Abstract
Introduction
The validated 40-gene expression profile (40-GEP) test independently stratifies risk of regional or distant metastasis for cutaneous squamous cell carcinoma (cSCC) tumors with high-risk clinicopathologic features. This study evaluated the stratification of risk by the 40-GEP test in a large cohort of tumors with one or more high-risk factors and in clinically relevant subgroups, including tumors within National Comprehensive Cancer Network (NCCN) high- and very-high-risk groups, lower-stage BWH T1 and T2a tumors, and patients > 65 years old.
Methods
This multicenter (n = 58) performance study of the 40-GEP included 897 patients. Kaplan-Meier analyses were performed to assess risk stratification profiles for 40-GEP Class 1 (low), Class 2A (higher) and Class 2B (highest) risk groups, while nested Cox regression models were used to compare risk prediction of clinicopathologic risk classification systems versus risk classification systems in combination with 40-GEP.
Results
Patients classified as 40-GEP Class 1, Class 2A, or Class 2B had significantly different metastatic risk profiles (p < 0.0001). Integrating 40-GEP results into models with individual clinicopathologic risk factors or risk classification systems (Brigham and Women’s Hospital, American Joint Committee on Cancer Staging Manual, 8th Edition) and NCCN demonstrated significant improvement in accuracy for prediction of metastatic events (ANOVA for model deviance, p < 0.0001 for all models).
Conclusion
The 40-GEP test demonstrates accurate, independent, clinically actionable stratification of metastatic risk and improves predictive accuracy when integrated into risk classification systems. The improved accuracy of risk assessment when including tumor biology via the 40-GEP test ensures more risk-aligned, personalized patient management decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Limitations of current risk classification systems have created a challenging environment where clinicians must determine whether the patient is at sufficient risk to warrant costly adjuvant therapies that may have side effects. |
The 40-gene expression profile (40-GEP) test was developed and validated to independently stratify metastatic risk for high-risk cSCC patients (those with one or more risk factors), and use of the test has led to clinical implementation to reduce both overtreatment of biologically low metastatic risk patients and undertreatment of patients with aggressive tumor biology. |
The goal of the current study was to determine whether, within a large cohort of patients who had been diagnosed with high-risk cSCC, the 40-GEP test would provide independent prognostic value for regional and distant metastasis when evaluated in the context of risk classification systems, individual risk factors and clinically relevant subgroups. |
The study demonstrates the ability of the 40-GEP test to be an independent predictor of metastatic risk when combined with National Comprehensive Cancer Network guidelines, Brigham and Women’s Hospital staging and American Joint Committee on Cancer Staging Manual, 8th edition, risk classification systems and that integration of the test with these systems significantly improved the accuracy of risk prediction. |
Accurate risk assessment is the foundation of clinical decision-making; therefore, incorporation of 40-GEP test results into standard clinical practice better guides patients to receive appropriate care and can positively impact healthcare resource utilization by allowing for better selection of patients that will benefit from treatment based on their biologic risk of metastasis. |
Introduction
Of the 1.8 million people in the US diagnosed with cutaneous squamous cell carcinoma (cSCC) each year [1,2,3], approximately 5–8% develop regional or distant metastasis, with annual mortality estimated to be higher than that for melanoma [4,5,6]. Consistent with cancer management in general, treatment pathways for cSCC are based upon population-based estimates of risk. Available risk classification systems for cSCC based on clinicopathologic features include National Comprehensive Cancer Network (NCCN) risk groups, Brigham and Women’s Hospital (BWH) staging and American Joint Committee on Cancer Staging Manual, 8th edition (AJCC8) [7,8,9]. BWH and AJCC8 use tumor characteristics to classify a patient’s risk of disease progression by T-stage, while NCCN includes both patient-specific and tumor factors to stratify risk to offer treatment options for prevention of local recurrence, metastasis or disease specific death. Unfortunately, several of these risk factors are subjective or difficult to measure (i.e., rapidly growing, clinical extent of tumor, neurologic symptoms) or have documented intra- and inter-rater histopathologic variability (grade of differentiation [10] and depth of invasion [11, 12]) equating to limited accuracy in determining metastatic risk [13, 14].
Gene expression profile (GEP) tests have been shown to improve risk stratification accuracy beyond clinicopathologic based staging systems for many types of cancer, including breast and prostate cancer, and cutaneous and uveal melanoma [15,16,17,18]. As there is currently no prognostic molecular signature for cSCC, the 40-gene expression profile (40-GEP) test was developed and validated to statistically and independently stratify metastatic risk for cSCC patients to inform treatment pathway decisions. The 40-GEP test uses predictive modeling to categorize patients diagnosed with primary cSCC having one or more high-risk factors (i.e., high-risk cSCC; the intended clinical use group comprised of an estimated 10–15% of all cSCC patients) into three risk categories with increasing metastatic risk, Class 1, Class 2A and Class 2B, with previously reported overall metastatic event rates of < 7%, 20% and > 50%, respectively [13, 14]. The intended use population is comprised of NCCN high or very high risk patients. Using an extended validation cohort, Ibrahim et al. [14], demonstrated the ability of the 40-GEP test to predict regional or distant metastasis in a cohort of 420 high-risk cSCC patients. Furthermore, 40-GEP class was an independent and significant predictor of metastasis when included in multivariate models adjusted for individual clinicopathologic risk factors or risk classification systems. Robust validation of the 40-GEP has led to clinical use of the test [19, 20] to improve precision risk stratification, resulting in reduction of potential overtreatment of biologically low-risk patients and undertreatment of patients with aggressive tumor biology. Algorithms have been developed that detail integration of test results into current treatment approaches [21].
Multiple studies have shown that clinicians use 40-GEP test results to improve treatment pathway decisions by incorporating tumor biology into risk classification systems so that patients move from population-based treatment pathway decisions to individual risk-based decisions informed by tumor biology [22,23,24]. Current areas of use include clinical decision-making for nodal assessment (primarily use of imaging), adjuvant radiation therapy (ART) and surgical or therapeutic intervention. These clinical usage management decisions demonstrate a risk-aligned reduction or de-escalation for low-risk, Class 1 patients and increased or escalation in higher-risk Class 2A and 2B patients [21, 23].
The goal of the current study was to present an independent validation of the 40-GEP test and then, after merging with a previous validation cohort, determine the performance of the 40-GEP test in providing independent prognostic value for the endpoints of regional and distant metastasis. A large cohort of high-risk cSCC further allowed for novel analyses within subgroupings of risk classification systems, individual risk factors and clinically relevant populations, which have not been assessed previously. Evaluation of a large cohort of patients enables a more robust establishment of the significant and independent contribution of the 40-GEP to risk stratification overall and within these novel subpopulations, which could not be achieved with a smaller cohort.
Methods
Study Cohort Enrollment and Data Acquisition
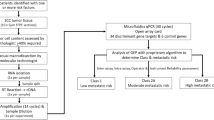
As previously described [14], archival formalin-fixed, paraffin-embedded (FFPE) cSCC tumor tissue, associated clinicopathologic and outcomes data, was obtained for each patient following institutional review board approval of the study protocol and waiver of patient consent. A consort diagram describing sample and patient inclusion and exclusion is shown in Fig. 1. Study inclusion/exclusion criteria were previously reported [14]. In short, patients were required to have at least 3 years of documented clinical follow-up if no metastatic events were observed. Patients with documented regional and/or distant metastasis within 3 years were required to have 2 years of clinical follow-up. Metastasis at the first location was recorded as the date of metastasis. Cases with prior history of cSCC, cutaneous basal cell carcinoma or melanoma in situ were permitted if prior malignancies were considered cured by the treating physician. The authors have obtained appropriate institutional review board (IRB) ethical approval via Western IRB (approval number 20162697), and a waiver of consent was obtained. The study adhered to the Declaration of Helsinki and its subsequent amendments.
The initial independent clinical validation cohort (n = 420) [14] was combined with a novel, independent performance cohort that used identical inclusion criteria (n = 534) (Supplemental Fig. 1A) for the purpose of creating a large, comprehensive study cohort that meets current clinical testing criteria and would enable test performance analysis within clinically relevant subgroups (n = 954) (Supplemental Fig. 1B). The final, primary cohort for analysis (n = 897) excluded patients who were treated with ART (n = 57) for the purpose of removing the bias on patient outcomes. All patient medical records were monitored for comprehensive clinicopathologic factors and staging, and a board-certified dermatopathologist independently reviewed tissue samples for tumor content and histologic risk factors. Assessed risk factors, based on medical records and/or the independent dermatopathologist review, were used to generate comprehensive risk classifications in accordance with each system’s criteria (AJCC8, BWH, NCCN Supplemental Table 1).
Gene Expression Analysis
All samples were analyzed using 40-GEP clinical testing standard operating procedures in a Clinical Laboratory Improvement Amendments (CLIA) certified, College of American Pathologists (CAP) accredited, New York State Department of Health permitted laboratory, as previously described [13, 14]. Laboratory personnel were blinded to patient outcomes. Samples with at least 40% tumor content were processed for real-time PCR and run in triplicate; duplicate sample runs were used to generate 40-GEP test results [25].
Statistics
The endpoint of metastasis-free survival (MFS), for regional (including nodal) or distant metastases, was used to analyze risk stratification by the 40-GEP test in this high-risk cSCC cohort. Kaplan-Meier plot and survival summaries, and associated log-rank tests, were used to determine significant stratification of risk. Association and interaction between 40-GEP and risk classification systems were assessed using the Cox model, and p value > 0.05 was defined as no significant interactions. Univariate and multivariate Cox regression analyses were also performed, and all variables were tested for violation of proportional hazards assumptions prior to modeling and were not in violation (p > 0.05). All clinicopathologic and patient-associated variables previously shown to contribute to risk assessment were included, regardless of significance of the variable in univariate analysis. Variables were interrogated for collinearity, and only modest relationships were noted (presence of perineural invasion with poor histologic differentiation and invasion of subcutaneous fat, R = 0.25 and 0.28, respectively). The likelihood ratio was calculated for each model and captures the relative amount of predictive power over a null model with no predictors. Models based on risk classification systems alone were compared to models that included 40-GEP results as nested Cox models via a model ANOVA (as analysis of deviance), based on the log likelihood of each model’s goodness of fit to the observed data. Positive (PPV) and negative (NPV) predictive values were calculated to assess the accuracy of metastasis-risk prediction for the 40-GEP in combination with clinical substages (BWH and AJCC8) or NCCN risk category.
Results
Cohort Demographics
The cohort's median age was 72 (range 26–95) years old (Table 1). The cohort included 25.6% (n = 230/897) immunosuppressed patients, of which 68.7% (n = 158/230) of immunosuppressed patients were transplant patients (data not shown). Sex, immunosuppression, location on the head or neck, type of surgery, tumor diameter, tumor thickness, poor differentiation status, presence of perineural invasion (PNI), lymphovascular invasion (LVI) and invasion beyond subcutaneous fat were significantly associated with metastasis (p < 0.01).
Association of NCCN Risk Categories and T-Stage with Metastatic Events
Separating cases into NCCN-defined (version 1.2024 [9]) risk groups (low risk, high risk and very high risk) identified 1.7% (n = 15) as low risk, 63.5% (n = 570) high risk and 34.8% (n = 312) very high risk (Table 1). Metastatic event rates within the high-risk and very-high-risk groups were 6.5% (37/570) and 26.0% (81/312), respectively (p < 0.001; no events were observed in the NCCN low-risk group). Similarly, both staging systems (BWH and AJCC8) demonstrated an association between T-stage and metastasis (p < 0.001), with event rates of 6.5% (29/444), 13.4% (45/335), 35.5% (33/93) and 44.0% (11/25) in BWH T1, T2a, T2b and T3 categories, respectively, and rates of 8.9% (44/496), 8.3% (18/216), 27.7% (46/166) and 52.6% (10/19) in AJCC8 T1, T2, T3 and T4 categories, respectively. Although the rates of metastasis increase with increasing stage within both BWH and AJCC, the majority of total number of metastatic events occurred in cSCC tumors categorized as low risk by BWH (T1/T2a) as well as AJCC8 (T1/T2).
Three-Year Metastasis-Free Survival (MFS) According to 40-GEP Risk Class
The MFS rate for the overall cohort was 87.5% at 3 years post-diagnosis, and the metastatic event rate inclusive of all follow-up times was 13.2% (Fig. 2). There was a total of 118 metastatic events: 83 were regional, 32 were regional/distant, and 3 were distant only. The majority of patients experiencing a distant metastatic event (32/35, 91%) also had a corresponding regional metastatic event.
Kaplan-Meier plot based on metastasis-free survival (MFS) for the combined cohort, demonstrating significant stratification by 40-GEP results (log-rank test). The table below the plot indicates the number of patients at risk annually for each 40-GEP risk class. The bottom table demonstrates 3-year MFS rates for each 40-GEP class and associated metastatic event rates, in addition to overall survival metrics for the cohort
The 40-GEP demonstrated clinically and statistically significant risk stratification for cSCCs classified as 40-GEP Class 1, Class 2A or Class 2B (log rank test, p < 0.001). The 3-year MFS rates were 94.1% for Class 1, 81.1% for Class 2A and 56.8% for Class 2B, and the metastatic event rates were 6.5%, 19.4% and 45.9% for Class 1, Class 2A and Class 2B, respectively. Most metastatic events (72%) occurred in Class 2A or Class 2B categories. The median time to regional and/or distant metastasis was 0.73 years (95th percentile: 3.27 years [data not shown]). Removal of the highest risk patients alone (LVI positive and BWH T3) did not change the findings or inflate the results (Supplemental Fig. 1C).
40-GEP Results Independently Improve Metastatic Risk Prediction Beyond Risk Classification Systems and Individual Clinicopathologic Risk Factors
Univariate analysis of 40-GEP risk prediction revealed that Class 2A and Class 2B groups had 3.2- and 9.4-fold higher risk of metastasis, respectively, compared to Class 1 (p < 0.001). Similar analysis for high-stage subsets for metastasis by BWH (T2b/T3), AJCC8 (T3/T4) and NCCN (very high) resulted in 4.8-, 4.0- and 4.6-fold higher risk of metastasis for each group, respectively, compared to lower staged subsets of BWH (T1/T2a), AJCC8 (T1/T2) and NCCN high risk (p < 0.001) (Table 2). Multivariate analysis including 40-GEP and risk classification systems was conducted to evaluate how these factors collectively predict metastatic events. Multivariate analysis demonstrated that clinical and biologic risk predictors were both independent and significant predictors of metastasis. When evaluated in the context of NCCN risk stratification, 40-GEP Class 2A and 2B had hazard ratios (HRs) of 2.4 and 6.0, respectively (p < 0.001); NCCN very high risk category had a HR of 3.6 (p < 0.001). Similar findings were observed when evaluating 40-GEP in the context of BWH and AJCC8 staging (Table 2). Importantly, when interaction terms between the 40-GEP test and binary risk classification (BWH, AJCC8 and NCCN) were added to the multivariate analysis, no significant interactions (p > 0.05) were observed, demonstrating both 40-GEP and risk classification systems are acting as independent prognosticators.
A second set of multivariate analyses was done to compare individual clinicopathologic risk factors with and without 40-GEP. For multivariate analysis without 40-GEP, clinicopathologic risk factors, including deep invasion (> 6 mm), poor differentiation and immunosuppression, were significant risk predictors, with HRs of 3.3, 3.2 and 2.1, respectively (p < 0.001; Table 3). Additionally, LVI, NCCN high-risk location (head and neck) and invasion beyond subcutaneous fat were also identified as significant risk factors for metastasis, with HRs of 2.7, 2.2 and 1.9, respectively (p < 0.01). Male patients and tumor diameter > 4 cm were also found to be significantly associated with a higher risk of metastasis, with HRs of 1.7 and 2.1, respectively (p < 0.05).
Inclusion of 40-GEP class into the multivariate analysis model described above (all factors included, see Methods) demonstrated Class 2A and Class 2B to be significant independent predictors of metastasis with HRs of 2.2 and 4.9, respectively (p < 0.001). The clinicopathologic risk factors of immunosuppression, poor differentiation and deep invasion (> 6 mm) also maintained significance with HRs of 2.2, 2.8 and 2.9, respectively (p < 0.001) (Table 3). Invasion beyond subcutaneous fat, LVI and location (head and neck) was also found to be significantly associated with a higher risk of metastasis, with HRs of 1.8, 2 and 1.92, respectively (p < 0.05).
40-GEP Significantly Improves Metastatic Risk Prediction When Combined with Risk Classification Systems
Given the independent metastatic risk prediction as demonstrated using multivariate analysis, we next evaluated the contribution of the 40-GEP to improving the accuracy compared to BWH, AJCC8 or NCCN risk classification systems. When risk classification alone models were compared to multivariate models that included the 40-GEP, a significant improvement in predictive accuracy was observed, with a significantly higher likelihood ratio when the 40-GEP is included with these systems (p < 0.0001 for all models) (Table 4). Furthermore, PPV and NPV were calculated within these risk classification systems to determine the accuracy of the 40-GEP test in predicting positive and negative outcomes (Table 5). When compared to each risk classification system alone, the addition of a 40-GEP Class 1 result both enhanced the NPV of these high-risk, yet lower staged (BWH T1/T2a; AJCC8 T1/T2; NCCN high-risk) cSCC patients and identified a significant NPV for higher staged (BWH T2b; AJCC8 T3; NCCN very-high-risk) patients. Additionally, a 40-GEP Class 2B result enhanced the PPV associated with higher staged patients and identified a significant PPV for lower staged patients. Specifically, incorporation of a Class 2B result was associated with PPVs ranging from 54 to 67%, while PPVs achieved by any of the clinicopathologic based risk classification systems ranged from 26 to 35%. This analysis shows that the inclusion of a 40-GEP result enhances the accuracy of metastatic risk prediction and indicates that the 40-GEP identifies a clinically actionable degree of metastatic risk when results are incorporated in the context of existing risk stratification frameworks used by clinicians treating patients with high-risk cSCC.
40-GEP Demonstrates Impactful Risk Stratification in Clinically Relevant Subgroups
NCCN Guideline Risk Groups
The risk stratification from NCCN into high- and very-high-risk groups is used to direct treatment pathways; patients in high-risk and very-high-risk subsets may be considered for more intensive surveillance, including use of imaging, consideration for adjuvant therapy, including ART and systemic therapies, or sentinel lymph node biopsy. As such, we aimed to evaluate the risk stratification value added by the 40-GEP within these two risk groups. For the NCCN defined high-risk subgroup, Kaplan-Meier analysis showed significantly different 3-year MFS rates for each 40-GEP class (97.0%, Class 1; 88.4%, Class 2A; 69.2%, Class 2B; p < 0.001; Fig. 3A). Class 2B cases have a fourfold increase in event rate compared to the overall cohort (30.7% vs 6.5%), while Class 1 cases exhibit a 50% reduction in event rates compared to the overall cohort (3% vs 6.5%). The 40-GEP also demonstrated significant risk stratification within the NCCN very-high-risk subgroup and identified groups with different MFS rates compared to the overall cohort rate of 76.0% (85.4%, Class 1; 72.2%, Class 2A; 50.0%, Class 2B; p < 0.001; Fig. 3B). Class 2B cases have a twofold increase in event rate compared to the overall cohort (54.1% vs 25.9%), while Class 1 cases exhibit a 35% reduction in event rate compared to the overall cohort (16.9% vs 25.9%).
Kaplan-Meier survival analysis subset by the National Comprehensive Cancer Network (NCCN, v1.2024) risk classification system demonstrated statistically significant 3-year metastasis-free survival (MFS) between all 40-GEP classes. A NCCN high risk, B NCCN very high risk; Tables as described in Fig. 2, p values indicate log-rank tests
Lower-Stage Tumors (BWH T1 and T2a)
While both BWH T1 and T2a staged tumors are expected to have a lower population-based rate of metastasis compared to higher staged tumors, all tested tumors in the cohort had one or more identifiable clinicopathologic risk factors, and 25% and 38% of metastatic events in this cohort were BWH T1 and T2a tumors, respectively (Supplemental Table 2). Risk stratification within BWH T1 tumors had an overall MFS rate of 93.7%, yet stratification by the 40-GEP test resulted in significantly different survival rates across 40-GEP classes within this T1 subset (97.3%, Class 1; 88.7%, Class 2A; 66.7%, Class 2B; p < 0.001; Fig. 4A). This stratification identified BWH T1 Class 2B tumors as having a > 5 × increase in event rate compared to the overall BWH T1 cohort (33.3% vs 6.5%) as well as identifying the BWH T1 Class 1 group as having a close to 50% reduction in the event rate (3.1% vs 6.5%).
Kaplan-Meier survival analysis of Brigham and Women’s Hospital (BWH) lower risk stages demonstrated statistically significant 3-year metastasis-free survival (MFS) between all 40-GEP classes. (A) BWH T1; (B) BWH T2a; Tables as described in Fig. 2, p values indicate log-rank tests
Clinically and statistically meaningful risk stratification was also seen within the BWH T2a tumors. Overall, BWH T2a tumors had an MFS rate of 87.2% and the 40-GEP classes also provided significant risk stratification within this group (93.1%, Class 1; 81.9%, Class 2A; and 63.6%, Class 2B; p < 0.001; Fig. 4B). This stratification identified a group of BWH T2a tumors with a > 2.5× increase in event rate for the Class 2B tumors compared to the overall BWH T2a cohort (36.4% vs 13.4%) as well as a close to 45% reduction in the event rate for Class 1 tumors (7.4% vs 13.4%). In both the BWH T1 and T2a subsets, a Class 2A result identified a level of metastatic risk that was similar to the contribution of an additional individual risk factor to metastatic prediction (based on HR; Table 3), with a 3.7- and 2.5-fold increase in metastasis over Class 1 results, respectively. Thus, the results support that 40-GEP stratifies risk for patients with metastatic event rates higher than, lower than and similar to the overall metastasis rate of the cohort, representing a clinically significant improvement in individualizing risk assessment for patients when informed by their biologic risk in addition to clinicopathologic factors used in risk classification.
Age-Associated Subsets
The average patient age at diagnosis of invasive cSCC for the overall cohort was 72 (range 26 to 95) years old. Given the advanced age of the majority of cSCC patients, the age-associated accumulation of comorbidities and accompanying complications is a distinct challenge to physicians when considering standard treatments. This risk-to-benefit calculation becomes even more challenging for intensive interventions used for those at a higher clinicopathologic-based risk. To address this, we tested the ability of the 40-GEP test to identify patients at low risk of metastasis who could safely forgo intensive treatments (a Class 1 result) or higher risk who may truly benefit from more invasive treatments (a Class 2A or 2B) at age-based quartile cut points (age ≥ 65 and ≥ 80 years old at diagnosis, 25th and 75th quartiles, respectively). For patients aged ≥ 65 years at the time of diagnosis, the 40-GEP test significantly stratified patient risk of metastasis, with MFS rates similar to the full cohort when younger patients were included (94.9%, Class 1; 82.1%, Class 2A; 55.6%, Class 2B [p < 0.001; Fig. 5A]). For patients ≥ 65 years of age at diagnosis, Class 1 patients showed a nearly 50% reduction in the event rate compared to the overall subset event rate, and Class 2A and 2B patients showed a nearly two- and fourfold increase in metastatic risk compared to the overall subset, respectively. For patients ≥ 80 years old at the time of diagnosis, growing concerns associated with comorbidities and invasive treatment options become even more central to risk-to-benefit calculations. Within this patient population, the 40-GEP significantly stratified metastatic risk, with MFS rates of 95.8% in Class 1, 80.9% in Class 2A and 61.5% in Class 2B (p < 0.001; Fig. 5B), again similar to the full cohort. The improved risk stratification demonstrated here identifies a subset of patients who could safely forgo potentially unnecessary adjuvant interventions for whom comorbidities and complications are of substantial concern [26].
Kaplan-Meier survival analysis of the ≥ 65 (25th quartile) and ≥ 80 (75th quartile) years of age at diagnosis subsets demonstrated statistically significant 3-year metastasis-free survival (MFS) between all 40-GEP classes. Tables as described in Fig. 2; p value indicates log-rank tests
Discussion
We have previously shown that the 40-GEP test provides clinically and statistically significant stratification of metastatic risk in cSCC patients with one or more clinicopathologic high-risk factors in both development and validation cohorts [13, 14]. Importantly, these studies also demonstrated that the 40-GEP provides independent prognostic value in multivariate models with clinicopathologic risk classification systems as well as individual risk factors. The analysis of a larger high-risk cSCC cohort enabled the current study to demonstrate a stronger validation of the performance of the 40-GEP. This cohort allowed for evaluation of test performance in novel, clinically meaningful subpopulations and enabled detailed analysis of the assay in combination with clinicopathologic risk stratification systems, an accomplishment not possible with smaller cohorts of patients.
The NCCN guidelines provide treatment pathway recommendations using traditional clinicopathologic risk factors. Recommendations include guidance for clinical follow-up and referrals, nodal assessment decisions (clinical exam, imaging using ultrasound or advanced imaging modalities, sentinel lymph node FNA or biopsy), ART and immunotherapy. The 40-GEP test improves the accuracy of risk stratification and enables personalized treatment and surveillance decisions. These refinements can result in escalation or de-escalation of treatment intensity.
Patients categorized as NCCN high or very high risk are eligible for consideration of ART; yet, as a group, most of these patients will not experience metastasis and will not benefit from radiation therapy. As demonstrated within this study, patients categorized as NCCN high risk with a Class 2B test result had an event rate of 30.7%, which is 19% higher than the NCCN very-high-risk group without 40-GEP. By comparison, a NCCN high-risk patient with a Class 1 test result had an event rate of 3.0%, which is 6% lower than the NCCN high-risk group without 40-GEP. Different treatment pathways may be considered as the study results suggest that the Class 2B patients would benefit from ART [27] and the Class 1 patients have a low likelihood of developing metastatic disease [14]. These results are consistent with previous studies and have led to the development of physician-derived NCCN-aligned patient management pathways that integrate the 40-GEP test with clinicopathologic features [11, 20, 21].
BWH T1 and T2a tumors lack the clinical factors, or a number of clinical factors, that are considered very high risk within the framework of this staging system but can occur in the setting of other high-risk features, including those identified as high risk or very high risk by NCCN guidelines (e.g., location on the head or neck, immunosuppression and LVI, among others) that are clinically concerning and confer eligibility for 40-GEP testing. Risk assessment in these lower staged subsets is important because as many as one-third of all metastatic events in cSCC patients have been observed in T1-staged patients alone because of the high denominator of patients diagnosed with T1 tumors [28]. Within this study, patients with BWH T1 or T2a tumors and Class 1 results experienced low event rates compared to patients with Class 2A or Class 2B tumors. In these patient subsets, the 40-GEP also identified patients who are considered low risk by current staging but harbor aggressive tumor biology with a substantial increase in metastatic risk associated with a Class 2B result. The Class 2A result adds a similar degree of metastatic risk as if a patient presented with an additional clinicopathologic risk factor. Consistent with clinical utility studies, the Class 1, 2A and Class 2B results each lead to risk-aligned changes in patient management (including ART, imaging and follow-up visit frequencies), demonstrating that clinical adoption of risk-aligned treatment plans informed by clinical, pathologic and 40-GEP data together results in comprehensively risk-aligned treatment plans for the benefit of patients [23, 24].
Test performance in the advanced age populations is particularly significant, given that patients generally have multiple age-associated comorbidities, increasing the need for improved risk stratification to identify patients who can safely forgo more invasive treatments given the increased potential for complications and substantial additions to healthcare costs, including Medicare. Removing younger patients did not impact the significant stratification of risk by the 40-GEP test, demonstrating strong clinical utility for patients diagnosed at ≥ 65 years of age, when comorbidity concerns can start to pose a particular challenge to clinicians. This also held true for patients diagnosed at ≥ 80 years of age, who more often present with additional age-related comorbidities. Thus, use of the 40-GEP can help clinicians identify patients in the population of patients of advanced age who can safely waive treatments and those who are at substantial risk of disease progression and therefore can benefit from more intensive treatment despite the potential for complications and increased cost. Risk-aligned management decisions about treatment options, such as ART [29,30,31] and immunotherapy [32,33,34], become increasingly important in the Medicare-eligible population as determining the risk-to-benefit ratio for a given patient can become increasingly complex. This highlights the need for additional risk stratification tools, such as the 40-GEP, to identify patients who can forgo treatments with high morbidity.
The multivariable analyses reported in Tables 2 and 3 and modeling reported in Table 4 show that using the 40-GEP test in combination with clinicopathologic risk assessment leads to more accurate determination of metastatic risk. Additionally, evaluation of accuracy metrics within the entire cohort of 897 cases demonstrated the improved PPV and NPV when including 40-GEP results with clinicopathologic factors and clinically actionable metastatic risk prediction within in stage or subset (Table 5). Based on the reported results, a Class 1 result in the context of a BWH stage T1, T2a or T2b diagnosis provides a level of metastatic risk that is lower than the risk that is expected based on staging alone, given the increased NPV for the combined result compared to staging alone. Conversely, when patients receive a Class 2B result, the observed increase in PPV compared to staging alone increases decision-making confidence to intensify management plans for the patient. Similar improvements in predictive values are also seen for both AJCC8 staging and NCCN risk groups.
A limitation of the study, and unfortunately, for the cSCC population as a whole, is that standard clinical and pathologic factors were not consistently recorded on pathology reports during the time period of this study. To address this limitation, histologic review was conducted on 100% of cases by an independent, board-certified dermatopathologist (blinded to patient outcome and 40-GEP result). Additionally, 100% monitoring of source documentation, including pathology reports, surgical reports and other medical records, was conducted.
Conclusion
In summary, this large, multicenter cohort study confirmed the performance of the 40-GEP test to provide clinically actionable and statistically significant risk stratification in patients diagnosed with high-risk cSCC defined as having one or more high-risk factors. The 40-GEP was an independent predictor of metastatic risk when combined with NCCN, BWH and AJCC8. In addition, the integration of the 40-GEP with NCCN, BWH or AJCC8 significantly improves the accuracy of risk stratification. The 40-GEP test allows clinicians to develop more precise treatment pathway decisions after considering the biology of the tumor in combination with clinicopathologic factors. Given the demonstrated improved outcomes in patients with high risk of metastasis from available treatment options, such as ART [29], management decisions that incorporate 40-GEP testing would be expected to positively impact healthcare resource utilization by allowing physicians to better select patients that will benefit from treatment based on their biologic risk of metastasis.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Skin Cancer Foundation. Our new approach to a challenging skin cancer statistic. The Skin Cancer Foundation; 2021. https://www.skincancer.org/blog/our-new-approach-to-a-challenging-skin-cancer-statistic/. Accessed June 14, 2021.
Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156(11):1192.
Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–7.
Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–66.
Soleymani T, Brodland DG, Arzeno J, Sharon DJ, Zitelli JA. Clinical outcomes of high-risk cutaneous squamous cell carcinomas treated with Mohs surgery alone: an analysis of local recurrence, regional nodal metastases, progression-free survival, and disease-specific death. J Am Acad Dermatol. 2023;88(1):109–17.
Marrazzo G, Zitelli JA, Brodland D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J Am Acad Dermatol. 2019;80(3):633–8.
Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on cancer, International Union against cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. JCO. 2014;32(4):327–34.
Amin MB, Edge S, Greene F, et al editors. AJCC cancer staging manual. 8th ed. Berlin: Springer International Publishing; 2017.
National Comprehensive Cancer Network. Squamous cell skin cancer (Version 1.2024). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed Nov 13, 2023.
Prezzano JC, Scott GA, Lambert Smith F, Mannava KA, Ibrahim SF. Concordance of squamous cell carcinoma histologic grading among dermatopathologists and Mohs surgeons. Dermatol Surg. 2021;47(11):1433–7.
Farberg AS, Fitzgerald AL, Ibrahim SF, et al. Current methods and caveats to risk factor assessment in cutaneous squamous cell carcinoma (cSCC): a narrative review. Dermatol Ther (Heidelb). 2022;12(2):267–84.
Yildiz P, Aung PP, Milton DR, et al. Measurement of tumor thickness in cutaneous squamous cell carcinomas: do the different methods provide better prognostic data? Am J Dermatopathol. 2020;42(5):337–42.
Wysong A, Newman JG, Covington KR, et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2021;84(2):361–9.
Ibrahim SF, Kasprzak JM, Hall MA, et al. Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test. Future Oncol. 2022;18(7):833–47.
Plasseraud KM, Cook RW, Tsai T, et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J Oncol. 2016;2016:5325762.
Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016;32(9):1599–604.
Scope A, Essat M, Pandor A, et al. Gene expression profiling and expanded immunohistochemistry tests to guide selection of chemotherapy regimens in breast cancer management: a systematic review. Int J Technol Assess Health Care. 2017;33:32–45.
Kohaar I, Petrovics G, Srivastava S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(8):1813.
Arron ST, Blalock TW, Guenther JM, et al. Clinical considerations for integrating gene expression profiling into cutaneous squamous cell carcinoma management. J Drugs Dermatol. 2021;20(6):5s-s11.
Farberg AS, Hall MA, Douglas L, et al. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: impact on patient management. Curr Med Res Opin. 2020;36(8):1301–7.
Singh G, Tolkachjov SN, Farberg AS. Incorporation of the 40-gene expression profile (40-GEP) test to improve treatment decisions in high-risk cutaneous squamous cell carcinoma (cSCC) patients: case series and algorithm. Clin Cosmet Investig Dermatol. 2023;16:925–35.
Litchman GH, Fitzgerald AL, Kurley SJ, Cook RW, Rigel DS. Impact of a prognostic 40-gene expression profiling test on clinical management decisions for high-risk cutaneous squamous cell carcinoma. Curr Med Res Opin. 2020;36(8):1295–300.
Hooper PB, Farberg AS, Fitzgerald AL, et al. Real-world evidence shows clinicians appropriately use the prognostic 40-gene expression profile (40-GEP) test for high-risk cutaneous squamous cell carcinoma (cSCC) patients. Cancer Invest. 2022;40(10):911–22.
Saleeby E, Bielinski K, Fitzgerald A, Siegel J, Ibrahim S. A Prospective, multi-center clinical utility study demonstrates that the 40-gene expression profile (40-GEP) test impacts clinical management for medicare-eligible patients with high-risk cutaneous squamous cell carcinoma (cSCC). J of Skin. 2022;6(6):482–96.
Borman S, Wilkinson J, Meldi-Sholl L, et al. Analytical validity of DecisionDx-SCC, a gene expression profile test to identify risk of metastasis in cutaneous squamous cell carcinoma (SCC) patients. Diagn Pathol. 2022;17:32.
Hirshoren N, Ruskin O, McDowell LJ, Magarey M, Kleid S, Dixon BJ. Management of parotid metastatic cutaneous squamous cell carcinoma: regional recurrence rates and survival. Otolaryngol Head Neck Surg. 2018;159(2):293–9.
Arron SA, Cañueto J, Siegel, J et al. Association of a 40-gene expression profile (40-GEP) with risk of metastatic disease progression of cutaneous squamous cell carcinoma (cSCC) and benefit of adjuvant radiation therapy (ART). In: Presented at American Society of Dermatologic Surgery Conference. November 2, 2023.
Eggermont C, Nené LEH, Koekelkoren FHJ, et al. The impact of routine ultrasonography on nodal metastasis in head and neck cutaneous squamous cell carcinoma: a retrospective multicentre cohort study. J Eur Acad Dermatol Venereol. 2023;37(9):e1136–40.
Ruiz ES, Kus KJB, Smile TD, et al. Adjuvant radiation following clear margin resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: a dual-center retrospective study. J Am Acad Dermatol. 2022;87(1):87–94.
Zhang J, Wang Y, Wijaya WA, Liang Z, Chen J. Efficacy and prognostic factors of adjuvant radiotherapy for cutaneous squamous cell carcinoma: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(9):1777–87.
Sahovaler A, Krishnan RJ, Yeh DH, et al. Outcomes of cutaneous squamous cell carcinoma in the head and neck region with regional lymph node metastasis: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2019;145(4):352–60.
Leiter U, Heppt MV, Steeb T, et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma (cSCC)—short version, part 2: epidemiology, surgical and systemic treatment of cSCC, follow-up, prevention and occupational disease. JDDG Journal der Deutschen Dermatologischen Gesellschaft. 2020;18(4):400–13.
Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305.
Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51.
Acknowledgements
The authors thank the following individuals and centers for their contributions to this project: David Beynet (VA-Los Angeles), Philip Scumpia (UCLA), Kimberly Brady (Roswell Park), David Pariser (Virginia Clinical Research, Inc), Ramona Behshad (Saint Louis University), Laura Ferris (University of Pittsburgh Medical Center), Mark Nestor (Skin and Cancer Associates), Thomas Knackstedt (MetroHealth-Cleveland), Augusti Toll (Hospital Clinic De Barcelona), Nima Gharavi (Cedars Sinai), Hugh Greenway (Scripps Health), Shawn Kwatra (Johns Hopkins), Kenneth Reed (Derm ASAP), Chrysalyne Schmults (Brigham and Women's Hospital), Ian Maher (University of Minnesota), Yang Xia (Brooke Army Medical Center), Leila Tolaymat (Mayo Clinic Florida), Charles Love, (Radiant Complexions-Iowa Dermatology), Joseph Curry (Thomas Jefferson University Hospital), Catherine Chung (Ohio State University), Diamondis Papadopoulos (Metro Derm- ACCR), Gene Kim (University of Southern California), Robert Bednarek (Parkview Research Center), Rogerio Neves (Penn State, Hershey), Christine Weinberger (University of Vermont), Hooman Khorasani (Mt Sinai), Martin Fleming (University of Tennessee Health Science Center), Simon Yoo (Northwestern University), James Lewis (University of Tennessee Medical Center-Knoxville), Keith Duffy (University of Utah), Evans Bailey (Naaman Clinic), Tim Hansen (McFarland Clinic), John Lyons (Mary Bird Perkins Cancer Center), Nathan Cleaver (Cleaver Dermatology), Manish Gharia (Ascension/Columbia St. Mary's), John Campana (Porter Adventist), Bruce Brockstein (Northshore University Health System), Emily Smith (University of Missouri). J Cañueto is partially supported by the (Gerencia Regional de Salud de Castilla y León (grants GRS2338/A/21 and GRS2549/A/22) and by the Instituto de Salud Carlos III through the project PI21/01207, co-funded by European Union. The authors also thank Sarah Kurley, PhD, for her contribution to the project. This work was conducted with support from Castle Biosciences, Inc. (CBI)
Medical Writing and Editorial Assistance.
The authors received no medical writing or editorial assistance for this article.
Funding
This study was supported by Castle Biosciences, Inc. (CBI), which provided funding for tissue and clinical data retrieval to contributing centers and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Sherrif Ibrahim, Sarah Arron and Ashley Wysong, Ally-Khan Somani contributed to the conception and design of the study. Sherrif Ibrahim, Julia Kasprzak, Aaron Farberg, Christie Regula, Anna Bar, David Brodland, Shlomo Koyfman, Ally-Khan Somani, Sarah Arron and Ashley Wysong, Javier Cañueto, contributed to acquisition of data. Alison Fitzgerald, Jennifer Siegel, Anesh Prasai and Matthew Goldberg contributed to the analysis and interpretation of data. Jennifer Siegel and Anesh Prasai performed statistical analysis of data. Sarah Arron, Anesh Prasai, Alison Fitzgerald, and Ashley Wysong drafted the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, have contributed important intellectual content for critically revising the manuscript, take responsibility for integrity of the data and approve the manuscript in its final form.
Corresponding author
Ethics declarations
Conflict of Interest
Ashley Wysong and Anna Bar receive research funding from CBI. Christie Regula, Ally-Khan Somani, and Sherrif Ibrahim are speakers and principal investigators for CBI. Aaron Farberg is consultant for CBI and has funding from Regeneron, Replimune, Enspectra Health, Gerson Lehrman Group. Alison Fitzgerald, Jennifer Siegel, Anesh Prasai, and Matthew Goldberg are employees, stock and options holders for CBI. Shlomo Koyfman is a consultant for CBI, Merck, BMS, Regeneron and Galera therapeutics; has research support from Merk, BMS and Regeron and honoraria from UpToDate and Varian. Sarah Arron is a paid consultant for Enspectra Health, CBI, and WorldCare Clinical; paid speaker bureaus for Regeneron and CBI; paid expert testimony from Forensis, Inc, and Muro & Lampe; travel expenses paid by CBI; paid participation on a data safety monitoring board for Replimune; paid advisory board participation for Regeneron, Dermatology Times/Multimedia Medical, and Matrix Medical; leadership in the International Transplant Skin Cancer Collaborative; stock holding in Rakuten Medical and Enspectra Health, with stock held by spouse in Genentech and 23andMe. All remaining authors participated as investigators for CBI during this study.
Ethical Approval
The authors have obtained appropriate institutional review board (IRB) ethical approval via Western IRB (approval number 20162697), and a waiver of consent was obtained. The study adhered to the Declaration of Helsinki and its subsequent amendments.
Additional information
Prior Presentation: Fall Clinical Dermatology Conference, Las Vegas, NV, October 19–22, 2023. American Society for Dermatologic Surgery, Chicago, IL, November 2–5, 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wysong, A., Somani, A., Ibrahim, S.F. et al. Integrating the 40-Gene Expression Profile (40-GEP) Test Improves Metastatic Risk-Stratification Within Clinically Relevant Subgroups of High-Risk Cutaneous Squamous Cell Carcinoma (cSCC) Patients. Dermatol Ther (Heidelb) 14, 593–612 (2024). https://doi.org/10.1007/s13555-024-01111-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01111-5