3 years. By shifting to prescribing patterns that represent the 20% most efficacious sequences, the model suggests that treatment failures would be reduced by 8,264 units, translating to savings of €5,593,039 over 3 years for the management of failures alone.
Impact of Optimized Prescribing in Italy
Under the assumption of the adoption of optimized prescribing in Italy, Fig. 5 shows the predicted impact on the estimated total number of failures over 3 years compared to anticipated outcomes resulting from the current practice. Importantly, the optimized practice model recognizes that clinicians require a level of flexibility when selecting treatments given the variability in patient needs; therefore, optimized practice assumes that prescribing would shift to a mix of the 20% most efficacious sequences. Results suggest that, over 3 years, 22.95% (from 36,003 to 27,739) of the treatment failures could be prevented by optimizing practice by leveraging the most efficacious treatment sequences. Similar findings were observed across each of the seven regions in Italy considered in the analysis (see the Electronic Supplementary Material).
Cumulative difference in the total number of treatment failures between the current practice and the optimized practice in Italy. Current-practice market shares are derived from the period 2019–2021, whilst the optimized practice was set up according to the discussed assumptions on optimized prescribing behavior
Table 8 shows the projected impact on expenditures resulting from adopting optimized prescribing in terms of cumulative costs for the full patient population over the 3-year time horizon (2022–2024). This summary also includes anticipated cost reductions resulting from avoided treatment failures, which contribute to offsetting the costs of increased prescribing of the most efficacious therapies. Over 3 years, a 2.27% increase in expenditure would result from adopting optimized prescribing based on this model. Similar outcomes would also be expected for each of the seven regions included in the analysis (see the Electronic Supplementary Material).
Discussion
The analysis presented here was conducted to investigate how adopting optimized, or “best-treatment-first,” treatment sequences impacts patient outcomes and the healthcare system in Italy. By further investigating and accounting for the cost associated with the management of treatment failures to the healthcare system, this analysis translates the value of avoided treatment failures to a metric that is relevant to the Italian NHS.
Our findings demonstrate that adopting optimized prescribing practices by leveraging more efficacious treatments earlier in the course of therapy leads to better outcomes for patients. For a chronic condition such as PsO, this allows for greater opportunities to minimize treatment failures while maximizing benefits to patients. In turn, this model helps to identify areas for improvement in current prescribing practice. The results presented here show that prioritizing the 20% most effective sequences, which accounts for varying patient needs, could lead to a 22.95% reduction in treatment failures with a modest 2.27% increase in expenditures.
In today’s environment of increasing pressures on healthcare budgets, treatment costs—rather than patient outcomes—often become the primary driver for treatment choice, especially for inflammatory immune-mediated diseases such as PsO, for which several treatment options are available. For example, some Italian regions impose prescribing constraints on dermatologists to contain drug costs; such constraints can include mandating the compulsory use of the least expensive yet less effective treatments for PsO. In recent years, understanding of the pathogenesis of PsO (in particular the role of the IL-23/Th17 pathway) has deepened, prompting the development of new innovative classes of biologics aimed at modulating the underlying molecular drivers of disease [35]. Within the context of healthcare budgetary pressures due to limited and strained resources, the differentiated value of innovative biologics, leading to better outcomes for patients, can easily be lost. While cost-containment policies might produce short-term savings, they heavily compromise long-term patient outcomes and will, in turn, have an overall negative impact on the NHS in the long term.
As described above, this analysis found that the most effective treatment sequences are based on using the most effective individual therapies early in the course of treatment (i.e., as first-line therapy); for PsO, the model identified sequences initiating with IL-23 inhibitors as the optimal first-line treatments. These results are in line with clinical data that support the notion that IL-23 inhibitors have a great immunomodulatory capability and allow long-term control of skin inflammation [36], given the role of IL-23 as a regulatory cytokine. As such, it has been suggested that IL-23 inhibitor treatment for PsO should be initiated in a timely manner and as early as possible [36].
Note that untreated or poorly treated PsO may evolve through progressive phases [36]. In addition to the worsening of signs and symptoms, several comorbidities, in particular psoriatic arthritis (PsA) [37,38,39], have been associated with PsO. Furthermore, it has been suggested that treatment with the newer, more innovative biologics may be associated with a lower risk of PsA incidence [36]. From a disease management perspective, PsO has also been associated with several other comorbidities, including intestinal bowel diseases [40], metabolic syndrome [41], diabetes mellitus [42], as well as cardio- and cerebrovascular diseases [43, 44]. From a patient perspective, PsO has been associated with a cumulative burden on patients’ psychological and social well-being [36, 45,46,47,48,49]. In this context, it has been shown that PsO may impact health-related quality of life to an extent similar to cancer and cardiovascular diseases [50].
New innovative biologics have demonstrated the ability to achieve substantial levels of skin clearance (e.g., PASI 90 and PASI 100) and lead to significant improvements in patients’ quality of life [36]. Increasing evidence suggests that early initiation of newer biologics could beneficially affect the clinical course of PsO at the molecular and genetic levels by preventing comorbidities, particularly PsA, and by improving quality of life and decreasing the cumulative impairment of patients’ lives [36]. From the clinical perspective, the authors believe there are limited possibilities to effectively treat PsO patients if less effective treatments are first initiated, especially when more effective options are readily available and their value is recognized by EMA and AIFA. At the very least, for patients with severe disease, it would seem appropriate to consider the early use of the latest generation of biologic treatments from both the clinical and economic resource perspectives.
Data on management of treatment failures in PsO and the associated costs are sparse, despite their implications for the utilization of of healthcare resources and impact on healthcare systems. Guerriero and colleagues [51] performed a retrospective analysis of administrative databases in southern Italy and found that the management of patient switching to another drug (where switching was considered a proxy for treatment failure, similar to in our study) resulted in a 30% increase in additional expenditures compared to patients who remained on treatment. To validate this finding and add more granularity to the actual costs, we collaborated with practicing expert dermatologists from northern, central, and southern Italy. Their input provided a breakdown of the various resource components required to manage a treatment failure, which ultimately included additional visits to healthcare professionals, laboratory tests, and additional treatments to gain short-term relief and disease control while patients waited to initiate their next line of treatment. The expert dermatologists further highlighted additional out-of-pocket costs for patients to cover over-the-counter topical treatments. Their insights resulted in an estimated increase in direct healthcare costs to the Italian NHS of €676.80 per patient per treatment failure; when out-of-pocket costs are considered, an additional €203.90 per patient per treatment failure can be factored in, bringing the predicted total cost of managing each individual PsO treatment failure to €880.69.
Budget constraints most often lead to prescribing restrictions because the short-term impact on expenditures for therapeutics is a relatively tangible and immediate result to track. However, the impacts of more treatment failures deriving from inadequate early-line treatment on the patient, the progression of disease, the healthcare system, and society as a whole are completely overlooked. By highlighting the substantial costs of managing treatment failures, we can begin to understand the wider and longer-term implications related to treatment choice for patients with a chronic condition such as PsO.
This study is not without limitations. Those from a methodology perspective are discussed in detail elsewhere [5]. From the perspective of the Italian case study presented here, it is important to flag that the model considers only a 3-year time horizon, which is not necessarily in line with the lifelong, chronic nature of PsO and the time dedicated by clinicians to managing it. However, as the objective of this study was to highlight the impact of treatment choice on patient outcomes and healthcare system costs, which are often restricted due to budget considerations, the 3-year timeframe aligns with usual budgetary planning timelines. Another limitation to consider is that the cost of treatment failure was determined based on a survey of a limited number of dermatologists; in future, the collection of more precise estimates that consider a broader range of stakeholders and data sources would add value to this analysis. Finally, the results of this analysis should be interpreted bearing in mind that there are variations in reimbursement and healthcare system costs in each country.
Conclusion
Our analysis suggests that, in Italy, there is a substantial opportunity to improve outcomes for patients through shifts in prescribing practice when managing PsO. Treatment sequences that leverage the most effective classes of therapy early in the course of treatment yield the best overall outcomes. When clinicians are given the freedom to prescribe based on their consideration of the best options, there are potentially impactful benefits to the patient, the NHS, and society as a whole. Additionally, avoiding treatment failures by shifting prescribing practice at a modest increase in cost is an important new perspective to consider. However, the short-term nature of this model does not fully capture the wider and longer-term benefits of optimized prescribing, which could help further minimize or neutralize the impact on budgets. This analysis provides valuable insight into potential differences between the current practice and a model for optimized practice in Italy that could assist policymakers and budget holders with implementing more effective prescribing practices that could reduce treatment failures and maximize patient outcomes with a modest impact on healthcare system resources.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85.
Ministero della Salute. Linea guida sulla psoriasi, presentato aggiornamento coordinato dall’ISS. 2013. https://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=null&id=1072. Accessed 2 Feb 2023.
Bardazzi F, Bonci C, Sacchelli L, et al. Suicide risk and depression in patients with psoriasis. Ital J Dermatol Venerol. 2022;157:497–501.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156:258–69.
Hart RJ, Hassan F, Alulis S, et al. Modelling treatment sequences in immunology: optimizing patient outcomes. Adv Ther. 2024. https://doi.org/10.1007/s12325-023-02766-w
Boer JH, Hassan F, Alulis S, Lee J, Lee D. Analysis of treatment sequences across seven immunological diseases and the variability in efficacy for patients per disease: opportunities for improvement. 2021. https://www.ispor.org/docs/default-source/euro2021/posc319-analysis-of-treatment-sequences-5nov21-pdf.pdf?sfvrsn=45cc4839_0. Accessed 18 Jan 2023.
Alulis S, Hassan F, Lee J, et al. The optimized patient treatment initiative (OPT-IN): choosing the right therapy, for the right patient, at the right time. ISPOR (International Society For Pharmacoeconomics and Outcomes Research). 2022. https://www.ispor.org/heor-resources/presentations-database/presentation/euro2022-3568/120331. Accessed 20 Jul 2023.
Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–17.
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017;76:418–31.
National Institute of Health and Care Excellence. Risankizumab for treating moderate to severe plaque psoriasis. Technology appraisal guidance [TA596]. 2019. https://www.nice.org.uk/guidance/ta596. Accessed 21 July 2023.
Lunder T, Zorko MS, Kolar NK, et al. Drug survival of biological therapy is showing class effect: updated results from Slovenian National Registry of psoriasis. Int J Dermatol. 2019;58:631–41.
IQVIA. Syndicated data: Italian market share and incident / prevalent population data. 2022.
IQVIA. IQVIA Use By Indication (UBI). https://www.iqvia.com/locations/united-kingdom/library/fact-sheets/iqvia-use-by-indication. Accessed 20 July 2023.
AIFA. Humira - siringhe preriempite/fiala + siringa (Adalimumab). Codifa. https://www.codifa.it/farmaci/h/humira-siringhe-preriempite-fiala-e-siringa-adalimumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Amgevita - siringhe preriempite/fiala + siringa (Adalimumab). Codifa. https://www.codifa.it/farmaci/a/amgevita-adalimumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Imraldi - soluzione (uso interno) (Adalimumab). Codifa. https://www.codifa.it/farmaci/i/imraldi-soluzione-uso-interno--adalimumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Hyrimoz - siringhe preriempite/fiala + siringa (Adalimumab). Codifa. https://www.codifa.it/farmaci/h/hyrimoz-adalimumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Idacio - soluzione (Adalimumab). Codifa. https://www.codifa.it/farmaci/i/idacio-soluzione-adalimumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Cimzia - soluzione (uso interno) (Certolizumab Pegol). Codifa. https://www.codifa.it/farmaci/c/cimzia-soluzione-uso-interno--certolizumab-pegol-immunosoppressori. Accessed 21 July 2023.
AIFA. Enbrel - soluzione (Etanercept). Codifa. https://www.codifa.it/farmaci/e/enbrel-soluzione-etanercept-immunosoppressori. Accessed 21 July 2023.
AIFA. Benepali - soluzione (uso interno) (Etanercept). Codifa. https://www.codifa.it/farmaci/b/benepali-soluzione-uso-interno-etanercept-immunosoppressori. Accessed 21 July 2023.
AIFA. Erelzi - soluzione (Etanercept). Codifa. https://www.codifa.it/farmaci/e/erelzi-etanercept-immunosoppressori. Accessed 21 July 2023.
AIFA. Remicade - Polvere (Infliximab). Codifa. https://www.codifa.it/farmaci/r/remicade-infliximab-immunosoppressori. Accessed 21 July 2023.
AIFA. Inflectra - preparazione iniettabile (Infliximab). Codifa. https://www.codifa.it/farmaci/i/inflectra-infliximab-immunosoppressori. Accessed 21 July 2023.
AIFA. Remsima - preparazione iniettabile (Infliximab). Codifa. https://www.codifa.it/farmaci/r/remsima-preparazione-iniettabile-soluzione-uso-interno--infliximab-immunosoppressori. Accessed 21 July 2023.
AIFA. Flixabi - preparazione iniettabile (Infliximab). Codifa. https://www.codifa.it/farmaci/f/flixabi-infliximab-immunosoppressori. Accessed 21 July 2023.
AIFA. Stelara - soluzione (uso interno) (Ustekinumab). Codifa. https://www.codifa.it/farmaci/s/stelara-preparazione-iniettabile-soluzione-uso-interno--ustekinumab-antipsoriasici. Accessed 21 July 2023.
AIFA. Tremfya - siringhe preriempite/fiala + siringa (Guselkumab). Codifa. https://www.codifa.it/farmaci/t/tremfya-guselkumab-antipsoriasici. Accessed 21 July 2023.
AIFA. Skyrizi - soluzione (Risankizumab). Codifa. https://www.codifa.it/farmaci/s/skyrizi-risankizumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Ilumetri - soluzione (Tildrakizumab). Codifa. https://www.codifa.it/farmaci/i/ilumetri-soluzione-tildrakizumab-antipsoriasici. Accessed 21 July 2023.
AIFA. Kyntheum - soluzione (Brodalumab). Codifa. https://www.codifa.it/farmaci/k/kyntheum-brodalumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Taltz - soluzione (uso interno) (Ixekizumab). Codifa. https://www.codifa.it/farmaci/t/taltz-soluzione-uso-interno--ixekizumab-immunosoppressori. Accessed 21 July 2023.
AIFA. Cosentyx - soluzione (uso interno) (Secukinumab). Codifa. https://www.codifa.it/farmaci/c/cosentyx-soluzione-soluzione-uso-interno--secukinumab-antipsoriasici. Accessed 21 July 2023.
AIFA. Otezla - compresse rivestite (Apremilast). Codifa. https://www.codifa.it/farmaci/o/otezla-apremilast-immunosoppressori. Accessed 21 July 2023.
Yang K, Oak ASW, Elewski BE. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am J Clin Dermatol. 2021;22:173–92.
Bellinato F, Chiricozzi A, Piaserico S, Targher G, Gisondi P. Could targeted pharmacotherapies exert a “disease modification effect” in patients with chronic plaque psoriasis? Int J Mol Sci. 2022;23:12849.
Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251.e19–65.e19.
Masson Regnault M, Konstantinou M-P, Khemis A, et al. Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol. 2017;31:1491–6.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318–28.
Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561–5.
Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
Ikumi K, Odanaka M, Shime H, et al. Hyperglycemia Is associated with psoriatic inflammation in both humans and mice. J Invest Dermatol. 2019;139:1329–38 (e7).
Hjuler KF, Böttcher M, Vestergaard C, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. 2015;128:1325-1334.e2.
Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2: e000062.
Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542–51.
Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984–91.
Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891–5.
Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
Schubert A, Villacorta R, Davila J, Cordon A, Afonso N. PSY7 Indirect cost burden of moderate-to-severe psoriasis in EU5 and the savings generated by choosing Guselkumab over Adalimumab. Value Health. 2019;22:S902.
Møller AH, Erntoft S, Vinding GR, Jemec GB. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167–77.
Guerriero F, Orlando V, Monetti VM, Russo V, Menditto E. Biological therapy utilization, switching, and cost among patients with psoriasis: retrospective analysis of administrative databases in Southern Italy. Clinicoecon Outcomes Res. 2017;9:741–8.
Medical Writing and Editorial Assistance
Publishing support and journal styling services were provided by Laura Fascio Pecetto (SEEd Medical Publishers, Torino) and funded by Janssen-Cilag Ltd. Statistical analyses were provided by Orietta Zaniolo and Lorenzo Pradelli (AdRes Health Economics & Outcomes Research, Torino) and funded by Janssen-Cilag Ltd.
Funding
The model was built by Janssen-Cilag Ltd, which also funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Conceptualization: Fareen Hassan, Sarah Alulis; data curation: Pier Cesare Francesa Morel, Ottavio Secchi; formal analysis: Fareen Hassan, Sarah Alulis, Pier Cesare Francesa Morel, Ottavio Secchi; funding acquisition: Ottavio Secchi; investigation: Fareen Hassan, Sarah Alulis, Pier Cesare Francesa Morel, Ottavio Secchi; methodology: Fareen Hassan, Sarah Alulis; project administration: Pier Cesare Francesa Morel; resources: Fareen Hassan, Sarah Alulis; software: Fareen Hassan, Sarah Alulis; supervision: Pier Cesare Francesa Morel; validation: Fareen Hassan, Sarah Alulis; visualization: Pier Cesare Francesa Morel; writing—original draft preparation: Pier Cesare Francesa Morel; writing—review & editing: Sarah Alulis, Nicoletta Bernardini, Martina Burlando, Antonio Costanzo, Pier Cesare Francesa Morel, Paolo Gisondi, Francesco Loconsole, Matteo Megna, Giovanni Pellacani, Stefano Piaserico, Francesca Prignano, Ottavio Secchi, Nevena Skroza, Fareen Hassan.
Corresponding author
Ethics declarations
Conflict of Interest
Sarah Alulis, Fareen Hassan, Pier Cesare Francesa Morel, and Ottavio Secchi are employees of Janssen-Cilag. Sarah Alulis and Fareen Hassan are also shareholders of Janssen-Cilag. Nicoletta Bernardini served as an advisory board member and consultant for AbbVie, Almirall, Boehringer Ingelheim, LEO Pharma, Lilly, Janssen, Novartis, and UCB. Martina Burlando acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, and UCB Pharma. Antonio Costanzo has served as an advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, LEO Pharma, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. Paolo Gisondi is an editorial board member of Dermatology and Therapy. Paolo Gisondi was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Paolo Gisondi served as a speaker for Amgen, Almirall, AbbVie, Eli Lilly, Novartis, UCB, Sanofi, LEO pharma, and Jannsen. Francesco Loconsole has no conflict of interest outside the submitted work. Matteo Megna acted as a speaker or consultant for AbbVie, Eli Lilly, LEO Pharma, Novartis, UCB, Janssen, and Almirall. Giovanni Pellacani acted as scientific consultant/speaker/clinical study investigator for AbbVie, Jansenn, LEO Pharma, Lilly, Novartis, Pfizer, and UCB. Stefano Piaserico acted as a scientific consultant/speaker/clinical study investigator for AbbVie, Celgene, Galderm, Janssen, LEO Pharma, Lilly, MSD, Novartis, and Pfizer. Francesca Prignano served as an advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, LEO Pharma, Lilly, Janssen, Novartis, Biogen, Sanofi Genzyme, UCB, and Boehringer Ingelheim. Nevena Skroza has participated in clinical trials, as a member of the advisory board, and as a consultant on behalf of AbbVie, Boehringer Ingelheim, Galderma, Janssen, LEO Pharma, L'Oréal, Novartis, and Sanofi.
Ethical Approval
This study did not involve any human subject and no ethical approval was needed due to the secondary nature of data analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alulis, S., Bernardini, N., Burlando, M. et al. OPT-In; Optimized Patient Treatment Outcomes in Plaque Psoriasis: A 3-Year State-Transition Treatment-Sequencing Model in the Italian Setting. Dermatol Ther (Heidelb) 14, 1273–1291 (2024). https://doi.org/10.1007/s13555-024-01170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01170-8