Abstract
Introduction
The recurrent nature of hidradenitis suppurativa (HS), even under maintained systemic treatment, makes it necessary to have effective local treatments; however, the response to these therapies is variable (44–81%). The application of galvanic current (GC) has demonstrated its utility in humans in treating lesions structurally similar to those of HS. With this background, the main objective of this study was to evaluate the efficacy and safety of ultrasound-guided percutaneous GC in inflamed and/or draining tunnels of HS.
Methods
This was an open study (one-way repeated measures design over time). Patients were evaluated at 4 and 12 weeks after receiving GC. A combined clinical response at week 12 (absence of suppuration/inflammation on examination and clinical interview) was considered the principal variable of efficacy. Adverse effects potentially associated with GC were reported by telephone and at each visit.
Results
Twenty-six patients were included, with a male/female ratio of 5:8. The mean age was 35.84 (13.14) years. At 12 weeks after the administration of GC, a complete response was achieved in 77% (20/26) of the treated lesions. No serious adverse effects were observed, and the mean procedural pain assessed by the numeric rating scale was 0.03 (0.2).
Conclusion
GC has proven to be effective and well tolerated in inflamed and draining tunnels of patients with HS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Local therapies in hidradenitis suppurativa are necessary because of the recurrent nature of the disease. Currently, there are no intralesional therapies capable of physically eliminating the inflamed tunnels associated with hidradenitis suppurativa. |
The study aimed to evaluate the effectiveness and safety of percutaneous ultrasound-guided galvanic current (GC) therapy in HS tunnels, utilizing a 12-week repeated measures design. Effectiveness was assessed on the basis of symptom reduction and lesion dimensions via ultrasound, while safety was monitored for adverse effects related to GC administration. |
Significant symptom reduction and lesion dimension decrease were observed over time, with most patients achieving complete response by week 12 and experiencing mild, reversible adverse effects. |
Digital Features
This article is published with digital features, including an animation to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.25425787.
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory disease affecting the apocrine sweat glands located in the large skin folds [1, 2]. The estimated prevalence is around 0.7–1.2% in our environment, it is three times more frequent in women and starts in adolescence in about 50% of cases [1, 3].
Early lesions, inflammatory nodules, may evolve into abscesses and, if the inflammatory response is perpetuated, into draining tunnels [1, 4]. A key role in this response is played by the innate immunity pathway dependent on the NLRP3 inflammasome [5], which initiates the inflammatory process and is the stimulus for the release of cytokines involved in HS: tumour necrosis factor alpha (TNFα), interleukins (IL)-1β, 17 and 23 [6, 7]. On the other hand, an alteration of the local microbiota and the existence of bacterial biofilms inside the tunnels could act as an antigenic stimulus facilitating flares of suppuration and malodour [1, 2, 8].
Therapeutic options for HS differ according to severity and they range from topical antibiotics to surgical removal of the affected tissue [1, 2, 9, 10]. Biologic drugs that block molecular targets involved in the disease (TNFα, Il-17) are now available [1, 2, 9]. Unfortunately, classical intralesional therapies, necessary given the recurrent nature of HS (even under systemic therapy), have erratic resolution rates varying from 44% to 81% [10], an even more limited efficacy in draining tunnels [10].
In the absence of optimal intralesional therapy, interest arose in percutaneous galvanic current (GC). Its mechanism of action is based on the production of a stimulus that induces a new activation of the NLRP3 inflammasome to reinitiate the control of inflammation as well as the elimination of bacterial biofilms [5, 8]. GC causes a decrease in the release of IL-1β in macrophages in vitro [5], this interleukin being responsible for the transition of the abscess to tunnel formation [4, 6].
The administration of GC to fistulizing lesions using percutaneous needles has been effective in reducing inflammation in mamillary tunnels structurally similar to those that can be identified in HS [11].
Against this background, the main objectives of the study were to evaluate the effectiveness and safety of ultrasound-guided percutaneous GC in inflammatory and draining tunnels of HS.
Methods
Experimental Design
A one-way repeated measures design over time was used. Measures were obtained pre-intervention (time 1) and at 4 weeks (time 2) and 12 weeks (time 3) of follow-up. The study included patients older than 18 years with a clinical diagnosis of HS, presenting inflamed or draining tunnels in the areas affected by HS, in the absence of systemic treatment initiated in the last month. The candidate lesion to be treated had to be a single, structurally simple tunnel that did not communicate with other tunnels. Only one lesion was treated per patient. The study was approved by the Clinical Investigation Ethics Committee of the ‘Virgen de Las Nieves’ University Hospital (Granada, Spain).
Objectives
The aim of our study was to evaluate the effectiveness and safety of percutaneous ultrasound-guided GC administration in inflamed and/or draining tunnels of HS. Secondary objectives were to evaluate the ultrasound reduction of lesions treated with GC and to evaluate the persistence of remission in lesions in which a complete clinical response was achieved at week 4 (time 2).
Study Procedures
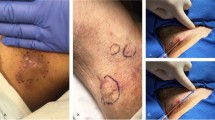
The Galvani-K® GC generator was used to administer GC, which consists of an adapter to which an Abbocath catheter (Fig. 1) is attached, and a contact socket to be held by the patient. The process begins with the administration of tumescent local anaesthetic in the area to be treated (100 ml of physiological saline, 2 ml of mepivacaine 2%, 1 ml of bupivacaine 0.5% with adrenaline, and 2 ml of bicarbonate 1 molar). Subsequently, the 14G Abbocath needle is inserted into the tunnel under ultrasound guidance, which is coupled to the negative electrode of the Galvani-K® GC generator equipment. Then, between 5 and 10 applications of polar, continuous and uninterrupted GC is performed with a constant intensity and a duration of 5 s. A second cycle of GC is administered after 4 weeks in case of no complete response (see Sect. “Complete Responses Achieved”). The procedure can be visualized in the audiovisual material provided (Video).
Galvani-K® device and ultrasound-guided procedure. a Galvanic current generator with built-in Abbocath catheter adapter and contact socket. b HS inflammatory tunnel before starting treatment. c Abbocath catheter incorporated inside the tunnel under ultrasound guidance. d Hyperechoic line of sclerosis after administration of 5 cycles of galvanic current and removal of the Abbocath catheter. e Reduction in perilesional edema, longitudinal diameter, and depth of the treated lesion after 12 weeks of treatment
Selection of Lesion to be Treated
Assessment of the lesion was based on physical and ultrasound examination. The tunnel must present spontaneous pain, pain on palpation, or active drainage 4 weeks prior to inclusion. If the patient has several lesions that meet the first criterion, the following criteria will be used to select the draining fistula in descending order of priority:
-
1.
Inflammatory or draining tunnel located over a Hurley II vs. Hurley III area.
-
2.
Inflammatory or draining tunnel that presents one path vs. several subcutaneous paths.
-
3.
Inflammatory or draining tunnel that does NOT have other lesions typical of the disease (inflammatory nodules, abscesses and other fistulas) in its proximity.
-
4.
Inflammatory or draining tunnel that, in the cutaneous ultrasound in mode B, does NOT present destructuring of the lower wall. Findings suggestive of deep extension and potential for associated abscess formation.
-
5.
Inflammatory or draining tunnel that, in the opinion of the investigator, is anatomically more accessible to perform the study procedures (ultrasound evaluation and follow-up) (Fig. 2).
Ultrasound-guided percutaneous galvanic current procedure. Abbocath needle is observed inside an inflammatory tunnel; as the galvanic current is applied, the Abbocath must be mobilized to contact the physical limits of the fistula, as it is withdrawn to cover the entire length of the tunnel (AVI 4783 KB)
Outcome Measures
Effectiveness
At each visit, pain, suppuration, malodour and pruritus were evaluated by means of a clinical interview; all of these features were scored by the patient on an 11-point numerical rating scale (NRS, with 0 equalling “no symptoms” and 10 equalling “very severe symptoms” used as anchors). Physical examination was used to evaluate the local inflammatory activity of the tunnel, assessing suppuration on vigorous digital compression, pain on palpation, or erythema.
The main outcome of the study was complete response at week 12, which was considered if no inflammatory signs were identified in the treated lesion (no erythema, no suppuration on vigorous digital compression and no pain on manipulation) and the patient reported no suppuration or pain in the previous week (0 out of 10 in NRS in the last week).
Those lesions in which a complete response was achieved at week 4 were re-evaluated by examination and clinical interview at week 12 to assess whether the remission achieved was maintained over time.
Other secondary outcome measures were the longitudinal and transverse diameters, the volume and the depth of the lesion, all of them determined by ultrasonography at each visit.
The Hurley stage and the International Hidradenitis Suppurativa Severity Score System-4 (IHS4) scales were used to determine structural damage of the treated area and overall disease inflammatory severity. Both Hurley and IHS4 were evaluated at the baseline visit and at week 12 post-treatment.
Safety
Adverse effects were assessed by telephone call 48 h after the intervention, as well as at each face-to-face visit after the first GC administration (weeks 4 and 12). Among the adverse effects under study, both local (haematoma, infection, paraesthesia, recurrence) and systemic (syncope, bleeding with anaemia, sepsis) complications were studied, any unfavourable event potentially related to the administration of GC was considered an adverse effect.
Ethical Approval
This study was conducted in accordance with the Helsinki Declaration of 1964 and was approved by the Ethics Committee for Biomedical Research of the Province of Granada (Internal code 1239-N-22). The participants in this study have given their informed consent for the publication of the details of their cases.
Statistical Analysis
Descriptive statistics were used to evaluate the characteristics of the sample. The Shapiro–Wilk test was used to assess the normality of the variables. Continuous variables are expressed as mean and standard deviation (SD). Qualitative variables are expressed as relative and absolute frequency distributions. Multiple analysis of variance (MANOVA) tests were performed to analyse the differences in ultrasound measurements and symptom intensity according to NRS at each visit. Statistical significance was considered if p values were less than 0.05. Statistical analyses were performed using JMP version 9.0.1 (SAS institute, North Carolina, USA).
Results
The baseline characteristics of the sample are summarized in Table 1.
Clinical Response
The results from MANOVA showed there were differences over time in the combined dependent variable from symptoms, p < 0.01 (Table 2). When the results were considered separately, the univariate analysis showed significant differences in suppuration, pain, itching and malodour. Figure 1 shows p values from univariate analyses and means scores for each symptom over time. In this regard, suppuration showed a mean reduction of 70% (1.34/4.62) at week 4 and 88.53% (0.53/4.62) at week 12, compared to baseline NRS values. Pain was reduced by 77.81% (0.69/3.11) at week 4 and by 85.21% (0.46/3.11) at week 12. Itching decreased 58.56% (1.5/3.62) at week 4 and 58.08% (1.53/3.65) at week 12. For malodour, a reduction from baseline values of 84.25% (0.57/3.62) was achieved at week 4 and 98.07% (0.07/3.62) at the final visit at week 12.
Ultrasound Response
In terms of ultrasound response, MANOVA tests showed differences over time in the combined dependent variable of ultrasound measurements. Significant differences were observed when analysing each of the ultrasound response variables independently by univariate analysis. Thus, longitudinal diameter decreased by 36.7% (18.06/28.53 mm) at week 4 and by 71.47% (13.2/28.53 mm) at week 12. The transverse diameter decreased by 25.4% (12.95/17.36 mm) at week 4 and by 52.59% (8.23/17.36 mm) at week 12. The reduction in depth achieved at week 4 was 32.81% (3.01/4.48 mm) and 40% (2.69/4.48 mm) at week 12. Volume experienced a 58.82% reduction (1103.58/2679.62 mm3) at week 4 and was reduced by 60.57% (1056.46/2679 mm3) at week 12.
Complete Responses Achieved
At week 4, 57.69% (15/26) of patients had achieved complete response and the remaining 11 patients required a second cycle of GC. At the week 12 visit, five of the 11 patients who required a second application of GC had made a complete clinical response, implying that, finally, 77% (20/26) of the patients in the sample had achieved complete response at week 12.
Finally, the 15 patients who achieved complete response at the week 4 visit persisted without clinical signs of inflammation and without pain or suppuration in the previous week, i.e. the treated lesions persisted in remission.
Overall Disease Inflammatory Severity
Ten out of the 26 patients in the GC group transitioned from being classified as Hurley stage II to Hurley stage I owing to the physical and sonographic disappearance of the fistulous structure. The mean baseline IHS4 score was 5.9; at 12 weeks, the mean IHS4 score was 2.5, indicating a reduction of 3.4 (0.72) points from baseline (p < 0.01).
Safety
Regarding safety, most patients experienced no adverse effects. In the telephone interview 48 h after the first cycle of GC, some patients reported dysaesthesia (15.4%; 4/26) or mild pain (15.4%; 4/26). The most frequent adverse effect referred by patients at the week 4 and week 12 visits was the development of a breakout of suppuration of the lesion, experienced by 15.4% (4/26) of patients at both visits (Fig. 3). The mean procedural pain assessed by NRS was 0.03; only one patient gave a pain score of 1 for the procedure, and the remaining 25 reported that the procedure was painless (they gave a pain score of 0 for the procedure). No serious or unexpected adverse effects were identified during the procedure. All adverse effects reported were mild, reversible and did not lead to discontinuation of treatment or additional medical care.
Ultrasonographic Factors of Response
A univariate analysis was designed to identify potential predictors of response. Ultrasonographic predictors of response to GC were thus identified: greater baseline lesion depth was associated with higher NRS pain scores at week 12 and a lower probability of complete response at weeks 4 and 12. Higher volume was also associated with higher NRS pain scores at the final visit.
Discussion
The present study evaluated the effectiveness and safety of the administration of GC in inflammatory and draining tunnels in patients diagnosed with HS, by means of a pilot study. The baseline sociodemographic and clinical features are similar to those of other studies that have analysed intralesional treatment options in HS (female predominance, mean age close to 35 years, high prevalence of smoking) [10, 12, 13]. In our study, 12 weeks after initiation of treatment, complete response (absence of signs of inflammation on physical examination and absence of pain or suppuration reported by the patient in the last week) was achieved in 77% of patients (20/26); most of these complete responses were achieved after a single administration of GC: 57.69% (15/26) of complete responses after 4 weeks of initiating GC. Most of the treated lesions experienced progressive ultrasound reduction with significant differences at each visit.
In a systematic review of 15 studies evaluating the different intralesional treatment options in HS, infiltration with intralesional triamcinolone acetonide obtained complete response of lesions in 44–70% of cases [10]. The variability in the efficacy obtained could be explained by the heterogeneity of the response assessment methods in each study, although the clinical criteria of absence of suppuration was the most frequently adopted element of response to therapy [10]. Another factor to take into account when comparing therapies is the nature of the lesions treated: in most studies, inflammatory nodules, abscesses and tunnels were included simultaneously [10]. Regarding this last point, a multicentre study of 135 individual lesions treated with intralesional infiltration of triamcinolone acetonide achieved complete response in 37.5% (9/24) of the treated tunnels, using a sample and criteria of response to treatment similar to our study (significant reduction of the lesion on clinical or ultrasound examination) [12].
Photodynamic therapy is another modality of intralesional therapy that has been classically employed. A case series of 38 patients achieved complete remission in 76.3% of the lesions treated [13], although the percentage of response is similar to that obtained in our work, the response achieved exclusively in tunnels is not specified.
In terms of safety, the administration of GC was considered painless by the patients; the most frequent adverse effect 48 h after the first cycle was mild, self-limited dysaesthesia of the treated area, and the most frequent at subsequent visits was an outbreak between cycles. The safety profile is similar to that obtained with intralesional corticosteroid therapy, and no atrophic scarring was recorded in our study [10]. Adverse effects derived from the administration of GC were mild and reversible. The tolerability described by patients treated with GC seems to exceed that reflected in studies evaluating the administration of photodynamic therapy or intralesional laser, where serious adverse effects (abscesses, fever, cellulitis) have also been described [10, 13].
A univariate analysis identified that the ultrasound parameters of depth and volume could potentially serve as predictors of response. However, the sample size of this study did not permit definitive conclusions to be drawn from these findings, and future larger studies are necessary.
In the present pilot study, GC appears to be an effective therapeutic alternative in inflammatory and draining HS tunnels, with a high response rate with a single administration of treatment. The fact of being a physical therapy, without the use of drugs with potential systemic absorption gives GC a favourable safety profile. As it does not require general anaesthesia, it can be applied in the office with a low cost in consumables.
The main limitations of our study are the lack of a control group in the design, the small sample size and the absence of follow-up of lesions beyond 12 weeks.
Based on the results of this study, our future research perspectives include the development of clinical trials in order to confirm our results and compare them with other classical intralesional treatments.
Conclusion
Intralesional galvanic therapy could be an effective and safe physical therapy in the treatment of inflamed or draining tunnels of HS.
Data Availability
All data generated or analyzed during this study are included in this published article. Data supporting the conclusions of this study are available upon request from the corresponding author.
References
Nguyen TV, Damiani G, Orenstein LAV, Hamzavi I, Jemec GB. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35(1):50–61. https://doi.org/10.1111/jdv.16677.
Scala E, Cacciapuoti S, Garzorz-Stark N, et al. Hidradenitis suppurativa: where we are and where we are going. Cells. 2021. https://doi.org/10.3390/cells10082094.
Molina-Leyva A, Cuenca-Barrales C. Adolescent-onset hidradenitis suppurativa: prevalence risk factors and disease features. Dermatology. 2019;235(1):45–50. https://doi.org/10.1159/000493465.
Dajnoki Z, Somogyi O, Medgyesi B, et al. Primary alterations during the development of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2022;36(3):462–71. https://doi.org/10.1111/jdv.17779.
Peñin-Franch A, García-Vidal JA, Martínez CM, et al. Galvanic current activates the NLRP3 inflammasome to promote type I collagen production in tendon. Elife. 2022. https://doi.org/10.7554/eLife.73675.
Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. https://doi.org/10.3389/fimmu.2018.02965.
Smith SDB, Okoye GA, Sokumbi O. Histopathology of hidradenitis suppurativa: a systematic review. Dermatopathol (Basel). 2022;9(3):251–7. https://doi.org/10.3390/dermatopathology9030029.
Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176(4):993–1000. https://doi.org/10.1111/bjd.15007.
Tchero H, Herlin C, Bekara F, Fluieraru S, Teot L. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85(3):248–57. https://doi.org/10.4103/ijdvl.IJDVL_69_18.
Cuenca-Barrales C, Montero-Vílchez T, Sánchez-Díaz M, et al. Intralesional treatments in hidradenitis suppurativa: a systematic review. Dermatology. 2022;238(6):1084–91. https://doi.org/10.1159/000524121.
de Berná-Serna JD, García-Vidal JA, Escolar-Reina MP, et al. A new treatment for mammillary fistulas using ultrasound-guided percutaneous needle electrolysis. J Clin Med. 2020. https://doi.org/10.3390/jcm9030649.
García-Martínez FJ, Vilarrasa Rull E, Salgado-Boquete L, et al. Intralesional corticosteroid injection for the treatment of hidradenitis suppurativa: a multicenter retrospective clinical study. J Dermatol Treat. 2021;32(3):286–90. https://doi.org/10.1080/09546634.2019.1655524.
Suárez Valladares MJ, Eiris Salvado N, Rodríguez Prieto MA. Treatment of hidradenitis suppurativa with intralesional photodynamic therapy with 5-aminolevulinic acid and 630nm laser beam. J Dermatol Sci. 2017;85(3):241–6. https://doi.org/10.1016/j.jdermsci.2016.12.014.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors had full control of the and made the final decision on all aspects of this publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest associated with the publication of this article and have no relevant financial interest in the findings of this manuscript. Alejandro Molina-Leyva is an Editorial Board member of Dermatology and Therapy. Alejandro Molina-Leyva was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This study was conducted in accordance with the Helsinki Declaration of 1964 and was approved by the Ethics Committee for Biomedical Research of the Province of Granada (CEI/CEIM Granada). The participants in this study have given their informed consent for the publication of the details of their cases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Soto-Moreno, A., Cuenca-Barrales, C., Arias-Santiago, S. et al. Safety and Effectiveness of Percutaneous Ultrasound-Guided Galvanic Current in Tunnels of Patients with Hidradenitis Suppurativa: A Pilot Study. Dermatol Ther (Heidelb) 14, 1115–1125 (2024). https://doi.org/10.1007/s13555-024-01149-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01149-5