Abstract
Introduction
Psoriasis is a chronic inflammatory condition affecting the skin, joints, and several other organ systems with significant disease burden. Bimekizumab is the first monoclonal antibody targeting both interleukin (IL)-17A and interleukin-17F and has demonstrated efficacy for treating moderate to severe psoriasis. Limited guidelines exist for incorporating this drug into clinical practice. The purpose of this study was for a panel of experts in psoriasis management to synthesize current literature and provide consensus statements with guidance on use of bimekizumab.
Methods
A comprehensive literature search of PubMed, Scopus, and Google Scholar was completed for English-language original research articles on the use of bimekizumab for moderate to severe psoriasis and psoriatic arthritis. A panel of nine dermatologists with significant expertise in treatment of psoriasis gathered to review the articles and create consensus statements on this new medication. A modified Delphi process was used to approve each statement and a strength of recommendation was assigned using Strength of Recommendation Taxonomy criteria.
Results
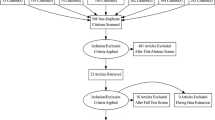
The literature search produced 102 articles that met criteria. A thorough screening of the studies for relevance to the research question resulted in 19 articles. These were distributed to all panelists for review prior to a roundtable discussion. The panel unanimously voted to adopt 14 consensus statements and recommendations, 12 of which were given a strength of “A”, one of which was given a strength of “B”, and one of which was given a strength of “C”.
Conclusion
Bimekizumab results in rapid and long-lasting clinical improvement for patients with moderate to severe plaque psoriasis and psoriatic arthritis. It has demonstrated superior efficacy when compared to several other biologics. The safety profile is consistent with other biologics, except for an increased incidence of oropharyngeal candidiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Psoriasis is a chronic condition, and many patients continue to search for a regimen that adequately treats their disease and offers long-term disease control. |
Bimekizumab results in rapid and long-lasting clinical improvement for patients with moderate to severe plaque psoriasis and psoriatic arthritis. |
Head-to-head trials of bimekizumab with other biologics demonstrate its greater efficacy and faster response. |
Bimekizumab has a durable response overtime with a sustained response if drug withdrawal is required. |
Introduction
Plaque psoriasis is an immune-mediated inflammatory condition affecting skin, joints, and several other systems [1]. As a result of its chronic relapsing–remitting course, psoriasis has a considerable effect on quality of life [1, 2]. Psoriasis affects 1–3% of the worldwide population [3] and has an incidence of 63.8 per 100,000 person-years in the USA [4]. There are a variety of treatments available, including both topical and systemic agents [1]. Biologic therapies targeting tumor necrosis factor (TNF), interleukin (IL)-12/23, IL-17, and IL-23 have shown efficacy in the management of moderate to severe plaque psoriasis [5].
The IL-17 family of cytokines are key mediators of disease in psoriasis [6]. Specifically, IL-17A and IL-17F share high homology and are frequently co-expressed, elevated in psoriatic vs. non-psoriatic skin, and are primarily responsible for pathogenesis of psoriatic changes [6, 7]. Currently approved IL-17 biologic therapies include the IL-17A inhibitors secukinumab and ixekizumab [8, 9] and the IL-17RA inhibitor brodalumab, which targets a common receptor subunit for multiple IL-17 family cytokines [10]. Bimekizumab is the first monoclonal IgG antibody to target both IL-17A and IL-17F [11, 12], and recently received approval by the US Food and Drug Administration (FDA) for treatment of moderate to severe plaque psoriasis. In multiple phase II and III trials, bimekizumab led to rapid and long-lasting clinical improvement for patients with moderate to severe plaque psoriasis and psoriatic arthritis (PsA) [13,14,15,16,17,18,19,20,21]. It has a safety profile consistent with other biologics, apart from an increased incidence of oropharyngeal candidiasis [22]. Real-world data also supports the efficacy of bimekizumab for plaque psoriasis [23] and there is case evidence for its use in erythrodermic psoriasis [24].
Despite the advancements in biologic therapies, there remain patients with plaque psoriasis that continue to struggle with achieving and maintaining adequate disease control [1, 25]. Given clinical evidence of bimekizumab’s efficacy in psoriasis, it may be a valuable alternative for patients with moderate to severe plaque psoriasis. The purpose of this study was for a panel of experts in psoriasis management to synthesize current literature and provide consensus statements with guidance on use of bimekizumab.
Methods
Literature Search and Study Selection
A comprehensive literature search of PubMed, Scopus, and Google Scholar was completed on July 17, 2023, using the keywords “psoriasis,” “IL-17,” and “bimekizumab” along with the Boolean term “AND” for English-language original research articles, systematic reviews, and meta-analyses without date restrictions. This study did not require institutional review board approval. Articles were screened for relevance to the treatment of moderate to severe psoriasis and PsA with bimekizumab. A nine-person consensus panel was selected for their expertise in the management of plaque psoriasis. The experts in the panel included Benjamin Lockshin, MD, Jeff Crowley, MD, Joseph F. Merola, MD MMSc, Ken Gordon, MD, Mona Shahriari, MD, Neil J. Korman, MD, PhD, Raj Chovatiya, MD, PhD, Robert Kalb, MD, Mark Lebwohl, MD.
The articles that met inclusion criteria were distributed to the panelists, and each member of the panel reviewed the selected studies and assigned them a level of evidence based on Strength of Recommendation Taxonomy (SORT) criteria [26]. These levels include level 1 (good-quality patient-oriented evidence), level 2 (limited-quality patient-oriented evidence), or level 3 (other evidence such as consensus guidelines, usual practice, opinion, or disease-oriented evidence) [26]. Importantly, for retrospective studies or basic science articles that focus on disease states, a respective level 2 or 3 designation is required and does not necessarily stipulate a deficient study.
Development of Consensus Statements
The panel consisted of nine dermatologists with expertise in treatment of psoriasis. The panel convened on August 25, 2023, to review and discuss the studies and create consensus statements with guidance on the use of bimekizumab for psoriasis. In order to reach consensus for each statement, a modified Delphi process was utilized [27]. This process requires supermajority approval for adoption of a recommendation through multiple rounds of real-time voting and is a regularly utilized method to create expert recommendations in dermatology [28,29,30,31]. All panel experts involved in the study are authors of the manuscript and were informed of the study’s objectives. All of the experts support the submission of this manuscript to a peer-reviewed journal.
Results
Literature Search and Study Selection
The literature search resulted in 102 articles that met the search criteria. After a comprehensive screening process, 19 articles were selected as relevant to the research questions. These articles were distributed to the panelists for evaluation prior to the roundtable discussion.
Levels of Evidence Designation
For the 19 articles that were evaluated, the panel assigned level 1 evidence to 16 articles, level 2 evidence to one article, and level 3 evidence to two articles (Table 1).
Consensus Statements
The panel developed 14 consensus statements regarding bimekizumab and the treatment of moderate to severe psoriasis and PsA. Of the 14 statements, 13 received a unanimous (9/9) vote for adoption, and one statement received 7/9 votes for adoption. SORT criteria were utilized to assign a strength of recommendation for each of the statements and recommendations (Table 2).
Statement 1: There is an unmet need for additional treatments for psoriasis. (SORT Level A)
Psoriasis is a chronic condition, and many patients continue to search for a regimen that adequately treats their disease and offers long-term disease control [1, 25]. Patients seek alternative therapies for a variety of reasons, including loss of response to current therapy, failure of medications in the same class or alternative classes of biologics, worsening disease severity, and/or desire for faster results in response to treatment [32, 33]. With bimekizumab, over 50% of patients achieve Psoriasis Area and Severity Index (PASI) 100 at the end of the placebo-controlled period, which has not been reported with any prior psoriasis therapies [34].
In addition, there is a need for more effective treatments for PsA [35, 36]. For patients on advanced therapies, including biologic disease-modifying antirheumatic drugs (DMARDs) and conventional synthetic DMARDs, more than 40% report moderate or severe PsA [35]. While many biologics demonstrate efficacy in treating psoriasis and PsA, patients who are diagnosed with both psoriasis and psoriatic arthritis require more effective treatment options [37].
Statement 2: Data suggests benefit of targeting IL-17F in addition to IL-17A to treat psoriasis. (SORT Level B)
The IL-17 cytokine family is a key component in the pathogenesis of psoriasis [6]. IL-17A is the most potent cytokine within this family, but there is a higher concentration of IL-17F present in psoriatic lesions [6, 38]. These two isoforms share 55% homology and are often co-expressed, promoting skin inflammation and bone remodeling [6]. In vitro studies have demonstrated that IL-17F stimulates a similar pattern of genes as IL-17A, although to a weaker extent, and is implicated in the disease pathway for psoriasis [6, 38].
Bimekizumab is the first monoclonal antibody to target both IL-17A and IL-17F and has demonstrated high efficacy in treatment of moderate to severe psoriasis [13,14,15, 20]. In addition, bimekizumab has shown superior clinical efficacy in a head-to-head trial with secukinumab, which only targets IL-17A [34, 39]. While clinical data has revealed bimekizumab’s effectiveness, there remains the question of whether this is attributable to blockade of both cytokines, higher affinity of bimekizumab for the 1L-17A cytokine in comparison to secukinumab, or superior drug delivery. Currently available data suggests a benefit to targeting both IL-17A and IL-17F for the treatment of psoriatic disease.
Statement 3: Bimekizumab is highly effective in the treatment of moderate to severe psoriasis. (SORT Level A)
The efficacy of bimekizumab for treatment of moderate to severe psoriasis has been evaluated in phase II and phase III trials. In a phase IIa trial, patients received bimekizumab at weeks 0 and 4, and then either bimekizumab or placebo at week 16 [15]. This trial found 47% of patients who received bimekizumab achieved PASI 100 by week 8, peaking at 57% by week 12 [15]. At week 16, PASI 75 was reached by 92%, PASI 90 was achieved by 80%, and PASI 100 was achieved by 49% [15]. Patients who received an additional dose of bimekizumab at week 16 demonstrated maintenance of PASI 90 at week 28, while those treated with placebo had a decreased response [15]. This same study found that bimekizumab resulted in normalization of the psoriasis transcriptome at week 8 to near non-lesion levels [15]. In a phase IIb trial, PASI 90 response in all dosing groups (64 mg, 160 mg, 160 mg (320 mg loading dose), 320 mg, 480 mg) was significantly greater at week 12 compared to placebo (46.2–79.1% vs. 0%, p < 0.0001) [20]. In this study, PASI 75 (61.5–93.0% vs. 4.8%; p < 0.0001) and PASI 100 (27.9–60.0% vs. 0%, p < 0.0002) were also achieved by a higher percentage of patients with bimekizumab at week 12 compared to placebo [20]. Patients who achieved PASI 90 in the phase IIb trial maintained PASI response out to week 60 which demonstrates a durability of response over time (PASI 90, 80–100%; PASI 100, 69–83%) [13].
In a phase III trial comparing bimekizumab 320 mg every 4 weeks to placebo, 91% patients who received bimekizumab achieved PASI 90 at week 16 compared to 1% of patients who received placebo (p < 0.0001) and 93% achieved an Investigator’s Global Assessment (IGA) score of 0 or 1 with at least a 2-grade improvement from baseline compared to 1% of patients who received placebo (p < 0.0001) [14]. In addition, PASI 100 was achieved by 68% of patients taking bimekizumab (p < 0.0001) and an IGA score of 0 was achieved by 70% of patients (p < 0.0001) [14]. At week 16, significantly more patients receiving bimekizumab had clinically meaningful improvement in the Psoriasis Symptoms and Impacts Measure. Significantly more patients in the bimekizumab group also reported a Dermatology Life Quality Index of 0 or 1 (76% vs. 6%, p < 0.0001) [14]. Bimekizumab recently received FDA approval for treatment of moderate to severe plaque psoriasis.
Statement 4: Based on head-to-head studies, bimekizumab is more effective at treating moderate to severe psoriasis than secukinumab, adalimumab, and ustekinumab. (SORT Level A)
Several clinical trials have compared efficacy of bimekizumab to other biologics for moderate to severe psoriasis, including secukinumab, adalimumab, and ustekinumab [39,40,41]. Secukinumab is a selective IL-17A inhibitor for moderate to severe psoriasis [42]. In a phase IIIb trial, bimekizumab was found to be non-inferior and superior to secukinumab [39]. Significantly more patients receiving bimekizumab achieved PASI 100 versus secukinumab at week 16 (61.7% vs. 48.9%, p < 0.001) and week 48 (67% bimekizumab vs. 46.2% secukinumab, p < 0.001) [39]. The results for PASI 90 at week 16 were similar between the two drugs (85.5% bimekizumab vs. 74.3% secukinumab) [39].
Adalimumab is a TNF inhibitor approved for the treatment of moderate to severe psoriasis [43]. In a clinical trial comparing bimekizumab to adalimumab, a greater number of patients who received bimekizumab achieved PASI 90 (86.2% vs. 47.2%, p < 0.001) and PASI 100 (60.8% vs. 23.9%, p < 0.001) at week 16 [40]. In addition, significantly more patients in the bimekizumab group had an IGA of 0 or 1 at week 16 (85.3% vs. 57.2%, p < 0.001) [40]. On the basis of this data, bimekizumab was determined to be non-inferior and superior to adalimumab [40].
Bimekizumab was also studied in comparison to ustekinumab [39], an IL-12/IL-23 inhibitor approved for treatment of psoriasis [44]. The clinical trial revealed that significantly more patients who received bimekizumab achieved PASI 90 compared to ustekinumab at week 16 (85% vs. 50%, p < 0.001) [41]. A similar result was seen with IGA response at week 16 (84% bimekizumab vs. 53% ustekinumab, p < 0.0001). These results were maintained out to week 52 [41].
One limitation is that the lack of clinical trials comparing the efficacy of bimekizumab to ixekizumab (IL-17A inhibitor), brodalumab (IL-17 receptor blocker), IL-23 inhibitors, or other TNF blockers. Nevertheless, a network meta-analysis demonstrated statistical superiority of bimekizumab over all biologics in achieving PASI 90 and PASI 100 at 10–16 weeks [34]. It should be noted that this network meta-analysis compared drug efficacy over the short term, so the long-term comparative efficacy of bimekizumab to other biologics is less clear [34].
Statement 5: Optimal dosing for bimekizumab for psoriasis is 320 mg every 4 weeks for 16 weeks, then every 8 weeks. For selected patients, every 4-week dosing can be continued after week 16. (SORT Level A)
Patients receiving 320 mg of bimekizumab every 4 weeks have demonstrated the highest clinical responses (PASI 90 or IGA 0 or 1) compared to other dosages over 12 weeks [20]. In a phase III clinical trial, patients were given 320 mg every 4 weeks for 16 weeks, then continued on a schedule of every 4 weeks or every 8 weeks for 56 weeks [14]. More patients receiving the dose every 8 weeks achieved PASI 90 (91.0 vs. 86.8%) and PASI 100 (83.0% vs. 70.8%) compared to every 4 weeks [14]. In another study, at week 48, 73.5% receiving bimekizumab 320 mg every 4 weeks and 66% receiving it every 8 weeks achieved PASI 100 [39]. A long-term study evaluating dosing over 3 years showed that 320 mg every 4 weeks for 16 weeks, then every 8 weeks had high levels of sustained response, and was consistent with data for all bimekizumab-treated patients [45].
For bimekizumab treatment of psoriatic arthritis, clinical trials demonstrated efficacy utilizing dosing of 160 mg every 4 weeks [17, 18]. In patients with both psoriasis and psoriatic arthritis, the appropriate dosing for bimekizumab is still unknown and should be investigated further. However, clinical trials have suggested that dosing every 4 weeks is optimal.
Statement 6: Based on head-to-head studies, bimekizumab works more quickly than secukinumab, adalimumab, and ustekinumab for psoriasis. (SORT Level A)
Bimekizumab has consistently demonstrated a rapid clinical response. Whereas other biologics have a loading dose, bimekizumab-treated patients have had significant improvements in the signs and symptoms of plaque psoriasis several weeks after initiation without a loading dose.
In a phase I trial evaluating bimekizumab for mild psoriasis, Glatt et al. found that bimekizumab had clinically meaningful results as early as week 2 (> 80% mean reduction in Lesion Severity Score from baseline, > 65% mean reduction in PASI) [12]. In a phase III trial, after only 4 weeks (one dose of bimekizumab), PASI 75 was achieved by 76% of patients (vs. 1% in placebo group, p < 0.0001) with moderate to severe psoriasis [14].
When the speed of response of bimekizumab was compared to that of secukinumab at week 4, 71% of those receiving bimekizumab achieved PASI 75 versus 47.3% of those receiving secukinumab (p < 0.001) [37]. For comparison to adalimumab, PASI 75 was reached at week 4 by 76.5% receiving bimekizumab versus 31.4% receiving adalimumab (p < 0.001) [40]. Similar results were seen when bimekizumab was compared to ustekinumab. At week 4, PASI 75 was achieved by 77% receiving bimekizumab versus 15% receiving ustekinumab (p < 0.0001) [41].
Statement 7: Bimekizumab is effective long term for moderate to severe psoriasis. (SORT Level A)
As part of the bimekizumab clinical trial program, the long-term management (> 1 year) of moderate to severe psoriasis with bimekizumab has been evaluated. In one study, PASI 100 responses were maintained to week 96 in 70.8% of patients [21]. At this same endpoint, 90.5% of patients maintained PASI 90 responses [21]. For patients that were switched from secukinumab to bimekizumab, PASI 100 rates increased from 52.8% at week 48 to 76.6% at week 96 [21]. With regard to an IGA score of 0 or 1 for those given bimekizumab, the proportion was 94% at week 48 and 90.9% at week 96 [21]. In another study, when responses at week 104 were compared to week 16, PASI 90 was achieved by 91.2% and PASI 100 was achieved by 72.3% [46]. There was no significant variation in the PASI 90 or PASI 100 response after 2 years based on dosing every 4 weeks or every 8 weeks [21, 46].
Maintenance of bimekizumab response for moderate to severe psoriasis through 3 years was reported by Strober et al. Of patients that achieved PASI 90 and PASI 100 by week 16 of treatment, 93% maintained PASI 90 and 80.8% maintained PASI 100 at the end of 3 years [45]. The authors of that study used a modified non-responder imputation analysis, in which patients who discontinued because of a lack of efficacy or treatment-related adverse event (TRAE) were counted as non-responders [45]. There was no significant variation in the PASI 90 or PASI 100 response after 3 years based on dosing schedule [45]. This evidence demonstrates the durability of complete clearance results for at least 3 years on bimekizumab.
Statement 8: Bimekizumab’s effect on psoriasis is sustained after discontinuation. (SORT Level A)
Psoriasis is a chronic disease that requires long-term management to successfully control the disease. However, some patients, for a variety of reasons, may need to pause treatment. Patients who received bimekizumab experienced sustained efficacy for an extended period after treatment withdrawal [14]. In a phase III trial, Gordon et al. demonstrated that the median time to relapse (defined as PASI < 75) was 32 weeks from the last dose of bimekizumab (Fig. 1) [14]. No adverse events of psoriasis rebound were reported [14]. While the maintenance effect of bimekizumab is stronger when treatment is continued compared to withdrawal, this data is promising for patients that may need to temporarily stop treatment.

Used with permission from Gordon et al. [14]
Median time to relapse. Median time to relapse (defined as not achieving a > 75% improvement from baseline in PASI [PASI 75] at week 20 or later) in patients who were randomly assigned again to placebo at week 16. Crosses represent patients who were censored at that timepoint. Patients who completed the randomized withdrawal period without relapsing are censored at the date of the week 56 visit. PASI = Psoriasis Area and Severity Index, CI = confidence Interval. Patients randomly assigned to bimekizumab 320 mg every 4 weeks who achieved PASI 90 at week 16 were randomly assigned again for maintenance treatment or for placebo; for patients randomly assigned again to placebo, the last dose of bimekizumab was at week 12; missing data were imputed with non-responder imputation.
Statement 9: In head-to-head studies, bimekizumab’s safety profile is similar to that of other biologics, with the exception of an increased incidence of oropharyngeal candidiasis. (SORT Level A)
Bimekizumab’s safety has been evaluated in head-to-head trials with secukinumab, adalimumab, and ustekinumab [39,40,41]. In all three head-to-head trials, there was no difference between bimekizumab and either secukinumab, adalimumab, and ustekinumab with regards to overall rate of TRAE [39,40,41]. The most common TRAE with bimekizumab included oral candidiasis, nasopharyngitis, and upper respiratory tract infections [39,40,41]. Rates of oral candidiasis with bimekizumab when compared to secukinumab were 19.3% vs. 3%, respectively [39]. When bimekizumab was studied against adalimumab, the rate of oral candidiasis was 9.5% vs. 0%, respectively [40]. Lastly, bimekizumab had an oral candidiasis rate of 15% when compared to ustekinumab (1%) [41]. The infections caused by oral candidiasis were mostly mild or moderate in all head-to-head trials of bimekizumab with other biologics [39,40,41]. The incidence of serious adverse events and those leading to drug discontinuation were similar between bimekizumab and secukinumab, adalimumab, and ustekinumab [39,40,41].
Long-term safety data for bimekizumab is limited and future studies should explore the rate of adverse events after long-term exposure to bimekizumab.
Statement 10: In clinical trials of bimekizumab, more than 99% of candidiasis infections were mild to moderate and rarely led to discontinuation. (SORT Level A)
Bimekizumab was well tolerated in a pooled analysis from eight randomized clinical trials; however, there was an increased incidence of mild to moderate oral candidiasis [22]. Oral candidiasis rates ranged from 4% to 13.4% in phase II and III trials [13,14,15, 20]. The overall exposure-adjusted incidence rate (EAIR) of opportunistic infections was 1.2 per 100 person-years [22]. Almost all were localized mucocutaneous fungal infections (EAIR 20.1 per 100 person-years), of which most were Candida infections (EAIR 14.2 per 100 person-years) [22]. Oral candidiasis occurred at an EAIR of 12.6 per 100 person-years and decreased with longer duration of bimekizumab exposure [22]. Most oral candidiasis cases were mild or moderate and the majority of patients who had an infection reported one instance [22]. The rate of oral candidiasis did not differ greatly between dosing of bimekizumab 320 mg every 4 weeks or every 8 weeks (12.9% vs. 16.7%, respectively) [39]. Only three patients (0.2%) discontinued bimekizumab as a result of oral candidiasis [22].
Statement 11: Candidal infections can be managed easily according to standard protocols in patients on bimekizumab. (SORT Level C)
In clinical trials for bimekizumab, most patients who developed oral candidiasis had mild or moderate infection [22]. Most often, oral candidiasis was treated with nystatin and/or fluconazole and the majority of infections were reported to resolve with treatment (median duration of 12 days) [22]. This is consistent with prior literature on treatment of oral candidiasis resulting from anti-IL-17 therapy for psoriasis [47,48,49]. In general, treatments for oral candidiasis are effective and have low resistance rates [50]. Certain patient populations are at greater risk of developing candida infections. Predisposing risk factors include immunodeficiency (e.g., HIV, chemotherapy), antibiotics, older age, endocrine disorders, nutritional deficiencies, smoking, steroid inhalers, poor oral hygiene, and salivary hypofunction [49]. As such, candidal infections from bimekizumab can be effectively treated with current standard of care.
Statement 12: Bimekizumab is effective for the treatment of psoriatic arthritis across all disease domains. (SORT Level A)
Efficacy of bimekizumab for treatment of PsA has been studied in several clinical trials. A phase II trial found that significantly more patients treated with bimekizumab achieved American College of Rheumatology criteria (ACR; ACR50) compared to placebo at 12 weeks [19]. As dosage of bimekizumab increased, more patients achieved ACR50 response, and those in the 160 mg group were more likely to achieve ACR70 [19]. In a phase III trial, patients who were naïve to biologic DMARDs were either given bimekizumab, placebo, or adalimumab (reference group) [17]. After 16 weeks of treatment, patients receiving bimekizumab had a significantly higher rate of reaching ACR50 compared to placebo (ACR50 44% vs. 10%, p < 0.0001; adalimumab 46%) [17]. Clinical effectiveness was seen as early as week 2 (after one dose of bimekizumab), with 27% of patients reaching ACR20 (vs. 8% with placebo) [17]. In another study, Merola et al. explored the efficacy of bimekizumab in patients with PsA that had a history of inadequate response or intolerance to treatment with up to two TNFα inhibitors [18]. ACR50 was reached by 43% of patients receiving bimekizumab versus 7% of patients receiving placebo (p < 0.0001) [18], demonstrating the value of bimekizumab for PsA regardless of prior DMARD use.
Pertaining to PsA disease domains, minimal disease activity response was reached by significantly more patients receiving bimekizumab compared to placebo after 16 weeks [17, 18]. Bimekizumab significantly decreased radiographic progression of joint disease and resulted in complete resolution of enthesitis in a greater proportion of patients [17, 19]. In addition, a larger number of patients with baseline dactylitis achieved complete resolution after 16 weeks [17]. Patients also experienced significant improvements in physical function, pain, and fatigue with bimekizumab [17, 18]. Further, bimekizumab provided effective and rapid improvement of axial spondyloarthritis in trials for treatment of ankylosing spondylitis [51].
Statement 13: Bimekizumab can be used as first-line therapy for moderate to severe psoriasis, with or without psoriatic arthritis. (SORT Level A)
Clinical trials of bimekizumab have demonstrated its efficacy in treating moderate to severe psoriasis and psoriatic arthritis in patients who are not only systemic naïve but also in patients who are systemic experienced and even non-responders to other biologics [14, 17, 19, 20]. Additionally, studies have shown bimekizumab is able to effectively treat non-responders to other biologics. A study by Kokolakis et al. found that patients who did not respond to either secukinumab, adalimumab, or ustekinumab and were switched to bimekizumab achieved rapid and durable clinical improvements [16]. For PASI 90 non-responders who were switched to bimekizumab, a majority achieved PASI 90 after 4 weeks (secukinumab 53%, adalimumab 67%, ustekinumab 79%) [16]. This improvement became more pronounced after 48 weeks of bimekizumab, as 79%, 91%, and 90% of PASI 90 non-responders achieved PASI 90 switching from secukinumab, adalimumab, and ustekinumab, respectively [16].
The appropriate selection for first-line therapy should be made on an individualized basis for each patient. The evidence for bimekizumab as a first-line therapy is substantial, as it has consistently exhibited efficacy for moderate to severe psoriasis with or without PsA across multiple randomized clinical trials. However, as a result of the higher rate of candidal infections, some panel participants recommended a risk–benefit discussion when deciding on when to use bimekizumab relative to other biologics.
Statement 14: In patients with inflammatory bowel disease and moderate to severe psoriasis, other options should first be considered. (SORT Level C)
Prior literature has established the similarities in the pathogenesis of psoriasis and inflammatory bowel disease (IBD) [52]. There are several classes of biologics that are approved for IBD and psoriasis, including Janus kinase inhibitors, IL-12/23 blockers, and TNF inhibitors [53]. It has been previously reported that patients with IBD may experience worsening of symptoms due to IL-17 blockade [54]. As such, patients with symptomatic IBD were excluded from clinical trials of bimekizumab [22]. Across clinical trials evaluating bimekizumab for moderate to severe psoriasis, the EAIR of IBD was 0.1 per 100 person-years, and the incidence did not increase with longer exposure to treatment [22]. There were a total of four cases of new-onset IBD reported, three of which led to discontinuation of bimekizumab [22]. Although this rate is low, caution is advised when using bimekizumab in patients with IBD as prior literature on IL-17 inhibitors has shown exacerbation of this condition; therefore, other treatment options should first be considered.
Strengths and Limitations
There are both strengths and limitations to the use of the Delphi process. Limitations typically include the use of clinical opinion to develop consensus statements rather than evidence in the literature. However, this study utilized high-quality published clinical articles to establish consensus statements. In this case, the Delphi process is a systematic method well suited to develop clinical recommendations.
Conclusion
There remains a need for additional treatments for moderate to severe plaque psoriasis. Current evidence suggests that targeting both IL-17A and IL-17F with bimekizumab is a highly effective means of reducing the signs and symptoms of psoriasis and PsA. Based upon a comprehensive review of the literature, these 14 consensus statements related to the utilization of bimekizumab for moderate to severe psoriasis and PsA will be helpful in guiding clinician management. Head-to-head trials of bimekizumab with other biologics demonstrate its greater efficacy and faster response. Bimekizumab has also been shown to have a durable response overtime with a sustained response if drug withdrawal is required. This expert panel concluded that this drug has a safety profile consistent with other biologics, and although there is a higher incidence of oropharyngeal candidiasis, most cases are mild or moderate and can be treated successfully.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427. https://doi.org/10.3390/ijms18112427.
Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–92. https://doi.org/10.2165/00128071-200506060-00005.
Raho G, Koleva DM, Garattini L, Naldi L. The burden of moderate to severe psoriasis: an overview. Pharmacoeconomics. 2012;30(11):1005–13. https://doi.org/10.2165/11591580-000000000-00000.
Burshtein J, Strunk A, Garg A. Incidence of psoriasis among adults in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2021;84(4):1023–9. https://doi.org/10.1016/j.jaad.2020.11.039.
Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD011535.pub2.
Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front Immunol. 2018;9:1682. https://doi.org/10.3389/fimmu.2018.01682.
Soderstrom C, Berstein G, Zhang W, et al. Ultra-sensitive measurement of IL-17A and IL-17F in psoriasis patient serum and skin. AAPS J. 2017;19(4):1218–22. https://doi.org/10.1208/s12248-017-0094-4.
Pariser D, Frankel E, Schlessinger J, et al. Efficacy of secukinumab in the treatment of moderate to severe plaque psoriasis in the North American subgroup of patients: pooled analysis of four phase 3 studies. Dermatol Ther. 2018;8(1):17–32. https://doi.org/10.1007/s13555-017-0211-4.
Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31. https://doi.org/10.1136/annrheumdis-2019-215386.
Farahnik B, Beroukhim K, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther. 2016;6(2):111–24. https://doi.org/10.1007/s13555-016-0121-x.
Reis J, Vender R, Torres T. Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs. 2019;33(4):391–9. https://doi.org/10.1007/s40259-019-00361-6.
Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. https://doi.org/10.1111/bcp.13185.
Blauvelt A, Papp KA, Merola JF, et al. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J Am Acad Dermatol. 2020;83(5):1367–74. https://doi.org/10.1016/j.jaad.2020.05.105.
Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–86. https://doi.org/10.1016/S0140-6736(21)00126-4.
Oliver R, Krueger JG, Glatt S, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double-blind multicentre study. Br J Dermatol. 2022;186(4):652–63. https://doi.org/10.1111/bjd.20827.
Kokolakis G, Warren RB, Strober B, et al. Bimekizumab efficacy and safety in patients with moderate-to-severe plaque psoriasis who switched from adalimumab, ustekinumab or secukinumab: results from phase III/IIIb trials. Br J Dermatol. 2023;188(3):330–40. https://doi.org/10.1093/bjd/ljac089.
McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet. 2023;401(10370):25–37. https://doi.org/10.1016/S0140-6736(22)02302-9.
Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet. 2023;401(10370):38–48. https://doi.org/10.1016/S0140-6736(22)02303-0.
Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–40. https://doi.org/10.1016/S0140-6736(19)33161-7.
Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277-286.e10. https://doi.org/10.1016/j.jaad.2018.03.037.
Strober B, Paul C, Blauvelt A, et al. Bimekizumab efficacy and safety in patients with moderate to severe plaque psoriasis: two-year interim results from the open-label extension of the randomized BE RADIANT phase 3b trial. J Am Acad Dermatol. 2023;89(3):486–95. https://doi.org/10.1016/j.jaad.2023.04.063.
Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158(7):735. https://doi.org/10.1001/jamadermatol.2022.1185.
Gargiulo L, Narcisi A, Ibba L, et al. Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a real-life multicenter study-IL PSO (Italian landscape psoriasis). Front Med (Lausanne). 2023;10:1243843. https://doi.org/10.3389/fmed.2023.1243843.
Megna M, Battista T, Potestio L, et al. A case of erythrodermic psoriasis rapidly and successfully treated with bimekizumab. J Cosmet Dermatol. 2023;22(3):1146–8. https://doi.org/10.1111/jocd.15543.
Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–40. https://doi.org/10.1038/jid.2015.208.
Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Med. 2004;17(1):59–67. https://doi.org/10.3122/jabfm.17.1.59.
Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. In: Practical assessment, research, and evaluation, vol. 12, Article 10. 2019. https://doi.org/10.7275/pdz9-th90.
Berman B, Ceilley R, Cockerell C, et al. Appropriate use criteria for the integration of diagnostic and prognostic gene expression profile assays into the management of cutaneous malignant melanoma: an expert panel consensus-based modified delphi process assessment. SKIN J Cutan Med. 2019;3(5):291–306. https://doi.org/10.25251/skin.3.5.1.
Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the global alliance to improve outcomes in acne. J Am Acad Dermatol. 2018;78(2):S1–23. https://doi.org/10.1016/j.jaad.2017.09.078.
Richard MA, Barnetche T, Rouzaud M, et al. Evidence-based recommendations on the role of dermatologists in the diagnosis and management of psoriatic arthritis: systematic review and expert opinion. J Eur Acad Dermatol Venereol. 2014;28:3–12. https://doi.org/10.1111/jdv.12560.
Zakria D, Brownstone N, Berman B, et al. Incorporating prognostic gene expression profile assays into the management of cutaneous melanoma: an expert consensus panel. SKIN J Cutan Med. 2023;7(1):556–69. https://doi.org/10.25251/skin.7.1.1.
Bayaraa B, Imafuku S. Sustainability and switching of biologics for psoriasis and psoriatic arthritis at Fukuoka University Psoriasis Registry. J Dermatol. 2019;46(5):389–98. https://doi.org/10.1111/1346-8138.14834.
Özkur E, Kıvanç Altunay İ, Oğuz Topal İ, et al. Switching biologics in the treatment of psoriasis: a multicenter experience. Dermatology. 2021;237(1):22–30. https://doi.org/10.1159/000504839.
Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther. 2022;12(8):1777–92. https://doi.org/10.1007/s13555-022-00760-8.
Gottlieb A, Gratacos J, Dikranian A, et al. Treatment patterns, unmet need, and impact on patient-reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatol Int. 2019;39(1):121–30. https://doi.org/10.1007/s00296-018-4195-x.
Michelsen B, Diamantopoulos AP, Høiberg HK, Soldal DM, Kavanaugh A, Haugeberg G. Need for improvement in current treatment of psoriatic arthritis: study of an outpatient clinic population. J Rheumatol. 2017;44(4):431–6. https://doi.org/10.3899/jrheum.160973.
Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020;21(5):1690. https://doi.org/10.3390/ijms21051690.
Cole S, Manghera A, Burns L, et al. Differential regulation of IL-17A and IL-17F via STAT5 contributes to psoriatic disease. J Allergy Clin Immunol. 2023. https://doi.org/10.1016/j.jaci.2023.03.035.
Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52. https://doi.org/10.1056/NEJMoa2102383.
Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–41. https://doi.org/10.1056/NEJMoa2102388.
Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98. https://doi.org/10.1016/S0140-6736(21)00125-2.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis — results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. https://doi.org/10.1056/NEJMoa1314258.
Leonardi C, Papp K, Strober B, et al. The long-term safety of adalimumab treatment in moderate to severe psoriasis: a comprehensive analysis of all adalimumab exposure in all clinical trials. Am J Clin Dermatol. 2011;12(5):321–37. https://doi.org/10.2165/11587890-000000000-00000.
Zaghi D, Krueger GG, Callis DK. Ustekinumab: a review in the treatment of plaque psoriasis and psoriatic arthritis. J Drugs Dermatol JDD. 2012;11(2):160–7.
Strober B, Tada Y, Mrowietz U, et al. Bimekizumab maintenance of response through 3 years in patients with moderate-to-severe plaque psoriasis: results from the BE BRIGHT open-label extension trial. Br J Dermatol. 2023;188(6):749–59. https://doi.org/10.1093/bjd/ljad035.
Thaçi D, Vender R, De Rie MA, et al. Safety and efficacy of bimekizumab through 2 years in patients with moderate-to-severe plaque psoriasis: longer-term results from the BE SURE randomized controlled trial and the open-label extension from the BE BRIGHT trial. Br J Dermatol. 2023;188(1):22–31. https://doi.org/10.1093/bjd/ljac021.
Rodríguez-Cerdeira C, González-Cespón JL, Martínez-Herrera E, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Ital J Dermatol Venereol. 2021. https://doi.org/10.23736/S2784-8671.20.06580-3.
Armstrong AW, Bukhalo M, Blauvelt A. A clinician’s guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol. 2016;17(4):329–36. https://doi.org/10.1007/s40257-016-0206-4.
Armstrong AW, Blauvelt A, Mrowietz U, et al. A practical guide to the management of oral candidiasis in patients with plaque psoriasis receiving treatments that target interleukin-17. Dermatol Ther. 2022;12(3):787–800. https://doi.org/10.1007/s13555-022-00687-0.
Kessler SQS, Lang PM, Dal-Pizzol TS, Montagner F. Resistance profiles to antifungal agents in Candida albicans isolated from human oral cavities: systematic review and meta-analysis. Clin Oral Investig. 2022;26(11):6479–89. https://doi.org/10.1007/s00784-022-04716-2.
Van Der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023. https://doi.org/10.1136/ard-2022-223595.
Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417. https://doi.org/10.1001/jamadermatol.2018.3631.
Hedin CRH, Sonkoly E, Eberhardson M, Ståhle M. Inflammatory bowel disease and psoriasis: modernizing the multidisciplinary approach. J Intern Med. 2021;290(2):257–78. https://doi.org/10.1111/joim.13282.
Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700. https://doi.org/10.1136/gutjnl-2011-301668.
Funding
This study was funded in part by an unrestricted educational grant from UCB Pharma. The journal’s Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Joshua Burshtein, Milaan Shah, Danny Zakria, Benjamin Lockshin, Jeff Crowley, Joseph F. Merola, Ken Gordon, Mona Shahriari, Neil J. Korman, Raj Chovatiya, Robert Kalb, Mark Lebwohl all contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Benjamin Lockshin has served as an advisor, speaker, and/or investigator for Abbvie, Novartis, Eli Lilly, Sanofi, Regerneron, Incyte, Leo, UCB, Strata Skin Sciences, Arcutis, Dermavant, BMS, Dermtech, and as a consultant for Abbvie, Novartis, Eli Lilly, DermTech, Strata Skin Sciences, CorEvitas Registry, Leo, UCB, Boehringer Ingelheim, Dermavant, BMS, Regeneron, Sanofi. Investigator for Abbvie, Novartis, Eli Lilly, Sanofi, Regerneron, Leo, Pfizer, Franklin Bioscience, Trevi Therapeutics, Inc., Vanda, Celgene, Strata Skin Sciences, Galderma, Amgen, Dermira, and Castle. Jeff Crowley has served as consultant, speaker, and investigator for Abbvie, Lilly, Janssen, Regeneron, Sanofi, UCB, Boerhinger Ingelheim, Bristol Myers Squibb, and Arcutis. Joseph F. Merola is a consultant and/or investigator for Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Abbvie, Dermavant, Eli Lilly, Incyte, Novartis, Janssen, UCB, Sanofi-Regeneron, Sun Pharma, Biogen, Pfizer and Leo Pharma. Ken Gordon has received honoraria and/or research support from AbbVie, Amgen, BMS, Boehringer Ingelheim, Dermavant, Dice, Eli Lilly, Incyte, Janssen, Novartis, MoonLake, Pfizer, Protagonist, UCB, Union. Mona Shahriari has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Arcutis, Bristol Myers Squibb, Dermavant, Janssen, Leo Pharma, Lilly USA, Novartis, Ortho Dermatologics, Sanofi-Genzyme, Regeneron, UCB, Pfizer, CorEvitas Psoriasis Registry, Dermira, Cara, Union, and Mindera. Neil J. Korman has served as an advisor, consultant, speaker, or investigator for: AbbVie, Abcentra, Amgen, Argenx, Astrazeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Castle Bioscience, Celgene, Chemocentryx, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Hakko Kirin Pharma, Leo Pharma, Menlo Therapeutics, Novartis, Olix, Pfizer, Principia, Regeneron, Sun Pharma, Trevi, UCB, XBiotech. Raj Chovatiya has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Apogee Therapeutics, Arcutis, Argenx, ASLAN Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Eli Lilly and Company, FIDE, Galderma, Genentech, GSK, Incyte, Janssen, LEO Pharma, L’Oréal, Nektar Therapeutics, Opsidio, Pfizer Inc., Regeneron, RAPT, Sanofi, and UCB. Robert Kalb has received research funding from AbbVie, Amgen, CorEvitas LLC, Janssen Ortho Inc, UCB and the University of Pennsylvania. He has been on the data safety monitoring board for studies with Eli Lilly and the pharmaceutical product development group. He has been a consult for Janssen Ortho and UCB. These grants are paid to his institution. Mark Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen Research & Development, LLC, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, and UCB, Inc., and is a consultant for Almirall, AltruBio Inc., AnaptysBio, Apogee, Arcutis, Inc., AstraZeneca, Atomwise, Avotres Therapeutics, Brickell Biotech, Boehringer-Ingelheim, Bristol-Myers Squibb, Castle Biosciences, Celltrion, Corevitas, Dermavant Sciences, EPI, Evommune, Inc., Facilitation of International Dermatology Education,Forte biosciences, Foundation for Research and Education in Dermatology, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. Joshua Burshtein, Milaan Shah and Danny Zakria have nothing to disclose.
Ethical Approval
All panel experts involved in the study are authors of the manuscript and were informed of the study’s objectives. All of the experts support the submission of this manuscript to a peer-reviewed journal.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burshtein, J., Shah, M., Zakria, D. et al. The Efficacy and Safety of Bimekizumab for Plaque Psoriasis: An Expert Consensus Panel. Dermatol Ther (Heidelb) 14, 323–339 (2024). https://doi.org/10.1007/s13555-024-01099-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01099-y