Abstract
Introduction
Vitiligo is a chronic autoimmune disease characterized by destruction of melanocytes, leading to skin depigmentation. Vitiligo can have a high quality-of-life burden and profound impact on psychosocial well-being. The objectives of this study were to describe the self-reported patient burden among patients with nonsegmental vitiligo with ≤ 10% affected body surface area, summarize the physician-reported psychosocial and psychological impact of vitiligo on patient lives, and describe disease characteristics and treatment history, goals, and satisfaction.
Methods
Data were drawn from the Adelphi Vitiligo Disease Specific Programme™, a real-world, cross-sectional survey with retrospective data collection of physicians and patients with vitiligo, collected in the United States between October 2021 and April 2022. Separate surveys for dermatologists and patients contained questions on clinical and demographic characteristics of patients with vitiligo and burden of vitiligo. Treatment history, goals, and satisfaction were assessed together with the impact of vitiligo on quality of life.
Results
Sixty-one dermatologists provided data for 326 patients with ≤ 10% affected body surface area (adults, n = 221; adolescents, n = 105); 90 of those patients also responded to the survey. The most common treatments were topical corticosteroids, topical calcineurin inhibitors, and narrow-band ultraviolet-B phototherapy, with the main treatment goal being repigmentation. Physician-reported treatment satisfaction was 56%; 25% of patients reported frustration with treatment options. Physicians reported impact of vitiligo on everyday life in 46% of patients. Patients reported 12.7% overall work impairment; mean scores for Hospital Anxiety and Depression Scale anxiety and depression domains were 3.5 and 2.2, respectively, and mean Vitiligo-specific Quality of Life index score was 26.9. Patients with facial involvement experienced higher burden than those without.
Conclusion
A high patient burden was reported by dermatologists and their patients with vitiligo who had ≤ 10% affected body surface area, including psychosocial and psychological consequences. These findings highlight an unmet need in the treatment of vitiligo.
Plain Language Summary
Vitiligo is a chronic disease in which cells that produce the skin pigment melanin are attacked, causing patches of skin to lose color and become pale. Vitiligo can have emotional impacts such as social or psychological distress that can affect the day-to-day well-being of individuals. However, there is a lack of studies that assess the ways that vitiligo affects the everyday lives of people with the condition in the United States. Dermatologists and people with vitiligo answered survey questions on treatment goals, any vitiligo treatments currently and previously used, and how satisfied they were with the results of treatment. The surveys also contained questions that assessed the impact of vitiligo on everyday life. Sixty-one dermatologists answered questions about 326 patients and 90 of those patients also provided their own answers to the survey questions. Both dermatologists and patients reported that restoring color to patches of pale skin was their goal in treating vitiligo. However, dermatologists and patients both reported that they were dissatisfied with the results of available treatments. Dermatologists and patients both reported that vitiligo impacted aspects of everyday life. Emotional and psychological impacts such as anxiety and depression were reported, as well as negative effects on patients’ work and social lives due to vitiligo. These results confirm that vitiligo impacts the day-to-day well-being of patients. Furthermore, this study highlights that there is a need for improvements in the treatment of vitiligo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Vitiligo, a chronic autoimmune disease characterized by destruction of melanocytes and skin depigmentation, can have a high quality-of-life burden and profound impact on psychosocial well-being. |
Real-world studies examining the burden of vitiligo on US patients are lacking. |
The objective of this study was to assess the self-reported and physician-reported burden of disease among US patients with nonsegmental vitiligo. |
What was learned from the study? |
A high patient burden was reported by dermatologists and patients with nonsegmental vitiligo, including psychosocial and psychological consequences. |
These findings suggest a high unmet treatment need in the management of nonsegmental vitiligo. |
Introduction
Vitiligo is a chronic autoimmune disease of the skin that targets melanocytes, causing patches of skin depigmentation [1]. The diagnosed prevalence of vitiligo is estimated to be approximately 0.1–1.5% of the US population (approximately 1.9 million diagnosed US patients), with estimates suggesting that up to 40% of US adults with vitiligo may be undiagnosed [2, 3]. Most patients with vitiligo have lesions affecting ≤ 10% of their body surface area (BSA), with the face being a commonly affected region [4].
A high level of clinical and emotional burden has been previously associated with vitiligo. Studies have documented a high prevalence of low self-esteem [5, 6], social isolation, stigma [7, 8], disease-specific discrimination [9, 10], and anxiety, hopelessness, depression, and depressive symptoms [11] among patients with vitiligo. Patients report feeling stigmatized and experience unnecessary negative and antagonistic attention in public, which results in them feeling ostracized. Furthermore, the emotional distress patients with vitiligo experience has been shown to negatively impact their employment, cause social withdrawal, and lead to substance use disorders [12]. In dermatologic diseases, including atopic dermatitis and psoriasis, the presence of lesions in visible areas (e.g., face) has been associated with particularly high disease burden [13, 14].
There is currently no cure for vitiligo and a historical lack of efficacious treatment, leaving patients and physicians frustrated and dissatisfied with available treatment options [15]. In July 2022, ruxolitinib cream was approved by the US Food and Drug Administration (FDA) for the treatment of nonsegmental vitiligo (NSV) in patients aged 12 years and older, for application to lesions affecting ≤ 10% BSA [16]. This approval marks the first pharmacologic treatment approved for repigmentation of NSV and augments the current treatment landscape for the disease [17]. Understanding the disease burden and real-world implications across the spectrum of NSV, not solely among those with higher BSA involvement, is necessary to guide the future evaluation and further research of FDA-approved therapies and products in development for the treatment of NSV.
The objectives of this study were to describe the self-reported patient burden among patients with NSV affecting ≤ 10% BSA, who constitute the majority of real-world patients with vitiligo and would be candidates for ruxolitinib cream treatment. We summarize the physician-reported impact of vitiligo on their patients’ lives, focusing on psychosocial and psychological consequences among patients with and without facial involvement and describe patients’ disease characteristics and treatment history, goals, and satisfaction.
Methods
Study Design
This is a secondary analysis of data from the Adelphi Vitiligo Disease Specific Programme™ (DSP) collected in the United States between October 2021 and April 2022, before the FDA approval of ruxolitinib cream for the treatment of NSV. For these analyses, only patients who had NSV with ≤ 10% affected BSA were included.
Data Source
The Vitiligo DSP is a large, multinational, cross-sectional survey with retrospective data collection, including dermatologists and their patients presenting with NSV in a real-world clinical setting. The DSP describes current disease management, disease burden impact, and associated treatment effects, both clinical- and dermatologist-perceived. A complete description of the survey methodology has been previously published and validated [18,19,20].
A geographically diverse sample of dermatologists was recruited to participate in the DSP, which received ethics exemption from the Pearl Institutional Review Board (study protocol number #21-ADRW-122) based on the use of aggregated and de-identified patient data. Dermatologists were eligible to participate in the survey if they were personally responsible for treatment decisions and management of patients with vitiligo and had a minimum monthly workload of 6 patients diagnosed with NSV. Patients were eligible for inclusion in the Vitiligo DSP if they were aged ≥ 18 years (adult population) or between 12 and 17 years (adolescent population), had a physician-confirmed diagnosis of NSV, were not involved in ongoing clinical trials, and visited the dermatologist for consultation. Both physicians and patients consented to take part in the research.
Dermatologists were instructed to complete a patient record form (PRF) for their next 6 consecutively consulted patients who visited for routine care. This form contained detailed information including patient demographics, disease characteristics (including affected BSA and facial involvement), treatment history and satisfaction with current treatment regimen, and disease control achieved. Facial involvement was defined as vitiligo affecting the head and neck area including eyes, ears, scalp, and the rest of the face. Completion of the patient record form was undertaken through consultation of existing patient clinical records, as well as the judgment and diagnostic skills of the respondent physician, which is entirely consistent with how decisions are made in routine clinical practice. It should be noted that the survey was designed to facilitate understanding of real-world clinical practice, and thus dermatologists could only report on data they had at hand at the time of the consultation. Therefore, data collected represent the evidence that physicians hold when making any clinical treatment and other management decisions at the time of consultation.
All data were collected in such a way that patients and dermatologists could not be directly identified; all data were aggregated and de-identified before receipt. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act of 1996 [21] and Health Information Technology for Economic and Clinical Health Act legislation [22]. Each patient for whom the dermatologist completed a form was then invited to voluntarily complete a patient self-completed questionnaire (PSC). PSCs were completed by patients independently from their dermatologist and were returned in a sealed envelope, ensuring the patient response was kept confidential from their dermatologist. The PSC contained detailed questions on current symptomatic burden, disease characteristics (including comorbid conditions), heath-related quality of life, level of satisfaction with their treatment regimen and disease control achieved, over-the-counter therapies, and other patient-reported outcomes. PSCs included the Work Productivity and Activity Impairment (WPAI) questionnaire (completed by adult patients only), the Hospital Anxiety and Depression Scale (HADS), and the Vitiligo-specific Quality of Life instrument (VitiQoL). The WPAI measures time missed from work (absenteeism), impairment at work (presenteeism), and impairment of regular activities. WPAI scores are reported as percentage impairment due to the disease [23]. The HADS score details symptoms of anxiety and depression, using a 0–3 scale across 7 questions [24]. For HADS, a total score ≥ 8 out of a possible 21 indicates symptoms of anxiety or depression [24]. The VitiQoL is a 15-question patient-reported outcome measure assessing vitiligo-specific aspects of life quality using a 7-point Likert scale (0–6), which provides a scoring scale of 0–90; higher scores indicate poorer quality of life [25].
Data Analysis
For the analysis of patient characteristics, mean, standard deviation, and range were calculated for continuous variables, and frequency counts and percentages for categorical variables. All analyses were conducted in Stata v16 (StataCorp, Texas, USA) [26]. Missing data were not imputed. Data were analyzed using descriptive statistics.
Results
Study Population and Demographics
In total, 61 US physicians (all dermatologists) completed PRFs for 326 patients with NSV who had ≤ 10% affected BSA. Of these patients, 221 were adults (≥ 18 years) and 105 were adolescents (12–17 years; Table 1). Mean (SD) age of all patients was 30.7 (16.4) years, and 199 (61%) had Fitzpatrick skin types I–III. Mean (SD) time since diagnosis was 27.2 (40.8) months, with a mean (SD) affected BSA of 7.6% (5.7%) at diagnosis and 5.6% (3.1%) at time of analysis. Overall, 152 patients (47%) had facial involvement; characteristics for patients with facial involvement are detailed in Table 1.
A total of 90 patients who had NSV with ≤ 10% affected BSA completed a PSC, of whom 42 (47%) patients had facial involvement. Mean (SD) age of patients completing a PSC was 33.8 (15.9) years, and 65 (72%) of these patients had Fitzpatrick skin types I–III. Mean time since diagnosis was 30.6 (60.0) months, with a mean (SD) affected BSA of 7.3% (4.4%) at diagnosis and 5.5% (2.7%) at time of analysis.
Physician-Reported Regions Affected
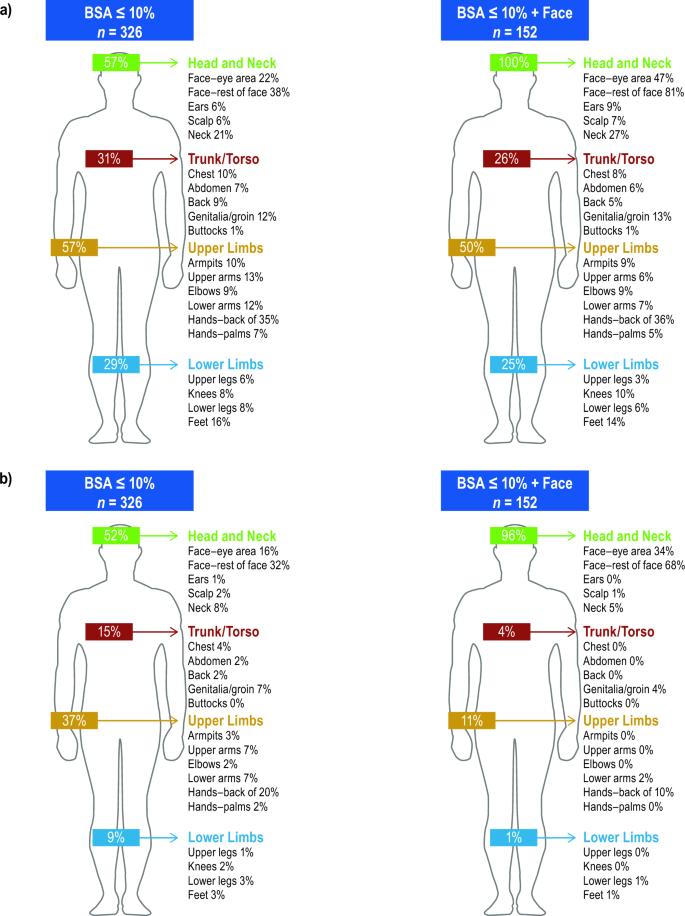
For 326 patients with ≤ 10% affected BSA (including those with facial involvement), the physician-reported body regions most affected were head and neck (57%), upper limbs (57%), trunk/torso (31%), and lower limbs (29%; Fig. 1a). Physicians considered the most bothersome regions to be head and neck for 52% of patients, followed by upper limbs (37%), trunk/torso (15%), and lower limbs for 9% of patients. Among patients with facial involvement, physicians considered the most bothersome regions to be head and neck for 96%, trunk/torso for 4%, upper limbs for 11%, and lower limbs for 1% (Fig. 1b).
Physician- and Patient-Reported Treatment
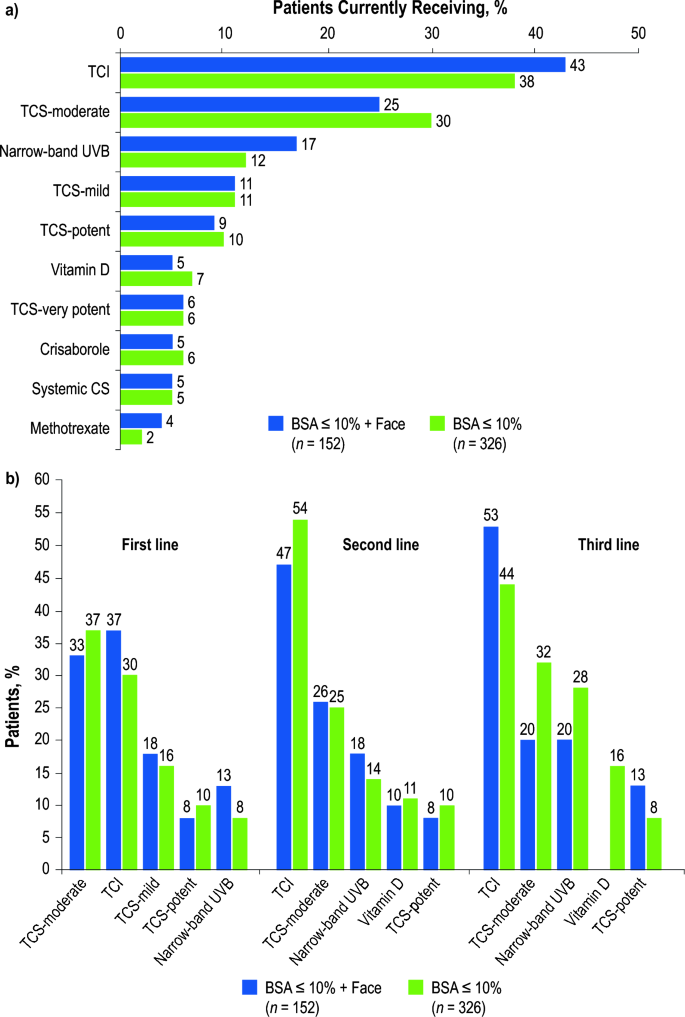
At the time of the survey, physicians reported that their 326 patients were receiving an average of 1.4 prescribed treatments, with 61% receiving monotherapy, 35% receiving combination therapy, and 4% not currently receiving prescribed treatment (i.e., untreated). Overall, 38% of patients were receiving topical calcineurin inhibitors (TCI), 30% were receiving moderate-potency topical corticosteroids (TCS), and 12% were receiving narrow-band ultraviolet-B (UVB) phototherapy. Among 152 patients with facial involvement, the prescription of TCIs and narrow-band UVB increased to 43% and 17%, respectively (Fig. 2a).
Physician-reported most commonly used treatment regimens a currently and b by treatment linea; BSA body surface area, CS corticosteroid, TCI topical calcineurin inhibitor, TCS topical corticosteroid, UVB ultraviolet-B phototherapy. aRuxolitinib cream was not approved in the United States at the time of the analysis
Overall, physicians reported that moderate-potency TCS were the most common first-line therapy (37%), and TCI were the most commonly prescribed second- (54%) and third-line therapies (44%; Fig. 2b). Among patients with facial involvement, TCI were the most commonly prescribed first- (37%), second- (47%), and third-line (53%) therapies. Additionally, prescription of narrow-band UVB increased with each line of therapy. Physicians reported that cosmetics or skin camouflage creams were used by 19% of all patients (facial involvement, 33%).
Furthermore, 37/90 (41%) patients reported using over-the-counter medications. Among these 37 patients, the most commonly reported treatments used were vitamins (38%), creams or oils (35%), and makeup/camouflage cream (22%). Patients also reported spending a mean (SD) of US$17.19 ($36.92) over the last month on pharmacy/drug store nonprescription medicines for their vitiligo.
Physician- and Patient-Reported Treatment Goals
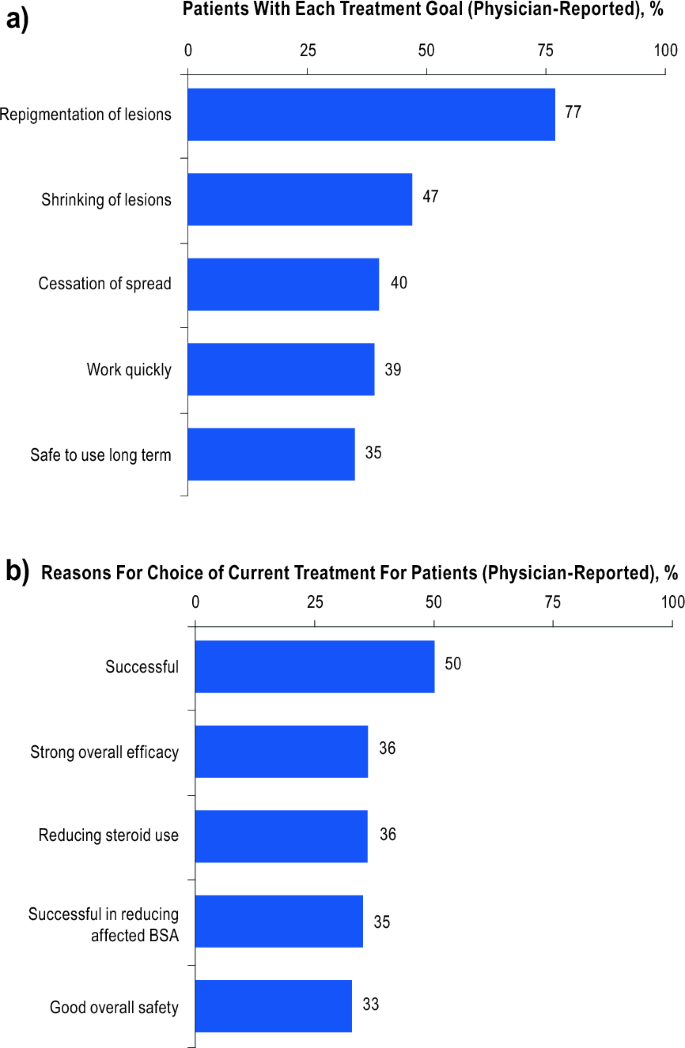
The most common physician-reported treatment goal was repigmentation of lesions for 77% (251/326) of patients, followed by shrinkage of lesions in 47% (154/326; Fig. 3a). Physicians reported long-term safety of treatment as a goal in 35% (113/326) of patients. For patients with physician-reported data available, repigmentation of lesions was the main physician-reported reason for prescribing the current treatment [50% of patients (146/290); Fig. 3b]. Reduction of steroid use (36%; 103/290) and overall safety (33%; 95/290) were also considered important reasons for treatment selection.
Repigmentation of lesions was the main treatment goal for 67% (60/90) of patients, which rose to 74% (31/42) for patients with facial involvement (Figure S1). Shrinkage of lesions was reported as the main treatment goal by 61% (55/90). Among all patients, 64% (58/90) wanted a treatment that worked quickly, and 54% (49/90) wanted a treatment to stop the spread of their vitiligo; of those patients with facial involvement, 60% (25/42) wanted a treatment that worked well to specifically shrink more noticeable areas. Overall, 42% (38/90) of patients also reported wanting a treatment that was safe to use long term, as did 52% (22/42) of patients with facial involvement.
Physician- and Patient-Reported Satisfaction with Current Treatment
Physicians were satisfied with current treatment outcomes for only 56% of their 326 patients and believed best realistic control had been achieved for only 51% (facial involvement, 45%). Top reasons for treatment dissatisfaction were not initially inducing repigmentation (66%), lack of sustained efficacy (24%), and treatment ineffectiveness for obtaining complete depigmentation (14%).
Among the 90 patients, 14% were dissatisfied with current treatment results (facial involvement, 24%; Figure S2a). Main reasons for dissatisfaction included not liking the way the treatment was taken (23%) and not improving quality of life (21%). For the 42 patients with facial involvement, not improving vitiligo noticeability (27%) and lack of improved quality of life (23%) were most frequently reported as reasons for dissatisfaction. Patients reported feeling frustrated with available treatment options (25%; facial involvement, 37%). Patients reported being fairly (50%) and completely (39%) happy with the way their physician treated their vitiligo (Figure S2b), and were very confident (20%) or somewhat confident (58%) in managing their condition (Figure S2c).
Physician-Reported Psychosocial Impact
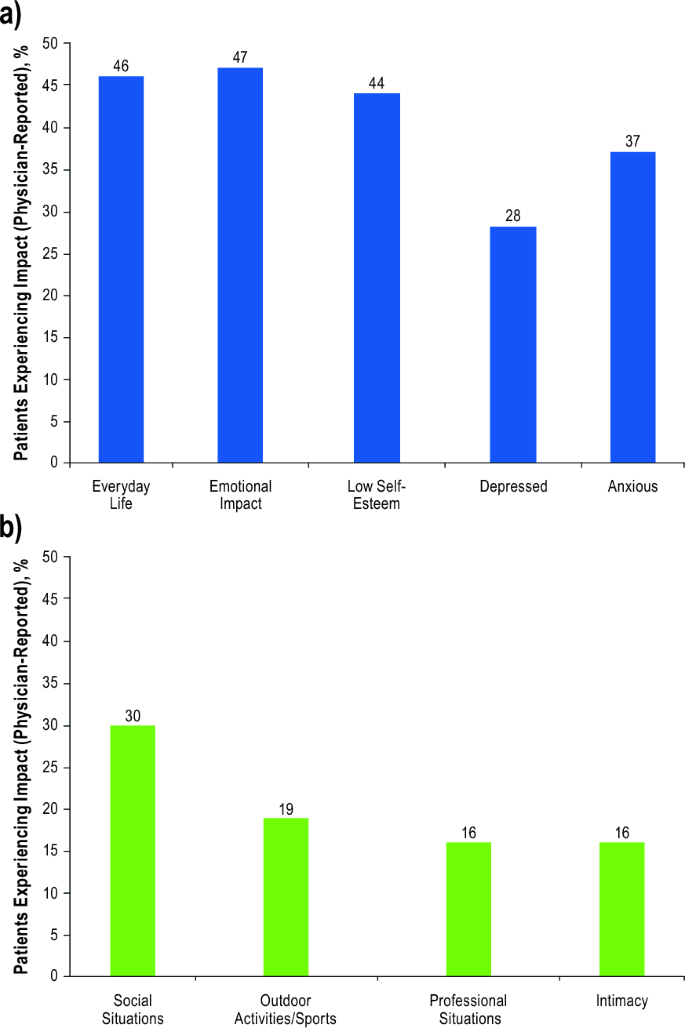
Physicians reported that 46% of their 326 patients were impacted by vitiligo in everyday life, with 47% experiencing emotional impact and 44% having low self-esteem (Fig. 4). Overall, physicians indicated that 30% avoided social situations, 19% avoided sports or other outdoor activities, and 16% avoided professional situations or intimacy. Physicians also reported that 21% felt socially isolated (facial involvement, 31%), and 14% experienced bullying or abuse (facial involvement, 22%). Physicians also reported that bullying, abuse, or social ostracization were experienced by 22% of all adolescent patients (facial involvement, 39%). Furthermore, physicians reported that 28% of their patients experienced depression and 37% experienced anxiety (facial involvement, 37% and 47%, respectively). Concerningly, 9% of all patients had suicidal ideation (facial involvement, 13%).
Patient-Reported Vitiligo Impact
When patients reported how they felt at the time they received their vitiligo diagnosis, 33% of the 90 patients worried what other people would think (facial involvement, 38%; Figure S3a). Patients reported that their physician understood the impact vitiligo had on their life “quite well” (54%) or “completely” (39%). Overall, 20% reported feeling socially isolated (facial involvement, 29%); however, only 6% reported experiencing bullying or abuse (facial involvement, 10%). Additionally, 53% self-reported having to cover or hide their vitiligo; 31% used makeup to cover vitiligo on their face, and 18% used makeup to cover vitiligo elsewhere on their body (Figure S3b). Patients spent a mean (SD) time of 57 (100) minutes per day covering vitiligo on their face and 30 (48) minutes covering vitiligo on their body. Overall, 21% reported depression (facial involvement, 26%), and 32% reported anxiety (facial involvement, 38%).
Patient-Reported Impact of Vitiligo on Quality of Life and Work Productivity
Work Productivity and Activity Impairment (WPAI)
According to the WPAI questionnaire, 43 patients reported a mean (SD) of 12.7% (15.7%) overall work impairment [facial involvement (n = 18), 13.5% (15.4%)], and 68 reported a mean (SD) of 14.1% (18.6%) activity impairment due to their vitiligo [facial involvement (n = 32), 16.6% (21.0%); Figure S4a].
Hospital Anxiety and Depression Scale (HADS)
For the anxiety domain of the HADS, the mean (SD) score for the 90 patients was 3.5 (3.6), with 4.2 (4.1) in patients with facial involvement (Figure S4b). However, 14% of patients reported a score ≥ 8, suggesting either borderline abnormal or abnormal levels of anxiety (facial involvement, 22%).
For the depression domain of the HADS, the mean (SD) score for all patients was 2.2 (3.2), with 2.9 (3.8) in patients with facial involvement. However, 9% of patients reported a score ≥ 8, suggesting either borderline abnormal or abnormal levels of depression (facial involvement, 14%).
Vitiligo-Specific Quality of Life (VitiQoL) Instrument
The mean (SD) score on the VitiQoL instrument for the 89 patients with data available and the 42 patients with facial involvement was 26.9 (22.9) and 32.5 (25.5), respectively (Figure S4c), indicating moderate quality-of-life impairment [27].
Discussion
This study highlights unmet needs in the treatment of vitiligo among patients with ≤ 10% affected BSA. Despite treatment, patients reported minimal or no changes in their vitiligo, exposing them to a high degree of emotional burden. Many patients experienced low self-esteem, avoided social situations, felt socially isolated or experienced bullying/abuse, and reported symptoms of depression and anxiety. Our study was conducted before FDA approval of ruxolitinib cream for topical treatment of NSV, and, in line with treatment guidelines [28, 29], physicians prescribed mainly TCI, moderate-potency TCS, or narrow-band UVB phototherapy.
In our study, both physicians and patients reported similar treatment goals, namely repigmentation, shrinkage of lesions (especially noticeable areas), and prevention of the spread of vitiligo. Patients also wanted a treatment that was safe long term and had few or no side effects. However, physicians and patients were dissatisfied with disease control and treatment options available at the time of the study due to a lack of sustained efficacy, including not inducing initial repigmentation and ineffective complete depigmentation. The limited long-term success of current management strategies and the lack of effective treatment options are current barriers in the treatment of vitiligo [30]. Dissatisfaction rates are often high among physicians and their patients with vitiligo [15, 31, 32], commonly due to issues with sustained repigmentation [33], as depigmentation recurs in ~ 40% of cases after therapeutic intervention [34]. Patients, especially those with facial involvement, were also frustrated with treatment outcomes; a quarter of patients reported no improvement in quality of life after treatment.
Nearly half of physicians reported that vitiligo had a negative impact on their patients’ day-to-day life, including low self-esteem, avoidance of social situations, social isolation, bullying, abuse, social ostracization, depression, anxiety, and suicidal ideation. Patients’ reports were consistent with those of their physicians. Recently, the emphasis placed on addressing the emotional burden of vitiligo [35] and integrating patient-oriented measures in diagnosis and treatment decisions [36] has been shown to increase treatment adherence, patient satisfaction, and quality of life [35]. In our study, patients were satisfied with their physician’s awareness of the impact vitiligo had on their daily life, felt fairly or completely happy with the way their physician treated their vitiligo, were involved in treatment decisions, and felt supported by their dermatologists in managing their condition. Patients resorted to over-the-counter medications and spent a significant amount of time using camouflaging makeup to cover or hide their vitiligo. Over a third reported being worried what people would think, and patient-reported low self-esteem, social isolation, and avoidance were high, indicating significant emotional burden. Social avoidance behavior is reported to stem from the fear of negative social encounters including rude remarks and questions about their disease [7, 8]. Patients with vitiligo also report feeling embarrassed and disfigured, leading to limited personal relationships [7, 9] and sexual disorders [37, 38].
Furthermore, our findings are consistent with previous studies indicating depression and anxiety to be the most common psychological comorbidities among patients with vitiligo. A meta-analysis of 29 studies, capturing data on 2530 patients with vitiligo with varying disease severity, found that a quarter of patients had depression and 1 in 7 had anxiety [10]. In our study, physicians reported depression in nearly one-third of patients and anxiety in two-fifths, with increased suicidal ideation among patients who had facial involvement. These percentages were higher than general population estimates from the United States, where depression and anxiety were reported to affect 19% and 15% of people, respectively [39]. It is also likely that the prevalence of depression and anxiety is underreported in vitiligo, consistent with other autoimmune conditions [40,41,42].
Most studies capturing data on the burden of vitiligo report on patients with a greater extent of affected BSA (often > 25%) [43], more noticeable lesions (Fitzpatrick skin types IV–VI or lesions on the face, neck, or hands), or those with genital involvement [44]. However, our study specifically evaluated patients with vitiligo who had ≤ 10% affected BSA and included those with and without facial involvement. As such, our data encompass an underreported cohort of patients with vitiligo and demonstrate that high levels of disease burden occur irrespective of disease extent. Our findings indicate that the disease burden associated with vitiligo is high even among those with a more limited disease extent and may be driven by a lack of efficacious treatment options. Physicians (all dermatologists) were aware of the high disease burden among their patients with vitiligo who had ≤ 10% affected BSA and were dissatisfied with disease control and treatments available at the time of the study. Although patients felt that their dermatologists understood the impact vitiligo had on their daily lives, they were also frustrated with current treatment outcomes and options.
Limitations of this study include that participating patients may not reflect the general population with vitiligo. Firstly, our study included only patients with ≤ 10% affected BSA, consistent with the labeling for ruxolitinib cream. Secondly, data collection was restricted to patients who visited their dermatologist for consultation during the study period. Thirdly, patients who visited their physician more frequently were more likely to be included in the survey than those not consulting their physician as frequently. Additionally, we acknowledge that the results may not be generalizable to patients who are treated by healthcare personnel other than dermatologists or to patients with > 10% affected BSA. To avoid selection bias, physicians were asked to provide data for a consecutive series of patients; however, there were no formal patient selection verification procedures. Recall bias is a common limitation of surveys; however, the data for these analyses were collected at the time of each patient’s appointment, an approach expected to reduce the likelihood of recall bias, particularly since reporting was about current clinical characteristics, treatment experience, and patient burden. Despite the limitations described above, the DSP sample is internally consistent and enables us to accurately describe any differences in clinical or health-related quality-of-life characteristics, management approach, treatment patterns, and burden for patients with vitiligo with or without facial involvement.
Further research would enable identification of additional factors that might be drivers of greater burden among patients with vitiligo. In the global VALIANT survey, patients with vitiligo reported substantial psychosocial burden, which was greatest among patients with > 5% affected BSA, Fitzpatrick skin types IV–VI, and facial or hand lesions versus their counterparts. However, patients with < 1% affected BSA, Fitzpatrick skin types I–III and no facial or hand lesions also reported considerable burden [45]. Investigation of these and other factors, including affected body regions, age groups, and sex, would help further our understanding of vitiligo burden.
Conclusions
Dermatologists and their patients reported a high patient burden associated with vitiligo limited to ≤ 10% affected BSA, including psychosocial and psychological consequences. Facial involvement was common, with head/neck lesions considered by physicians to be the most bothersome for patients. Repigmentation was the most common dermatologist- and patient-reported treatment goal; however, both dermatologists and patients reported being dissatisfied with disease control and available treatments at the time of the survey. Both dermatologists and patients reported bullying, abuse, and psychosocial comorbidities including depression. Patients also reported spending a considerable amount of time each day covering their vitiligo, experienced work productivity and activity impairment, and had reduced quality of life. Considered together, these findings suggest a significant unmet need concerning treatment and management of this chronic disease in the United States, warranting a focus on managing vitiligo using a full range of treatment options including those more recently approved by the FDA.
Data Availability
Simran Marwaha, James Piercy, Peter Anderson, and Jinan Liu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Peter Anderson at peter.anderson@adelphigroup.com.
References
Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1):1–13.
Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158(1):43–50.
Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–12.
Hamzavi IH, Bibeau K, Grimes P, et al. Exploring the natural and treatment history of vitiligo: perceptions of patients and healthcare professionals from the global VALIANT study. Br J Dermatol. 2023;189(5):569–77.
Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–92.
Porter JR, Beuf AH, Lerner A, Nordlund J. Psychosocial effect of vitiligo: a comparison of vitiligo patients with “normal” control subjects, with psoriasis patients, and with patients with other pigmentary disorders. J Am Acad Dermatol. 1986;15(2):220–4.
Krüger C, Schallreuter KU. Stigmatisation, avoidance behaviour and difficulties in coping are common among adult patients with vitiligo. Acta Derm Venereol. 2015;95(5):553–8.
Ongenae K, Beelaert L, van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol. 2006;20(1):1–8.
Hamidizadeh N, Ranjbar S, Ghanizadeh A, Parvizi MM, Jafari P, Handjani F. Evaluating prevalence of depression, anxiety and hopelessness in patients with vitiligo on an Iranian population. Health Qual Life Outcomes. 2020;18(1):20.
Osinubi O, Grainge MJ, Hong L, et al. The prevalence of psychological comorbidity in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018;178(4):863–78.
Lai Y, Yew Y, Kennedy C, Schwartz R. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177(3):708–18.
Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther. 2020;33(3): e13418.
Silverberg JI, Simpson B, Abuabara K, et al. Prevalence and burden of atopic dermatitis involving the head, neck, face, and hand: a cross sectional study from the TARGET-DERM AD cohort. J Am Acad Dermatol. 2023;89(3):519–28.
Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the understanding psoriatic disease leveraging insights for treatment (UPLIFT) survey. Dermatol Ther (Heidelb). 2022;12(1):61–78.
Narayan V, Uitentuis S, Luiten R, Bekkenk M, Wolkerstorfer A. Patients’ perspective on current treatments and demand for novel treatments in vitiligo. J Eur Acad Dermatol Venereol. 2021;35(3):744–8.
Opzelura™ (ruxolitinib cream). Full prescribing information. Incyte Corporation, Wilmington; 2023.
Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):e1–20.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes–a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8): e010352.
Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–80.
US Department of Health and Human Services. Summary of the HIPAA Privacy Rule. 2003 [cited 2023 June 19]; http://www.hhs.gov/sites/default/files/privacysummary.pdf
Health Information Technology (HITECH). Health Information Technology Act. 2009 [cited 2023 June 19]; https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Lilly E, Lu PD, Borovicka JH, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol. 2013;69(1):e11–8.
StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp LLC 2019.
Picardo M, Huggins RH, Jones H, Marino R, Ogunsola M, Seneschal J. The humanistic burden of vitiligo: a systematic literature review of quality-of-life outcomes. J Eur Acad Dermatol Venereol. 2022;36(9):1507–23.
Eleftheriadou V, Atkar R, Batchelor J, et al. British Association of Dermatologists guidelines for the management of people with vitiligo 2021. Br J Dermatol. 2022;186(1):18–29.
Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.986918.
Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110–20.
Eleftheriadou V, Thomas K, Whitton M, Batchelor J, Ravenscroft J. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167(4):804–14.
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84.
Kubelis-López DE, Zapata-Salazar NA, Said-Fernández SL, et al. Updates and new medical treatments for vitiligo. Exp Ther Med. 2021;22(2):1–11.
Abdel-Malek ZA, Jordan C, Ho T, Upadhyay PR, Fleischer A, Hamzavi I. The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res. 2020;33(6):778–87.
Huggins RH, Gardner J, Pandya AG. Patient support, education, and compliance. In: Gupta S, Olsson MJ, Parsad D, Lim HW, van Geel N, Pandya AG, eds. Vitiligo: medical and surgical management. Wiley; 2018. pp. 69–75.
van Geel N, Lommerts JE, Bekkenk MW, et al. Development and validation of a patient-reported outcome measure in vitiligo: the Self Assessment Vitiligo Extent Score (SA-VES). J Am Acad Dermatol. 2017;76(3):464–71.
Maamri A, Badri T. Sexual disorders in patients with vitiligo. Tunis Med. 2021;99(5):504.
Sarhan D, Mohammed GF, Gomaa AH, Eyada MM. Female genital dialogues: female genital self-image, sexual dysfunction, and quality of life in patients with vitiligo with and without genital affection. J Sex Marital Ther. 2016;42(3):267–76.
Olfson M, Shea S, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med. 2000;9(9):876–83.
Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ. 2019;22(4):372–8.
Peterson S, Piercy J, Blackburn S, Sullivan E, Karyekar CS, Li N. The multifaceted impact of anxiety and depression on patients with rheumatoid arthritis. BMC Rheumatol. 2019;3:43.
Sruamsiri R, Kaneko Y, Mahlich J. The underrated prevalence of depression in Japanese patients with rheumatoid arthritis—evidence from a Nationwide survey in Japan. BMC Rheumatol. 2017;1:5.
Bibeau K, Pandya A, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36(10):1831–44.
Grimes P, Miller M. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4(1):32–7.
Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159(10):1124–8.
Acknowledgements
The authors thank the physicians and their patients with vitiligo who participated in this study.
Medical Writing and Editorial Assistance
Medical writing support under the guidance of the authors was provided by Rebecca Charlton at Adelphi Real World (Bollington, UK) and was funded by Incyte Corporation (Wilmington, DE, USA), in accordance with Good Publication Practice (GPP) guidelines. Editorial assistance was provided by ICON (Blue Bell, PA, USA) and was funded by Incyte.
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Vitiligo Disease Specific Programme™ (DSP), a wholly owned Adelphi program. Incyte Corporation (Wilmington, DE, USA) are subscribers to the Adelphi Vitiligo DSP on which this analysis is based. Incyte did not influence the original survey through either contribution to the design of questionnaires or data collection. Sponsorship of the study and the journal’s Rapid Service Fee were funded by Incyte Corporation. Permission to use the Hospital Anxiety and Depression Scale (HADS) was granted prior to its inclusion in this study.
Author information
Authors and Affiliations
Contributions
David Rosmarin, Jennifer H. Lofland, Simran Marwaha, James Piercy, Peter Anderson, and Jinan Liu were involved in 1) conception or design, or analysis and interpretation of data; 2) drafting and revising the article; 3) providing intellectual content of critical importance to the work described; and 4) final approval of the version to be published, and therefore meet the criteria for authorship in accordance with the International Committee of Medical Journal Editors (ICMJE) guidelines. In addition, all named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
David Rosmarin has received honoraria as a consultant for AbbVie, Abcuro, AltruBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, Dermavant Sciences, Dermira, Incyte Corporation, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio; has received research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Dermira, Galderma, Incyte Corporation, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals; and has served as a paid speaker for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, and Sanofi. Jennifer H. Lofland and Jinan Liu are employees and stockholders of Incyte Corporation. Simran Marwaha, James Piercy, and Peter Anderson are employees of Adelphi Group, which was contracted by Incyte Corporation to perform this analysis.
Ethical Approval
The Adelphi Vitiligo Disease Specific Programme™ received ethics exemption from the Pearl Institutional Review Board (study protocol number #21-ADRW-122) based on the use of aggregated and de-identified patient data. All data were collected in such a way that patients and dermatologists could not be identified directly; all data were aggregated and de-identified before receipt. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act of 1996 and Health Information Technology for Economic and Clinical Health Act legislation. Both physicians and patients consented to take part in the research.
Additional information
Prior Presentation: This manuscript is based on work that has been previously presented at the following conferences: Rosmarin D, Liu J, Marwaha S, et al. Patient Burden of Nonsegmental Vitiligo: Perspectives From US Patients. Presented at Global Vitiligo Foundation Annual Scientific Symposium, March 16, 2023, New Orleans, LA, USA; Joish VN, Rosmarin D, Lofland JH, et al. Management and Impact of Vitiligo on Patient Lives: Survey of US Dermatologists. Presented at Maui Derm NP + PA Summer, June 22–25, 2022, Colorado Springs, CO, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rosmarin, D., Lofland, J.H., Marwaha, S. et al. Patient Burden of Nonsegmental Vitiligo: A US Real-World Survey of Dermatologists and Their Patients. Dermatol Ther (Heidelb) 14, 1531–1546 (2024). https://doi.org/10.1007/s13555-024-01165-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01165-5