Abstract
Introduction
Atopic dermatitis (AD) is a chronic immuno-inflammatory skin disease. Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 inhibitor approved for the treatment of mild to moderate AD. This post hoc analysis assesses the efficacy and safety of crisaborole in Chinese patients aged ≥ 2 years with mild to moderate AD.
Methods
We evaluated the efficacy and safety of crisaborole in Chinese patients from the vehicle-controlled, phase 3 CrisADe CLEAR study. Patients were randomly assigned 2:1 to receive crisaborole or vehicle twice daily, respectively, for 28 days. The primary endpoint was percent change from baseline in Eczema Area and Severity Index (EASI) total score at day 29. Key secondary endpoints were improvement in Investigator’s Static Global Assessment (ISGA), ISGA success, and change from baseline in weekly average Peak Pruritus Numerical Rating Scale (PP-NRS) score. Adverse events were documented.
Results
Of 391 patients in the overall study, 237 were from China, 157 assigned to crisaborole and 80 assigned to vehicle. A greater reduction in percent change from baseline in EASI total score at day 29 was shown in the crisaborole vs. vehicle group (least squares mean [LSM]: −66.34 [95% (confidence interval) CI −71.55 to −61.12] vs. −50.18 [95% CI −58.02 to −42.34]). Response rates for achievement of ISGA improvement (43.2% [95% CI 35.4–51.1] vs. 33.4% [95% CI 22.5–44.2]) and ISGA success (31.7% [95% CI 24.3–39.0] vs. 21.5% [95% CI 12.1–30.9]) at day 29 were higher in the crisaborole vs. vehicle group. A greater reduction in change from baseline in weekly average PP-NRS score at week 4 was observed in the crisaborole vs. vehicle group (LSM: −1.98 [95% CI −2.34 to −1.62] vs. −1.08 [95% CI −1.63 to −0.53]). No new safety signals were observed.
Conclusion
Crisaborole was effective and well tolerated in Chinese patients aged ≥ 2 years with mild to moderate AD.
Trial Registration
ClinicalTrials.gov, NCT04360187.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atopic dermatitis (AD) is a chronic immuno-inflammatory skin disease characterized by different phenotypes depending on age, disease chronicity, ethnicity, and underlying molecular mechanisms/endotypes which may influence response to treatments and could be related to treatment side effects |
Trials involving different racial and ethnic groups are important to achieve a more targeted, patient-specific therapeutic approach |
Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 inhibitor approved for the treatment of mild to moderate AD |
This analysis showed a greater reduction in Eczema Area and Severity Index (EASI) score and a greater treatment benefit per Investigator’s Static Global Assessment (ISGA) improvement and success and Peak Pruritus Numerical Rating Scale (PP-NRS) score in crisaborole- vs. vehicle-treated Chinese patients |
The potential role of genotypic and phenotypic differences in the manifestation of AD was evidenced by the higher incidence of administration site reactions in the Chinese cohort vs. Western cohorts in similar studies; no new safety signals were observed |
Introduction
Atopic dermatitis (AD), also called atopic eczema, is a common, chronic immuno-inflammatory skin disorder that commonly arises during early childhood and has a significant impact on the overall well-being of patients and their families. AD may precede several comorbid disorders, with allergy disorders being the most common. About 60% of patients with AD develop asthma and allergic rhinitis, with about 30% developing food allergies [1,2,3,4,5,6,7,8,9]. AD is typically characterized by dry skin, severe pruritus, and eczematic lesions [7, 10].
With an estimated global prevalence of 230 million, AD affects between 15–30% of the pediatric population and 2–10% of adults [4, 11]. In industrialized regions, the prevalence of AD has increased approximately two- to threefold during the past several decades [3]. Although the prevalence of AD varies globally, it has increased in certain regions, particularly the Asia-Pacific region [4, 12,13,14]. The overall prevalence of AD in Chinese children aged 1–7 years was reported to be approximately 12.9% (with the value ranging between 9.0% and 24.7% between metropolises) [15]. In a recent multicenter study of children aged 1–12 months conducted in 12 metropolitan areas in China, the overall prevalence of AD was 30.5% [16]. A study that examined 8758 Chinese adults with eczema found the prevalence of AD to be about 4.6% [17]. Another study in Guangzhou City that compared ISAAC phase 1 data and ISAAC phase 3 data in children showed an increase in the prevalence of AD from 1.7% in 1994–1995 to 3.0% in 2001 in children aged 13–14 years [18, 19]. Such increasing trends in AD prevalence were considered related to changing socioeconomic and environmental factors [4, 18, 20]. AD prevalence in Asian populations appears to be increasing in part because of the rapid urbanization observed throughout major metropolitan areas in Asia. For example, the population in urban areas of China has increased from 11.8% in 1950 to 49.2% in 2010 [4, 21].
AD is a complex disease characterized by different phenotypes that vary depending on age, disease chronicity, ethnicity/race, and underlying molecular mechanisms/endotypes [22]. These differences play an important role in the efficacy and safety of drug treatment [23]. Prior studies have revealed distinctions in the clinical presentation, genetic predisposition, and pathophysiology of AD in Asian patients, including Chinese patients, compared with other ethnicities [24]. Compared with White patients, Asian patients with AD are more likely to exhibit lesions with clear demarcation that sometimes closely resemble psoriasis plaques as well as more prominent scaling and lichenification [24]. Asian patients also exhibit a unique spectrum of gene variants associated with increased AD risk or severity, including null mutations in FLG, loss of function mutations in SPINK5, and polymorphisms of IL-4 and IL13/IL-13RA1 [24,25,26,27]. A consistent immune polarization to a T-helper (Th) 17/Th22 or blended AD-psoriasis endotype is also apparent in Asian patients with AD. [27, 28]. Despite these differences, patients of races and ethnicities other than White are often underrepresented in clinical trials for AD therapies [29, 30]. As the prevalence of AD increases in patients of races and ethnicities other than White, clinical trials that involve different racial and ethnic subgroups become more important to achieve a more targeted, patient-specific therapeutic approach [22, 29]. Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 inhibitor approved for the treatment of mild to moderate AD in multiple countries and regions [31,32,33]. In two identically designed, randomized, vehicle-controlled, double-blind phase 3 clinical studies, CORE 1 and CORE 2 [34,35,36], crisaborole showed an improvement in the ISGA score and an acceptable safety profile in patients ≥ 2 years of age with mild to moderate AD [35, 36]. The CrisADe CLEAR study analyzed the efficacy and safety of crisaborole in Chinese and Japanese patients aged ≥ 2 years with mild to moderate AD. Treatment with crisaborole was effective and well tolerated in Chinese and Japanese patients with mild to moderate AD [37]. This post hoc analysis of the CrisADe CLEAR study examines the efficacy and safety of crisaborole in Chinese patients with mild to moderate AD.
Methods
Study Design
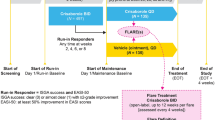
This is a post hoc analysis of the multicenter, randomized, double-blind, vehicle-controlled phase 3 study (CrisADe CLEAR; NCT04360187) that included Chinese and Japanese patients aged ≥ 2 years with mild to moderate AD involving a percentage of treatable body surface area (%BSA) ≥ 5. At baseline (day 1), patients were randomly assigned (2:1) to receive crisaborole or vehicle, respectively, twice daily (BID) for a 28-day treatment course. Patient follow-up was done on days 36 and 60 after the end of the treatment period (Fig. 1) [37].
Patients and Treatment
This analysis included only the subpopulation of Chinese patients enrolled in CrisADe CLEAR. All patients were aged ≥ 2 years at the time of informed consent and had a clinical diagnosis of AD at screening and at baseline (day 1) per Hanifin and Rajka criteria [38]. Patients had mild to moderate AD, defined as an ISGA score of 2 (mild) or 3 (moderate), as well as a %BSA involved (excluding the scalp) of ≥ 5% [37].
Patients and/or their parents/legal guardians were directed to apply the study treatment (either crisaborole or vehicle) to cover each lesion twice daily throughout the 28-day treatment period. Patients were also directed to apply their study drug to newly identified AD lesions that appeared following baseline (day 1). This included all treatable areas throughout the body, excluding the scalp, that were affected by AD. Patients and their parents/guardians were allowed to use emollients, sunscreen, and moisturizers during the study period to manage dry skin in areas surrounding, but not on or overlapping, the treatable areas affected by AD [37].
Endpoints and Assessments
Efficacy Assessments
Efficacy assessments were performed at baseline (day 1) and on days 8, 15, 22, and 29 (end of treatment) (Fig. 1). The primary endpoint was percent change from baseline in Eczema Area and Severity Index (EASI) score at day 29 [37]. The disease severity of AD is quantified by EASI based on the severity of lesion-related clinical signs and %BSA involved. EASI is a composite score of the degree of erythema, excoriation, induration/papulation, and lichenification (with each scored separately) for each of four body regions (upper limbs, lower limbs, head and neck, and trunk), with the adjustment for %BSA involved for each body region and for the proportion of the body region to the entire body [39].
ISGA assesses AD severity on a five-point clinician-reported scale, ranging from 0 (clear) to 4 (severe) [40]. Key secondary endpoints included the achievement of improvement in ISGA and the achievement of ISGA success. ISGA improvement is defined as an ISGA score of 0 (clear) or 1 (almost clear) at day 29. ISGA success is defined as an ISGA score of 0 or 1 with a ≥ 2-grade improvement from baseline at day 29.
Another key secondary endpoint was change from baseline on weekly average Peak Pruritus Numerical Rating Scale (PP-NRS; used with permission from Regeneron Pharmaceuticals, Inc., and Sanofi) score at week 4 (for patients aged ≥ 12 years). PP-NRS is an assessment of patient-reported pruritus in lesions in which the severity of pruritus over the past 24 h is rated on an 11-point scale of 0 (no pruritus) to 10 (most severe pruritus) [41].
Success in ISGA over time was assessed at days 8, 15, 22, and 29. Change from baseline in mean %BSA involved was assessed at day 29. Changes from baseline at day 29 in Children’s Dermatology Life Quality Index (CDLQI) score (used for patients aged 4–15 years), Dermatology Life Quality Index (DLQI) score (used for patients aged 16 years and older) score, and Dermatitis Family Index (DFI) score (used for parents/guardians of patients aged 2–17 years) were also examined [37].
Safety Assessments
Patients were assessed for treatment-emergent adverse events (TEAEs) during their study visits (Fig. 1). The study design allowed unscheduled safety assessments to be performed at any time during the study to assess potential safety concerns. TEAEs were defined as adverse effects with an onset on or after the day of the first study drug dose. TEAEs were classified as treatment-related if they were determined by the study investigator to be definitely, probably, or possibly related to the treatment with crisaborole or vehicle. AEs were recorded and classified according to Medical Dictionary for Regulatory Activities terminology [37].
Statistical Analysis
Efficacy analyses for the China subpopulation were performed on the full analysis set (FAS), which encompassed all patients who were randomly assigned to and received the study drug or vehicle, regardless of discontinuation. All randomly assigned patients who received one or more doses of the study drug were included in the safety populations. The subgroup analysis of Chinese patients was designed to evaluate the consistency of treatment efficacy by comparing the outcomes to those of the overall study. No hypothesis testing was prespecified for Chinese patients, and no P values will be reported for efficacy endpoints here.
Percent change from baseline in EASI total score at day 29 and change from baseline to week 4 in weekly average PP-NRS score (for patients aged ≥ 12 years) were analyzed using a linear mixed-effect model for repeated measures that included treatment group, visit, and treatment group-by-visit interactions as factors and baseline value as a covariate.
Percentages of patients achieving improvement or success in ISGA at day 29 were compared between the crisaborole and vehicle groups. The differences were tested based on normal approximation to response rates.
Secondary efficacy endpoints including change from baseline in %BSA involved was analyzed similarly to the primary efficacy endpoints using a linear mixed-effect model for repeated measures. Other secondary efficacy endpoints, including success in ISGA at all time points other than day 29, are analyzed using normal approximation to response rates.
DLQI, CDLQI, and DFI scores were summarized descriptively, and missing values were handled by following instrument-specific procedures when available.
Ethical Approval
This analysis of a previously conducted study was exempt from institutional review board approval. All patients or parents/guardians provided written informed consent for participation in the studies. The study was approved by the Quorum Review Institutional Review Board and was conducted in accordance with the ethical principles originating in the Declaration of Helsinki.
Results
Baseline Characteristics
Of the 391 patients in the overall population, 237 (60.6%) were from China and 154 (39.4%) from Japan. Here, we report data from the Chinese subpopulation only. Of a total of 237 patients in the Chinese subpopulation, 157 and 80 patients were randomly assigned to the crisaborole and vehicle groups, respectively (Fig. 1). Demographic and baseline characteristics were balanced between the two groups (Table 1). The proportion of patients in the 2-to-11-year group (50.6%) was similar to the proportion in the ≥ 12-year-old group (49.4%). The mean age (SD) was 19.5 (16.7) and 16.8 (14.9) years for the crisaborole and vehicle groups, respectively. The mean EASI scores (SD) were 8.7 (6.2) for the crisaborole group and 9.1 (7.2) for the vehicle group. In the crisaborole group, 40.1% of patients had an ISGA score of 2 (mild) and 59.9% of patients had an ISGA score of 3 (moderate). In the vehicle group, 42.5% of patients had an ISGA score of 2 (mild) and 57.5% of patients had an ISGA score of 3 (moderate). The mean PP-NRS scores (SD) were 5.4 (2.2) and 5.7 (2.2) for crisaborole and vehicle groups, respectively. The mean %BSA involved (SD) was 15.1 (12.8) and 15.9 (13.4) for the crisaborole- and vehicle-treated groups, respectively. Baseline CDLQI, DLQI and DFI scores were similar at baseline between the crisaborole- and vehicle-treated groups (Table 1).
Efficacy Endpoints
Primary Endpoint: Percent Change from Baseline in EASI Score at Day 29
Chinese patients treated with crisaborole showed a greater reduction vs. those who received vehicle in percent change from baseline in the EASI total score at day 29 (−66.34% [95% confidence interval (CI) −71.55 to −61.12]) vs. −50.18% [95% CI −58.02 to −42.34), respectively, with a LSM difference of −16.16; [95% CI −25.57 to −6.74]) (Fig. 2a).
Outcomes of efficacy endpoints. a Percent change from baseline in EASI total score at day 29, b ISGA improvement and success at day 29, c change from baseline in weekly average PP-NRS score (patients ≥ 12 years old) at week 4, d success per ISGA over time (days 8, 15, 22 and 29), and e change from baseline in mean %BSA at day 29 in the Chinese cohort. BID twice daily, BL baseline, %BSA percentage of treatable body surface area, EASI Eczema Area and Severity Index, ISGA Investigator’s Static Global Assessment, LSM least squares mean, PP-NRS Peak Pruritus Numerical Rating Scale. Improvement was defined as an ISGA score of 0 (clear) or 1 (almost clear). Success was defined as an ISGA score of 0/1 with a ≥ 2-grade improvement from baseline. %BSA was defined as the percentage of the patient’s total body surface area that was affected by AD, excluding the scalp
Key Secondary Endpoint: Achievement of Improvement and Success in ISGA Score at Day 29
Response rates for achievement of ISGA improvement at day 29 were higher for Chinese patients treated with crisaborole than for those who received vehicle (43.2% [95% CI 35.4–51.1] vs. 33.4% [95% CI 22.5–44.2]). The percentage of Chinese patients who achieved ISGA improvement was 9.9% higher for those treated with crisaborole vs. those who received vehicle (95% CI −3.5 to 23.3). A larger percentage of Chinese patients treated with crisaborole achieved ISGA success vs. those who received vehicle (31.7% [95% CI 24.3–39.0] vs. 21.5% [95% CI 12.1–30.9]). The percentage of Chinese patients who achieved ISGA success was 10.1% higher for those treated with crisaborole vs. those who received vehicle (95% CI −1.8 to 22.0) (Fig. 2b).
Key Secondary Endpoint: Change from Baseline in Weekly Average PP-NRS Score at Week 4 in Patients Aged ≥ 12 Years
Chinese patients treated with crisaborole showed a greater reduction vs. those who received vehicle in change from baseline in weekly average PP-NRS score at week 4 (−1.98 [95% CI −2.34 to −1.62] vs. −1.08 [95% CI −1.63 to −0.53]). The LSM of change from baseline in weekly average PP-NRS score was 0.9 lower for the crisaborole-treated group vs. the vehicle-treated group (95% CI −1.56 to −0.24) (Fig. 2c).
Secondary Endpoint: ISGA Success over Time (Days 8, 15, 22 and 29)
A higher percentage of Chinese patients treated with crisaborole achieved ISGA success at days 8, 15, 22, and 29 vs. those who received vehicle. The difference in ISGA success rate between the crisaborole- and vehicle-treated groups could be observed at day 8 and remained stable over time (Fig. 2d).
Secondary Endpoint: Change from Baseline in %BSA at Day 29
Chinese patients treated with crisaborole showed a greater reduction vs. those who received vehicle in change from baseline in mean %BSA involved (−8.57% vs. −5.6%) at day 29 (Fig. 2e).
Quality of Life Questionnaires: Change From BL in DLQI, CDLQI, and DFI Scores at Day 29
There was a greater mean reduction from baseline in CDLQI and DFI scores in Chinese patients treated with crisaborole vs. those who received vehicle (CDLQI: crisaborole, −4.1 and vehicle, −1.5; DFI: crisaborole, −4.5 and vehicle, −2.9) at day 29. There was no apparent difference in mean DLQI score decrease from baseline between the crisaborole- and vehicle-treated groups (crisaborole, −2.2; vehicle, −2.3) (Fig. 3a–c).
Outcomes of quality-of-life assessments. LSM of change from baseline in a CDLQI score at day 29 (patients aged 4–15 years), b DLQI score at day 29 (patients aged ≥ 16 years), c DFI score at day 29 (patients aged 2–17 years) in the Chinese cohort. BID twice daily, BL baseline, CDLQI Children’s Dermatology Life Quality Index, DFI Dermatitis Family Impact questionnaire, DLQI Dermatology Life Quality Index, LSM least squares mean
Safety
During the double-blind study treatment period, patient discontinuation rate among Chinese patients who received vehicle (18.8%) vs. those treated with crisaborole (2.5%) was examined. The most common reason for discontinuation was experiencing an AE (vehicle, 7.5%; crisaborole, 1.9%), followed by lack of efficacy (vehicle, 6.3%; crisaborole, 0.6%) (Table 2).
Overall, 50.3% and 45.0% of Chinese patients who were treated with crisaborole and received vehicle, respectively, experienced all-causality TEAEs. Most TEAEs were mild or moderate. The most frequently reported TEAE in both treatment groups was application site pain. Application site pain was experienced by 17.8% and 3.8% of the patients in the crisaborole- and vehicle-treated groups, respectively. Serious adverse events (SAEs) were reported in one patient each in the vehicle and crisaborole 2% BID groups (1.3% and 0.6%, respectively); neither of the SAEs were treatment-related (Table 3).
Treatment-related AEs occurred in 27.4% of Chinese patients treated with crisaborole and 22.5% who received vehicle; none were serious. The most frequently reported treatment-related AE in the crisaborole group was application site pain (17.8%) (Table 3). One patient in the vehicle group discontinued from the study because of an AE, and no patients in the crisaborole group discontinued because of treatment-related AEs (Table 2). No safety signals were identified from vital signs and laboratory testing in either group.
Discussion
This analysis addressed the efficacy and safety of crisaborole in Chinese patients aged ≥ 2 years with mild to moderate AD. Treatment with crisaborole demonstrated superior efficacy in the primary and key secondary endpoints vs. vehicle. Percent change from baseline in EASI score at day 29 was greater for Chinese patients treated with crisaborole vs. patients who received vehicle (LSM difference of −16.16 [95% CI −25.57 to −6.74]). Response rates for achievement of ISGA improvement and success at day 29 were higher in Chinese patients treated with crisaborole than in those receiving vehicle. Chinese patients treated with crisaborole also showed a greater reduction in change from baseline in weekly average of PP-NRS vs. those who received vehicle at week 4.
Crisaborole was well tolerated in Chinese patients ≥ 2 years of age with mild to moderate AD. Most TEAEs were mild to moderate. No significant difference in the percent of treatment-related AEs between the crisaborole- and vehicle-treated groups was identified. Although direct comparisons cannot be made between studies, reviewing safety data across the CORE 1/CORE 2 studies shows that the rates of overall TEAEs, skin and subcutaneous tissue disorders, general disorders and administration site conditions, and application site pain rates were numerically higher in the CLEAR study than in the CORE 1/CORE 2 studies [30, 32]. Application site pain may be mitigated by allowing time for damaged skin to heal prior to initiating crisaborole, applying the ointment on a small test area, and observing any reactions before applying it to affected areas [42, 43].
Overall, this analysis of the CrisADe CLEAR study and pooled CORE 1/CORE 2 studies had similar efficacy and safety results; however, the few differences might be explained by the differences in baseline characteristics and patient demographics. This analysis consisted of Asian patients of Chinese descent with mild to moderate AD. The skin of Asian patients has been noted to be more sensitive to chemical stimuli, potentially because of a higher sweat gland density or a thinner stratum corneum [44]. Previous studies have reported a higher level of intolerance to certain dermal preparations, with Asian patients having greater response rates than White patients [45, 46]. This may also be related to certain genotypic/phenotypic differences affecting the efficacy and tolerability of the topical products used [22, 23, 46]. The findings of this post hoc analysis further emphasize the potential role of genotypic and phenotypic differences in patients with AD regarding the development of intolerance to dermal preparations.
A potential limitation of the current analysis is that patients from Western countries were not included in this study; therefore, these results might not be reflective of Asian patients from Western populations. In addition, the study duration was not long enough to observe the long-term safety and efficacy of treatment in the population studied.
Conclusion
Because previous AD studies evaluated patients primarily across Western populations, it was important to evaluate the efficacy and safety of crisaborole in Asian populations, including Chinese patients. In this post hoc analysis of the Chinese population of the CrisADe CLEAR study, crisaborole showed greater efficacy in all primary and key secondary endpoints vs. vehicle. Crisaborole was effective and well tolerated in Chinese patients aged ≥ 2 years with mild to moderate AD with no new safety signals identified.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476–86.
Yang EJ, Sekhon S, Sanchez IM, Beck KM, Bhutani T. Recent developments in atopic dermatitis. Pediatrics. 2018;142(4):e20181102. https://doi.org/10.1542/peds.2018-1102.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:8–16.
Tsai TF, Rajagopalan M, Chu CY, Encarnacion L, Gerber RA, Santos-Estrella P, et al. Burden of atopic dermatitis in Asia. J Dermatol. 2019;46:825–34.
MedlinePlus. Atopic dermatitis. Bethesda: US National Library of Medicine; 2017 [updated 10/01/2017]. Available from: https://medlineplus.gov/genetics/condition/atopic-dermatitis/.
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–37.
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2.
Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56–66.
Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016;17:163–9.
Murota H, Inoue S, Yoshida K, Ishimoto A. Cost of illness study for adult atopic dermatitis in Japan: a cross-sectional web-based survey. J Dermatol. 2020;47:689–98.
Luger TA, Hebert AA, Zaenglein AL, Silverberg JI, Tan H, Ports WC, et al. Subgroup analysis of crisaborole for mild-to-moderate atopic dermatitis in children aged 2 to < 18 years. Paediatr Drugs. 2022;24:175–83.
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J Allergy Clin Immunol. 2009;124:1251-8.e23.
Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32:23–33.
Williams H, Stewart A, von ME, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–54.
Guo Y, Li P, Tang J, Han X, Zou X, Xu G, et al. Prevalence of atopic dermatitis in Chinese children aged 1–7 ys. Sci Rep. 2016;6:29751.
Guo Y, Zhang H, Liu Q, Wei F, Tang J, Li P, et al. Phenotypic analysis of atopic dermatitis in children aged 1–12 months: elaboration of novel diagnostic criteria for infants in China and estimation of prevalence. J Eur Acad Dermatol Venereol. 2019;33:1569–76.
Wang X, Shi XD, Li LF, Zhou P, Shen YW, Song QK. Prevalence and clinical features of adult atopic dermatitis in tertiary hospitals of China. Medicine (Baltimore). 2017;96: e6317.
Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE. 2012;7: e39803.
Wang HY, Zheng JP, Zhong NS. Time trends in the prevalence of asthma and allergic diseases over 7 years among adolescents in Guangzhou city. Zhonghua Yi Xue Za Zhi. 2006;86:1014–20.
Halvorsen JA, Lien L, Dalgard F, Bjertness E, Stern RS. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847–54.
Farrell K, Westlund H. China’s rapid urban ascent: an examination into the components of urban growth. Asian Geogr. 2018;35:85–106.
Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1–11.
Pincelli C, Pignatti M, Borroni RG. Pharmacogenomics in dermatology: from susceptibility genes to personalized therapy. Exp Dermatol. 2009;18:337–49.
Chiricozzi A, Maurelli M, Calabrese L, Peris K, Girolomoni G. Overview of atopic dermatitis in different ethnic groups. J Clin Med. 2023;12(7):2701.
Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449–55.
Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340–57.
Nomura T, Wu J, Kabashima K, Guttman-Yassky E. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840–52.
Gu C, Yao X, Li W. Burden of disease; the current status of the diagnosis and management of atopic dermatitis in China. J Clin Med. 2023;12(16):5370.
Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749–55.
Callender VD, Alexis AF, Stein Gold LF, Lebwohl MG, Paller AS, Desai SR, et al. Efficacy and safety of crisaborole ointment, 2%, for the treatment of mild-to-moderate atopic dermatitis across racial and ethnic groups. Am J Clin Dermatol. 2019;20:711–23.
Pfizer Inc. EUCRISA® (crisaborole) ointment, for topical use [prescribing information]. New York, United States: Pfizer Inc; 2020.
Pfizer Australia Pty Ltd. STAQUIS™ (crisaborole) [Australian product information]. Sydney, Australia: Pfizer Australia Pty Ltd; 2019.
Pfizer Canada Inc. PREUCRISATM Crisaborole ointment [product monograph including patient medication information]. Quebec, Canada: Pfizer Canada Inc.; 2018. Revised August 2023.
Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344–57.
Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
Eichenfield LF, Call RS, Forsha DW, Fowler J Jr, Hebert AA, Spellman M, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild-to-moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(641–9): e5.
Ma L, Zhang L, Kobayashi M, Tao X, Qian Q, Cheng H, et al. Efficacy and safety of crisaborole ointment in Chinese and Japanese patients aged ≥2 years with mild-to-moderate atopic dermatitis. J Dermatol. 2023;50(7):847–55.
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60:44–7.
Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol. 2000;136:763–9.
Simpson EL, Tom WL, Bushmakin AG, Cappelleri JC, Yosipovitch G, Ständer S, et al. Relationship among treatment, pruritus, investigator’s static global assessment, and quality of life in patients with atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:587–98.
Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbe A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761–9.
Madsen S, Price KN, Shi VY, Lio PA. Pearls in mitigating application pain of topical nonsteroidal agents. Dermatology. 2020;236:477–80.
Anzelc M, Burkhart CG. Crisaborole: application pain and prevention. Open Derm J. 2019;13:55–7.
Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79–93.
Robinson MK. Population differences in skin structure and physiology and the susceptibility to irritant and allergic contact dermatitis: implications for skin safety testing and risk assessment. Contact Dermatitis. 1999;41:65–79.
Robinson MK. Racial differences in acute and cumulative skin irritation responses between Caucasian and Asian populations. Contact Dermatitis. 2000;42:134–43.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance.
Editorial and medical writing support, under the guidance of the authors, was provided by Mark Bloom, PhD, and Chantell Hayward, PharmD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022; https://doi.org/10.7326/M22-1460).
Funding
The Rapid service fees was funded by the authors.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Lin Ma: Writing – Review and Editing. Xiaohua Tao: Resources, Writing – Review and Editing. Sujun Liu: Writing – Review and Editing. Hao Cheng: Investigation, Resources, Writing – Review and Editing. Ruihua Fang: Writing – Review and Editing. Yan Zhao: Data Collection, Writing – Review and Editing. Amy Cha: Conceptualization, Writing – Review and Editing. Gerardo A. Encinas: Writing – Review and Editing. Yangmei Zhou: Statistics and Data Analysis, Writing – Review and Editing. Yujie Deng: Conceptualization, Statistics and Data Analysis, Writing – Review and Editing. Jianzhong Zhang: Writing – Review and Editing.
Corresponding author
Ethics declarations
Conflict of Interest
Lin Ma, Xiaohua Tao, Hao Cheng, and Jianzhong Zhang have received fees for serving as consultants and speakers for Pfizer Inc. Sujun Liu, Ruihua Fang, and Yan Zhao had no conflict of interest to disclose. Amy Cha, Gerardo A. Encinas, and Yujie Deng are employees and shareholders of Pfizer Inc. Yangmei Zhou is an employee of Pfizer R&D China.
Ethics Approval
This analysis of a previously conducted study was exempt from institutional review board approval. All patients or parents/guardians provided written informed consent for participation in the studies. The study was approved by the Quorum Review Institutional Review Board and was conducted in accordance with the ethical principles originating in the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ma, L., Tao, X., Liu, S. et al. Efficacy and Safety of Crisaborole Ointment 2% in Chinese Patients Aged ≥ 2 Years with Mild to Moderate Atopic Dermatitis. Dermatol Ther (Heidelb) 14, 1229–1243 (2024). https://doi.org/10.1007/s13555-024-01156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01156-6