Abstract
Introduction
Involvement of the scalp is common in psoriasis and severely affects the quality of life of those affected. It is difficult to treat and places special demands on the galenics of a drug formulation. Tacrolimus is a calcineurin inhibitor and is approved as an ointment formulation for the treatment of atopic dermatitis. The efficacy and safety of topically applied tacrolimus have also been studied and proven for psoriasis. However, no proprietary pharmaceutical product is currently approved for this indication.
Methods
A multicenter, double-blind, vehicle-controlled phase 3 study was conducted to evaluate the efficacy and safety of 0.1% tacrolimus microemulsion when applied topically twice daily in 128 patients independently of sex with scalp psoriasis.
Results
The primary efficacy analysis showed a scalp Investigator Global Assessment (s-IGA) of 0 (absence of disease) or 1 (very mild disease) at 8 weeks in 28.6% of subjects in the tacrolimus group, indicating a significantly better response (p = 0.0476, chi-square test) versus 12.7% of subjects in the placebo group (difference of 15.9%-points). The Dermatology Life Quality Index (DLQI) improved over time and was more pronounced in the group treated with tacrolimus-containing microemulsion than in the placebo group, but showed no statistically significant difference after 8 weeks of use (p = 0.193, ANCOVA). The safety analysis revealed no evidence of cutaneous side effects other than those known. Toxicologically relevant serum levels of tacrolimus could be excluded.
Conclusion
The study data show that 0.1% tacrolimus microemulsion has good efficacy and safety in the treatment of scalp psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tacrolimus is a calcineurin inhibitor that is approved in topicals for atopic dermatitis, also has clinical data showing efficacy for the use in psoriasis, and has strong evidence regarding safety. |
Microemulsions are colloidal vehicles that are particularly suitable for stably dissolving tacrolimus and transporting it into the skin. Because of their low viscosity as well as their thermodynamic properties, they are particularly suited to the special features of the capillitium and epidermal hyperplasia in psoriasis. |
In a phase 3 study, the efficacy and safety of a 0.1% tacrolimus microemulsion was investigated in 128 subjects with mild to moderate scalp psoriasis testing a twice-daily application compared to vehicle. |
The primary efficacy analysis showed a s-IGA (0/1) at 8 weeks in 28.6% (95% confidence interval (CI) 17.42, 39.73) in the tacrolimus group, indicating a significantly better response (p = 0.0476, chi-square test) versus 12.7% (95% CI 4.48, 20.92) in the placebo group (difference of 15.9%-points). |
The safety analysis revealed no evidence of cutaneous side effects other than those known, and toxicologically relevant serum levels of tacrolimus could be excluded. |
The study data show that 0.1% tacrolimus microemulsion has good efficacy and safety in the treatment of scalp psoriasis. |
Introduction
Psoriasis is a T helper 1 (Th1)/Th17-mediated, chronic inflammatory systemic disease with phenotypic involvement of skin, skin appendages, joints, juxta-articular bones, and entheses [1]. Depending on the severity and duration of the disease as well as individual risk factors, comorbidity patterns may develop [2,3,4]. The prevalence of psoriasis in Europe is approximately 2% [5]. Topical, physical, and systemic therapeutic options are available for treatment, which have been evaluated by national and international guidelines. Depending on the severity of the disease, corresponding recommendations for action are outlined [6,7,8,9]. For the vast majority of patients with mild severity (approximately 80%), the use of topical agents is recommended as first-line therapy [7]. Topical agents are also used in combination with systemic therapies to accelerate the therapeutic response and to optimize local efficacy. For monotherapy, glucocorticoids of class II and III (according to Niedner), vitamin D derivatives, dithranol, tar preparations, and keratolytics are available [7]. In addition, combination preparations, especially of betamethasone dipropionate and calcipotriol or glucocorticoids and salicylic acid, have been proven effective for practical and pharmacological reasons.
Although topical monotherapy with calcineurin inhibitors is recommended in the guidelines, no preparation with a marketing authorization for the indication psoriasis is currently available [7]. The guideline recommendation for the use of tacrolimus is based on extensive data on the efficacy and safety of a 0.1% ointment preparation that is approved for atopic dermatitis [10,11,12,13,14]. These data are supplemented by study data on the therapy of psoriasis with experimental, partly higher-dose formulations [15,16,17]. From a pharmaceutical point of view, the problem with the tacrolimus formulations is predominantly the instability of the drug, which is thermally unstable and susceptible to hydrolysis. To circumvent this problem, an anhydrous vehicle in the form of a semi-solid ointment has been used so far. However, this vehicle does not meet the necessary requirements for a galenic therapy for psoriasis [18].
From a clinical point of view, affected areas of the body that do not sufficiently respond to conventional topical, and occasionally systemic therapy, are of particular interest. These “difficult to treat areas” include above all the scalp with a frequency of approximately 50% [19, 20]. Here, the capillitium, the adjacent forehead, and neck areas, but also the retroauricular area and the external auditory canals are relevant. A major reason for this insufficient response is the lack of suitability of the galenic formulations used, which do not correspond to the characteristics of follicle-rich, hairy skin [21]. The scalp features special conditions for the cutaneous bioavailability of topically applied drugs that are different from the penetration properties of interfollicular skin [22]. These result primarily from the pore pathway of diffusion, which is based on the anatomical conditions of the follicular structure. In this context, a low viscosity and the associated flow properties of the vehicle are of particular importance. When conventional vehicles are used for scalp psoriasis, two problems occur: the severe hyperkeratosis, which makes it difficult for the formulation to penetrate the follicular openings, as well as reduced contact between vehicle and scalp due to the adhesion of vehicle components to the hair shafts.
In order to fulfill the galenic requirements for the topical application of tacrolimus for the therapy of scalp psoriasis, a microemulsion system was developed that ensures a stable formulation of the drug tacrolimus. It has a low viscosity with good spreadability and allows the penetration of the drug not only by diffusion but also by the solvent drag effect. Additionally, the intrinsic effect of the vehicle reduces the hyperkeratosis of the psoriatic skin by its keratoemulsifying properties [18, 23, 24]. We hypothesized that tacrolimus 0.1% microemulsion will be more effective than tacrolimus-free vehicle.
Methods
Study Design
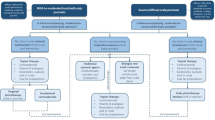
The study was a prospective, randomized, double blinded, placebo-controlled, multicenter, phase 3 study to evaluate efficacy and safety of a 0.1% tacrolimus-containing microemulsion in subjects with mild to moderate scalp psoriasis over an 8-week treatment period. After screening, subjects were randomized in a 1:1 ratio to tacrolimus-containing microemulsion twice daily or vehicle microemulsion without active ingredient twice daily. Thus 0.25 g per 1% body surface area (BSA), i.e., 5 to 6 drops of test preparation for an affected area equivalent to the size of the entire palm of the hand, was applied.
Ethical Approval
The study was performed in compliance with local laws and regulations, the Declaration of Helsinki, and the International Conference on Harmonization of Good Clinical Practice Guidelines. The study protocol was approved by the relevant independent ethics committees and national health authorities.
Subjects
The multicenter study was performed at 12 active sites in Germany (n = 9) and France (n =3) between July 2019 and August 2020. A total of 128 Caucasian subjects with mild to moderate scalp psoriasis were randomized after giving written informed consent. All subjects were in otherwise good health as determined by clinical and laboratory examinations at screening and eligible for study participation as assessed by predefined inclusion and exclusion criteria.
Study Assessments
Efficacy Parameters
The efficacy of the 0.1% tacrolimus-containing microemulsion on scalp psoriasis was evaluated by clinical assessments using the s-IGA scale (6-point scale of “absence of disease” (score 0), “almost clear” (score 1), “mild disease” (score 2), “moderate disease” (score 3), “severe disease” (score 4), and “very severe disease” (score 5)) as primary parameter, the scalp-modified Psoriasis Area and Severity Index (S-mPASI), the subject assessment of scalp pruritus (itch) using a 100-mm visual analogue scale (VAS), and the Dermatology Life Quality Index (DLQI) as secondary parameters [25,26,27,28].
Safety Parameters
Safety assessments included recording of adverse events (AEs), laboratory examinations including blood sampling for systemic bioavailability of tacrolimus (Synlab Analytics & Services Germany GmbH, München, Germany), and physical examinations.
Statistical Analyses
Data were summarized by means of summary statistics. Continuous data were presented with the number of observations, mean value, standard deviation (SD), minimum, 25th percentile, median, 75th percentile and maximum value. Categorical data were presented as counts and percentages. The data were presented for each treatment group by visit.
The primary endpoint, the proportion of subjects achieving an s-IGA after 8 weeks (visit 7) of treatment (tacrolimus-containing microemulsion vs. placebo) of 0 or 1, was analyzed using summary statistics: proportion of subjects in treatment group achieving s-IGA (0/1) as well as its 95% CI and the difference in proportions between the treatment groups. Furthermore, a continuity corrected chi-square test assessed whether the proportions of s-IGA (0/1) differed between the treatment groups. If the p value associated with this test was less than 0.05 and the proportion of subjects achieving an s-IGA (0/1) was greater in the group treated with the tacrolimus-containing microemulsion compared with the placebo treated group, it was concluded that the tacrolimus-containing microemulsion is efficacious.
The secondary efficacy analyses included the analysis of change from baseline for the ordered categorical variable s-IGA. Comparison of treatment group on each study visit was performed using Wilcoxon–Mann–Whitney test, as applicable. Comparison of category changes from baseline (visit 2) to each study visit by treatment groups was conducted using Friedman test.
Furthermore, the changes from baseline in the continuous variables S-mPASI, VAS (scalp pruritus), and DLQI were analyzed. Comparison of treatment group was performed using an analysis of covariance (ANCOVA). The ANCOVA model included treatment group and baseline value.
Non-compartmental analysis (NCA) was to be performed using tacrolimus plasma concentrations observed at visit 2 and visit 7, i.e., after the first and the last day of application of tacrolimus-containing microemulsion, respectively.
Results
Study Population
A total of 139 subjects were screened, resulting in the randomization of 128 subjects (32.0% male and 68.0% female subjects aged between 19 and 83) (Table 1). All 128 subjects received blinded study medication (tacrolimus-containing microemulsion or placebo) at least once applied to the affected areas of the scalp. A total of 103 subjects completed the study, whereas 25 subjects were prematurely discontinued. The primary reasons for premature discontinuation were lack of efficacy (6 subjects), AEs (3 subjects), withdrawal by subject (5 subjects), lost to follow-up (5 subjects), use of prohibited medication (1 subject), and other (5 subjects).
The data of all 128 randomized subjects were included in the safety analyses set. Data from 126 subjects (63 per treatment group) were valid for the primary analyses (full analyses set (FAS) using non-responder imputation (NRI)). The reason for exclusion from the FAS was that the respective subjects did not have at least one post-baseline assessment.
Primary Analysis Results of Efficacy
The primary endpoint of the study was the proportion of subjects achieving an s-IGA after 8 weeks (visit 7) of treatment of 0 (absence of disease) or 1 (very mild disease). In the primary efficacy analysis based on the FAS, the proportion of subjects achieving an s-IGA (0/1) was 28.6% (95% CI 17.42, 39.73) in the group treated with tacrolimus-containing microemulsion and 12.7% (95% CI 4.48, 20.92) in the placebo group, with a difference of 15.9%-points. The continuity corrected chi-square test indicates that the proportion of subjects with s-IGA (0/1) achieved under treatment with tacrolimus-containing microemulsion differed statistically significantly from the proportion under placebo treatment (p = 0.0476) and the proportion of subjects achieving an s-IGA (0/1) was greater in the group treated with tacrolimus-containing microemulsion compared with the placebo treatment group, showing efficacy of 0.1% tacrolimus-containing microemulsion.
The number of subjects with s-IGA (0/1) increased over time and was more pronounced in the group treated with tacrolimus-containing microemulsion compared with placebo group: after 8 weeks (visit 7) of treatment, there were 18 such subjects in the group treated with tacrolimus-containing microemulsion and 8 such subjects in the placebo group (Fig. 1).
Secondary Analysis Results
Secondarily the analysis of change from baseline for the ordered categorical variable s-IGA was performed. During the study, improvements in disease severity were obtained for both tacrolimus-containing microemulsion and placebo; however, improvements were more pronounced in the group treated with tacrolimus-containing microemulsion. After 8 weeks of treatment, a statistically significant difference (p = 0.0314) was achieved between the treatment groups. After 8 weeks of treatment (visit 7), the greatest proportion of subjects in the group treated with tacrolimus-containing microemulsion (38.5%) was in category 2 (mild disease), followed by category 1 (very mild disease; 30.8%). In the placebo group, these were 41.8% of the subjects in category 2 (mild disease) and 41.8% of the subjects in category 3 (moderate disease) (Fig. 2).
The S-mPASI improved over time and was more pronounced in the group treated with tacrolimus-containing microemulsion compared with placebo group. After 8 weeks of treatment (visit 7), the mean S-mPASI was 1.0 in the group treated with tacrolimus-containing microemulsion and 1.2 in the placebo group, which corresponds to mean changes from baseline of − 1.1 and − 0.9, respectively. The ANCOVA showed no significant difference between the treatment group on the S-mPASI score at visit 7 (p = 0.137).
The VAS (scalp pruritus) improved over time in both treatment groups and was more pronounced in the group treated with tacrolimus-containing microemulsion compared with placebo group. However, the rating at baseline differed between treatment groups: the mean VAS in the group treated with tacrolimus-containing microemulsion was 61.0 compared with 53.8 in the placebo group. After 8 weeks of treatment (visit 7), VAS was 32.6 in the group treated with tacrolimus-containing microemulsion and 33.8 in the placebo group, which corresponds to mean changes from baseline of − 27.8 and − 18.9, respectively. The ANCOVA for VAS (scalp pruritus) showed that there was no significant effect between the treatment group on the VAS score after 8 weeks (p = 0.357).
The DLQI improved over time and was more pronounced in the group treated with tacrolimus-containing microemulsion compared with placebo group. However, the rating at baseline differed between treatment groups: the mean DLQI in the group treated with tacrolimus-containing microemulsion was 8.3 compared with 6.7 in the placebo group. After 8 weeks of treatment (visit 7), mean DLQI was 3.8 in the group treated with tacrolimus-containing microemulsion and 3.8 in the placebo group, which corresponds to mean changes from baseline of − 4.6 and − 2.4, respectively. The ANCOVA for DLQI showed that there was no statistically significant difference of the DLQI at visit 7 between the treatment groups (p = 0.193).
Analysis of Safety Parameters
Overall, AEs were similarly distributed between treatment groups. A total of 140 AEs were reported in 71 (55.5%) subjects; 70 occurred in 35 (54.7%) subjects in the group treated with tacrolimus-containing microemulsion and 70 AEs in 36 (56.3%) subjects in the placebo group.
During the study no deaths and no serious adverse events (SAEs) related to treatment occurred. The proportion of subjects with AEs related to treatment was higher in the group treated with tacrolimus-containing microemulsion (18 AEs in 12 (18.8%) subjects) than in the placebo group (10 AEs in 8 (12.5%) subjects). The most frequent AEs that were related to the tacrolimus-containing microemulsion were skin and subcutaneous tissue disorders (tacrolimus-containing microemulsion, 8 AEs in 6 subjects (9.4%); placebo, 8 AEs in 7 subjects (10.9%)). The most frequently reported preferred term within this system organ class (SOC) was pruritus (tacrolimus-containing microemulsion, 6.3% of the subjects; placebo, 3.1% of the subjects). Furthermore, for one subject in the placebo group, application site pruritus (SOC: General disorders and administration site conditions) was reported.
Three AEs led to permanent discontinuation of treatment. All three were skin and subcutaneous tissue disorders (tacrolimus-containing microemulsion, exacerbation of body psoriasis and diffuse alopecia; placebo group, psoriasis flare-up).
For the large panel of safety laboratory parameters, no notable difference was observed between the two treatment arms and only single abnormal clinically significant measurements were observed during the study (four subjects) within both treatment groups. For vital signs and for the physical examination, no clinically significant findings were noticed during the study and only a single abnormal clinically significant finding for dermatologic examination of the skin (one in each treatment group) and general appearance (one subject in the group treated with tacrolimus-containing microemulsion).
The analysis of the systemic bioavailability of tacrolimus showed that concentrations observed both at visit 2 and at visit 7 were below the limit of quantification for all subjects and samples assessed. No subject showed blood concentrations greater than 1 ng/mL or greater than 5 ng/mL (the latter is associated with systemic immunosuppressive activity). Consequently, the presence of toxicologically relevant serum levels of tacrolimus could be excluded.
Discussion
The clinical need for effective and safe topicals for the treatment of scalp psoriasis is ongoing and is not adequately met by the currently available proprietary medicinal products [22]. As a result of the macro- and micromorphological characteristics of scalp altered by psoriasis, a galenic formulation specifically designed for this area of application is necessary in order to achieve sufficient cutaneous bioavailability of the applied drug. Also, patients expect formulations for use on hairy and scaly skin to have special physicochemical properties, so a vehicle that facilitates practical applicability increases adherence to therapy [29]. Therefore, formulations designed for psoriasis of the skin have very limited suitability for use on the scalp [30]. Despite numerous available treatment options for scalp psoriasis, therapeutic experience of patients and physicians is disappointing, sometimes even frustrating. This statement is confirmed by a survey of 17,990 patients with psoriasis in seven European countries [31]. The two main complaints were “time consuming” and “ineffective.” Also, most physicians underestimate the impact of scalp diseases on quality of life [32].
From a clinical perspective, the application of a tacrolimus-containing microemulsion not only offers optimized cutaneous bioavailability in the scalp area compared to conventional formulations but has also keratoemulsifying effects that significantly improve the diffusion conditions of the drug. As an ointment, tacrolimus is approved for the treatment of atopic dermatitis. In contrast to atopic dermatitis, plaques in psoriatic skin represent a larger barrier for the penetration of drugs and the physicochemical properties of tacrolimus itself (large molecular size, structure, and lipophilicity) are very disadvantageous for dermal drug delivery through the skin. Earlier studies found that tacrolimus as ointment was no more effective than placebo [33]. Therefore, a new formulation of tacrolimus with a significantly better skin penetration profile and a higher acceptance by patients would close the gap in the treatment of scalp psoriasis [18].
The main result of the current study was the statistically significant improvement of psoriatic lesions on the scalp with the topical administration of 0.1% tacrolimus-containing microemulsion. A significantly greater proportion of subjects achieved a s-IGA (0/1) in the group treated with tacrolimus-containing microemulsion compared with the placebo group (28.6% vs. 12.7%). Although 28.6% may seem low, it must be considered that this only includes patients who achieved a score of 0 or 1.
In atopic dermatitis, tacrolimus has been shown to be comparably effective to class II (according to Niedner) corticosteroids [34, 35]. For scalp psoriasis class III and IV (according to Niedner) are commonly used, very often as combination therapy with either calcipotriene or salicylic acid [32, 35]. Since prolonged treatment of scalp psoriasis with topical corticosteroids is not recommended because of the lack of data supporting the long-term safety and efficacy of topical steroids, the use of these combination therapies is limited [36]. Because of the lower percutaneous absorption of corticosteroids, however, the scalp is more resistant to atrophy, but it does occur (e.g., percutaneous absorption of cortisol is 6% on forehead, 3.5% on the scalp) [37]. Thus, continuous twice-daily application of a corticosteroid should be limited to a 2-week course, which highlights the need for therapeutic alternatives.
The secondary efficacy endpoint analysis comprised the change from baseline to the end of treatment after 8 weeks for the severity scores s-IGA, S-mPASI, and VAS (scalp pruritus) as well as the DLQI. Improvements over time were observed in all scores analyzed in both treatment groups and were more pronounced in the group treated with tacrolimus-containing microemulsion but surprisingly also in the placebo group. This underlines not only the benefit of the drug-related effect but also the improved outcome related to the vehicle itself, which has a keratoemulsifying effect, significantly improving mPASI by reducing scaling [23]. The keratoemulsifying effect of the vehicle is also a possible explanation for the relatively small difference between the treatment groups regarding the primary endpoint. The characteristics of the microemulsion also allow an effective administration to the hairy and psoriatic scalp. Visible ointment traces and oily and smeary hair that would further affect the quality of life are reduced, thereby improving the acceptance by patients.
In general, the results of this study are in line with a 2016 review of at least 23 studies involving more than 800 subjects evaluating topically applied tacrolimus, showing its general efficacy in treating different types of psoriasis in different areas [11, 38,39,40,41].
Study Limitations
As a result of the 8-week duration of the placebo-controlled phase 3 trial, the current analysis did not provide information about the long-term efficacy and safety of 0.1% tacrolimus-containing microemulsion in patients with mild to moderate scalp psoriasis. Also, no direct comparison to other treatments is possible as an active comparator arm was lacking in the study. Those aspects must be addressed in further studies. Furthermore, it has to be considered that the baseline values for S-mPASI, VAS, and DLQI differed between tacrolimus-containing microemulsion and vehicle, and that the number of subjects treated (64 per group) plus the defined inclusion and exclusion criteria cannot represent the average patient population.
Conclusion
Topical 0.1% tacrolimus-containing microemulsion appears to be an effective and safe option for treatment of scalp psoriasis.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because the data has not yet been released by the sponsor of the study.
References
Prinz I, Sandrock I, Mrowietz U. Interleukin-17 cytokines: effectors and targets in psoriasis-a breakthrough in understanding and treatment. J Exp Med. 2020. https://doi.org/10.1084/jem.20191397.
Branisteanu DE, Nicolescu AC, Branisteanu DC, et al. Cardiovascular comorbidities in psoriasis (Review). Exp Ther Med. 2022;23:152.
Branisteanu DE, Pirvulescu RA, Spinu AE, et al. Metabolic comorbidities of psoriasis (Review). Exp Ther Med. 2022;23:179.
Jalenques I, Bourlot F, Martinez E, et al. Prevalence and odds of anxiety disorders and anxiety symptoms in children and adults with psoriasis: systematic review and meta-analysis. Acta Derm Venereol. 2022;102:adv00769.
Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
Nast A, Altenburg A, Augustin M, et al. German S3-Guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm - Part 2: Treatment monitoring and specific clinical or comorbid situations. J Dtsch Dermatol Ges. 2021;19:1092–115.
Nast A, Altenburg A, Augustin M, et al. German S3-Guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm - Part 1: treatment goals and treatment recommendations. J Dtsch Dermatol Ges. 2021;19:934–150.
Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34:2461–98.
Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of psoriasis vulgaris - Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol. 2021;35:281–317.
De Simone C, Maiorino A, Tassone F, D’Agostino M, Caldarola G. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open-label study. J Eur Acad Dermatol Venereol. 2013;27:1003–6.
Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153–63.
Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double-blind clinical studies conducted in Japan. Clin Drug Investig. 2006;26:235–46.
Ring J, Mohrenschlager M, Henkel V. The US FDA “black box” warning for topical calcineurin inhibitors: an ongoing controversy. Drug Saf. 2008;31:185–98.
Huang X, Xu B. Efficacy and safety of tacrolimus versus pimecrolimus for the treatment of atopic dermatitis in children: a network meta-analysis. Dermatology. 2015;231:41–9.
Gabriel D, Mugnier T, Courthion H, et al. Improved topical delivery of tacrolimus: a novel composite hydrogel formulation for the treatment of psoriasis. J Control Release. 2016;242:16–24.
Jindal S, Awasthi R, Singhare D, Kulkarni GT. Topical delivery of tacrolimus using liposome containing gel: an emerging and synergistic approach in management of psoriasis. Med Hypotheses. 2020;142: 109838.
Lapteva M, Mondon K, Moller M, Gurny R, Kalia YN. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11:2989–3001.
Goebel AS, Neubert RH, Wohlrab J. Dermal targeting of tacrolimus using colloidal carrier systems. Int J Pharm. 2011;404:159–68.
Queiros CS, Duarte GS, Costa J, Vaz-Carneiro A. Analysis of the cochrane review: topical treatments for scalp psoriasis Cochrane Database. Syst Rev Acta Med Port. 2017;30:163–8.
Merola JF, Li T, Li WQ, Cho E, Qureshi AA. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486–9.
Kivelevitch D, Frieder J, Watson I, Paek SY, Menter MA. Pharmacotherapeutic approaches for treating psoriasis in difficult-to-treat areas. Expert Opin Pharmacother. 2018;19:561–75.
Wohlrab J, Michael J. Topical therapy of the scalp. Hautarzt. 2017;68:478–82.
Wohlrab J. Influence of keratolytics on cutaneous pharmacokinetics of glucocorticoids. J Dtsch Dermatol Ges. 2021;19:554–61.
Wohlrab J, Staubach P, Augustin M, et al. S2k-Leitlinie zum Gebrauch von Praparationen zur lokalen Anwendung auf der Haut (Topika). J Dtsch Dermatol Ges. 2018;16:376–92.
Jemec GB, Ganslandt C, Ortonne JP, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59:455–63.
van de Kerkhof PC, Hoffmann V, Anstey A, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. Br J Dermatol. 2009;160:170–6.
Krell J, Nelson C, Spencer L, Miller S. An open-label study evaluating the efficacy and tolerability of alefacept for the treatment of scalp psoriasis. J Am Acad Dermatol. 2008;58:609–16.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6.
Zampieron A, Buja A, Fusco M, et al. Quality of life in patients with scalp psoriasis. G Ital Dermatol Venereol. 2015;150:309–16.
Ghafoor R, Patil A, Yamauchi P, et al. Treatment of scalp psoriasis. J Drugs Dermatol. 2022;21:833–7.
Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155:729–36.
Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448–59.
Zonneveld IM, Rubins A, Jablonska S, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. a pilot study. Arch Dermatol. 1998;134:1101–2.
Svensson A, Chambers C, Ganemo A, Mitchell SA. A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. Curr Med Res Opin. 2011;27:1395–406.
Niedner R. Topically administered glucocorticosteroids. Part 2: dose effect–preparations. Fortschr Med. 1992;110:345.
Papp K, Berth-Jones J, Kragballe K, Wozel G, de la Brassinne M. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151–60.
Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181–3.
Wang C, Lin A. Efficacy of topical calcineurin inhibitors in psoriasis. J Cutan Med Surg. 2014;18:8–14.
Amiri D, Schwarz CW, Gether L, Skov L. Safety and efficacy of topical calcineurin inhibitors in the treatment of facial and genital psoriasis: a systematic review. Acta Derm Venereol. 2023;103:00890.
Dattola A, Silvestri M, Bennardo L, et al. Update of calcineurin inhibitors to treat inverse psoriasis: a systematic review. Dermatol Ther. 2018;31: e12728.
Guenther L, Lynde C, Poulin Y. Off-label use of topical calcineurin inhibitors in dermatologic disorders. J Cutan Med Surg. 2019;23:27S-34S.
Acknowledgements
The authors thank the participants of the study. Moreover, the authors wish to thank Dr. Walter Wigger-Alberti and Ragna Williams (bioskin GmbH, Hamburg, Germany) for involving in the background research and presentation of the study results on a contract research basis. Data analysis was performed by TFS Trial Form Support AB, Lund, Schweden.
List of the ScaTAC study group investigators: France: Prof. Dr. Carle Paul (Department of Dermatology, Larrey University Hospital, Toulouse, France), Prof. Dr. Thierry Passeron (Department of Dermatology, Archet 2 hospital, Nice, France), Dr. Pascal Reygagne (Centre d'étude de la peau et du cheveu, Hôpital Saint Louis, Paris, France), Dr. Mireille Ruer (Centre Hospitalier de Martigues, Martigues, France); Germany: PD Dr. Andreas Pinter (Department of Dermatology, University Hospital Frankfurt am Main, Frankfurt am Main, Germany), Prof. Dr. Stefan Beissert (Department of Dermatology, University Hospital Carl Gustav Carus Dresden, Dresden, Germany), Prof. Dr. Thomas Dirschka (Centroderm GmbH, Wuppertal, Germany), PD Dr. Sascha Gerdes (Psoriasis-Center Kiel, Department of Dermatology, University Medical Center Schleswig-Holstein, Kiel, Germany), Dr. Nicolas Leitz (Triderm Study Center, Stuttgart, Germany), Dr. Sylvia Pauser (Klinische Forschung Osnabrück (KliFOs), Osnabrück, Germany), PD Dr. Athanasios Tsianakas (Clinic of Dermatology, Fachklinik Bad Bentheim, Bad Bentheim, Germany), Dr. Oliver Weirich (Rosenpark Research GmbH, Darmstadt, Germany), Dr. Thomas Wildfeuer (Practice for Skin and venereal Diseases, Berlin, Germany), Dr. Andreas Kleinheinz (Dermatology Center, Elbe Kliniken, Buxtehude, Germany), Dr. Johannes Niesmann (Centre for clinical Studies, Skin Center Jahrhunderthaus, Bochum, Germany), Dr. (IM Temeschburg) Adrian Crainic and Dipl.-Med. Ridwan Weber (Group Practice for Skin and venereal Diseases, Augsburg, Germany). Carle Paul, Thierry Passeron, Pascal Reygagne, Mireille Ruer, Andreas Pinter, Stefan Beissert, Thomas Dirschka, Sascha Gerdes, Nicolas Leitz, Sylvia Pauser, Athanasios Tsianakas, Oliver Weirich, Thomas Wildfeuer, Andreas Kleinheinz, Johannes Niesmann, Adrian Crainic, Ridwan Weber.
Funding
The preparation of this article was supported by a grant from Bay Pharma GmbH, Hamburg, Germany.
Author information
Authors and Affiliations
Consortia
Contributions
AP: concept, study design, principal investigator, correction of manuscript. AT: co-investigator, correction of manuscript. AE: drafting manuscript, corresponding author.
Corresponding author
Ethics declarations
Conflict of Interest
All authors have nothing to disclose. Andreas Pinter acted as principal investigator and Athanasios Tsianakas as investigator in the presented trial.
Ethical Approval
The final study protocol (final draft V3.0, 17-Jan-2019) was approved by the responsible ethics committee of Medical Faculty, Johann Wolfgang Goethe University, Frankfurt/Main (vote of 17 May 2019; no. 75/19 F). Furthermore, the study was approved by the ethics committees of the different study groups. The study was conducted in compliance with the protocol, regulatory requirements, good clinical practice (GCP) and the ethical principles of the latest revision of the Declaration of Helsinki as adopted by the World Medical Association.
Additional information
For a complete list of ScaTAC study group investigators, see the Acknowledgments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pinter, A., Tsianakas, A., Eichner, A. et al. Efficacy and Safety of Topical Tacrolimus Microemulsion Applied Twice Daily in Patients with Mild to Moderate Scalp Psoriasis. Dermatol Ther (Heidelb) 14, 521–532 (2024). https://doi.org/10.1007/s13555-024-01102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01102-6