Abstract
Introduction
Limited data exist on skin cancer risk in patients with psoriasis using biologics. Here, we report treatment-emergent adverse events (TEAEs) of skin cancer in patients treated with ixekizumab from psoriasis clinical trials.
Methods
Integrated safety databases from 17 clinical trials of adults with moderate-to-severe psoriasis treated with ≥ 1 dose of ixekizumab for ≤ 5 years were used to analyze exposure-adjusted incidence rates (IRs) per 100 patient-years of exposure (PYE) and clinically characterize dermatologist-adjudicated skin cancer TEAEs.
Results
Of 6892 patients, 58 presented with ≥ 1 skin cancer TEAE (IR 0.3) with IRs remaining stable with longer ixekizumab exposure. Non-melanoma skin cancer (NMSC) was the most common event (IR 0.3) affecting 55 patients; of those, 44 had basal cell carcinoma (IR 0.2) and 16 had squamous cell carcinoma (IR 0.1). Two treatment-emergent melanoma events were identified; neither were classified as serious AEs.
Conclusions
Incidence of skin neoplasms in patients with psoriasis treated with ixekizumab for ≤ 5 years was low, and among those events, NMSC was most common. Limitations included that longer exposure may be required to confirm risk of skin cancer and that the study exclusion criteria of several studies, which excluded patients with skin cancer events within 5 years prior to baseline, might limit interpretation of skin cancer risk in this cohort. These findings support the safety profile of ixekizumab for patients requiring long-term psoriasis control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Limited evidence of skin cancer risk for newer biologic therapies are available. |
This study reports treatment-emergent adverse events of skin cancer in patients with moderate-to-severe psoriasis treated with ixekizumab for up to 5 years from 17 clinical trials. |
Longer-term ixekizumab exposure is not associated with an increased risk of cutaneous malignancies, although longer-term data are needed. |
Most patients had NMSCs, with a squamous cell carcinoma:basal cell carcinoma (BCC:SCC) ratio consistent with the general population. |
These findings support the safety profile of ixekizumab for patients requiring long-term psoriasis control. |
Introduction
Patients with psoriasis, a chronic immune-mediated disease, have increased risk to develop NMSC, especially squamous cell carcinoma (SCC), which increases with disease severity. This seems mainly related to UV exposure, specifically to phototherapy with psoralen and ultraviolet A (PUVA), with increased risk related to cumulative dose and increased in patients also treated with cyclosporine [1,2,3,4,5,6,7]. Similarly, reports investigating the contribution of TNF-inhibitors (TNFi) to skin cancer risk report increased rates of NMSC, largely driven by SCC and linked to prior phototherapy [8,9,10]. Conflicting evidence exists for TNFi regarding the risk of melanoma in patients with psoriasis [8, 9]. Indeed, PUVA has been associated with development of melanomas years after treatment [11]. A recent long-term safety analysis of adalimumab in patients with psoriasis reported increased standardized incidence rates (IRs) for SCC and melanoma [12].
Limited evidence of skin cancer risk is available for newer biologic therapies including interleukin (IL)-17 inhibitors. However, given the chronicity of psoriasis, information on skin cancer risk with long-term treatments are essential to informing long-term disease management.
The safety and tolerability of ixekizumab, a high-affinity monoclonal antibody that specifically neutralizes IL-17A, has been established for the treatment of patients with psoriasis for up to 5 years [13,14,15,16]. We report the rates and clinical characteristics of malignant skin neoplasms, including but not limited to NMSC and melanoma, in a large safety cohort of patients with psoriasis treated with ixekizumab from 17 clinical trials.
Objective
This post hoc analysis aimed to evaluate skin-cancer-treatment-emergent adverse events (SC-TEAE) in adult patients with psoriasis treated with ixekizumab for up to 5 years, from 17 clinical studies.
Methods
Patients and Study Design
This analysis pooled cumulative safety data from 17 clinical studies in adult patients with psoriasis (Figure S1). The study designs have been published elsewhere (references [16,17,18,19,20,21,22,23,24,25,26,27,28], NCT02993471, NCT03073213, NCT03364309). The trial registration numbers for the studies discussed are the following: UNCOVER-1, UNCOVER-2, UNCOVER-3, UNCOVER-A and UNCOVER-J, and NCT01474512, NCT01597245, NCT01646177, NCT01777191 and NCT01624233, respectively; IXORA-P, IXORA-S, IXORA-Q, and IXORA-R, and NCT02513550, NCT02561806, NCT02718898 and NCT03573323, respectively; RHBN, NCT03073213, RHBO, NCT02387801; RHBZ, NCT02634801; RHAJ, NCT01107457; RHBU, NCT02993471; RHBH, NCT03364309; and RHCV, NCT03942042.
Patients who developed a malignancy during the trials were required to discontinue from study treatment, however, patients were allowed to continue treatment if they developed ≤ 2 NMSCs over any 12-month study period. Exclusion criteria related to patient history of skin neoplasms are depicted in Figure S1. Skin cancer events encoded within the Medical Dictionary for Regulatory Activities (MedDRA) High-Level Group Term “Skin neoplasms malignant and unspecified” were extracted, evaluated on the basis of dermatologist adjudication, and frequencies were reported for: NMSC, SCC, and basal cell carcinoma (BCC), melanoma, and other skin neoplasms. Baseline patient characteristics, descriptive analyses of treatment-emergent skin cancers, therapies of treatment-emergent skin cancers, and implications on study continuation were presented.
Compliance with Ethics Guidelines
Protocols for all studies included in this analysis were approved by the Institutional Review Board or Ethics Committee at each participating site. All studies included in this analysis were conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all eligible patients before undergoing study-related procedures.
Statistical Analyses
All randomized patients who received ≥ 1 dose of the study drug in 17 clinical trials were included in the safety analysis population. Frequencies or exposure-adjusted IRs of adverse events (AEs) were summarized. IRs were expressed as number of unique patients with a particular category of event per 100 patient-years of ixekizumab exposure (PYE). Multiple AEs that occurred in different intervals were counted multiple times. As long-term control data were not available, previously published data for placebo and ixekizumab groups from the large Phase 3 UNCOVER studies were reported as time-adjusted IRs.
Results
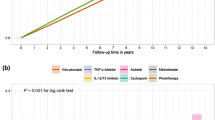
Overall, 58 patients with ≥ 1 skin cancer TEAEs (88 events total) in 6892 ixekizumab-treated patients (18,025.7 PYE) were identified, corresponding to an IR/100 PYE of 0.3. Of those, most patients reported ≥ 1 NMSC event (n = 55 patients with 85 events, IR 0.3). Among those, 44 patients had a BCC (IR 0.2) and 16 patients had a SCC (IR 0.1), and 5 of these patients had both SCC and BCC events (Table 1). Two patients were identified with a melanoma (IR 0.03) and one patient with a dermatofibrosarcoma protuberans (DFSP; IR 0.01; details below). When analyzed in yearly intervals, most skin cancer events were identified within the first year of exposure to ixekizumab (n = 34, IR 0.6). IRs remained stable with longer ixekizumab exposure (Table 2; Figure S2).
Patient Characteristics
Patients with ≥ 1 skin cancer TEAE tended to be older (median age 58.5) and to have a longer duration of psoriasis (median 27.0 years) compared with all ixekizumab-treated patients (median age 46.0 years with 16.7 years duration). All those patients were of white ethnicity and from North America, Europe, or Australia. A total of 69.0% had received prior systemic therapy, and 37.9% had received another biologic before receiving ixekizumab. Rates of prior phototherapy (37.9% versus 38.7%) and specifically PUVA therapy, as well as any systemic therapy (69.0% versus 64.1%) were similar between those with versus without skin cancer TEAE, although more patients with ≥ 1 skin cancer TEAE were pretreated with biologics, compared with those without skin cancer TEAEs (37.9% versus 28.9%; Table 3).
Of the 58 ixekizumab-treated patients who developed treatment-emergent skin cancer events, 12.1% (7/58) had a reported history of skin cancer (4 with history of SCC, 2 with history of BCC, 1 with melanoma history). Neither of the two patients who developed a treatment-emergent melanoma reported skin cancer history. Conversely, of all patients treated with ixekizumab in the clinical trial program, 0.7% (50/6892) had skin cancer history prior to 5 years before baseline, as set out by the exclusion criteria. Of those, most (86%, 43/50) did not develop skin cancer while on ixekizumab treatment.
Nonmelanoma Skin Cancers—BCC events
There were 56 BCC events in 44 patients, most commonly on sun-exposed body areas. In total, 24 (42.9%) events occurred on the head/neck region, 10 (17.9%) on the back or chest, 5 (8.9%) on the extremities and in 17 (30.4%) cases no location was reported. Most BCC events were of mild (41.1%) or moderate (53.6%) severity as reported by the investigator and no patients discontinued treatment due to BCC (Table 4). Median (IQR) time to onset for BCC events was 412.5 (123.5–1007.5) days (Table S1). A total of four events (9.1%) were classified as serious adverse events (SAE; three patients required hospitalization and one was considered medically significant, requiring intervention; Table 5) and most patients (92.9%) had recovered by time of data abstraction. Of the 44 patients who developed BCC, 4 (9.1%) had previous history of skin cancer (2 with history of SCC, 1 each with BCC or melanoma history),20 (45.5%) received prior phototherapy (11 UVB, 4 PUVA, 3 both UVB & PUVA, 2 not specified), and 16 (36.4%) patients were biologic experienced (including 6 TNFi; Table 4). Of those with known Psoriasis Area and Severity Index (PASI) at the time of BCC event, 88.2% (30/34) had PASI ≤ 5 and 67.6% (23/34) had PASI ≤ 2, indicating that detection of BCC was linked in most cases to high skin clearance.
Nonmelanoma Skin Cancers—SCC events
There were 29 SCC events in 16 patients, most commonly on sun-exposed body areas: 13 (44.8%) events occurred on the extremities, 8 (27.6%) occurred on the head, lips, or neck, 3 (10.3%) occurred on the back or chest, and 5 (17.2%) were unspecified. Most SCC events were rated mild (51.7%) or moderate (44.8%) in severity as reported by the investigator and no patients discontinued due to SCC (Table 4). Median (IQR) time to onset for SCC events was 309.0 (84.5–1019.0) days (Table S1). SCC subtypes included 20 events of SCC of the skin in 13 patients, 4 events of Bowen’s disease in 4 patients, 3 events of lip SCC in 2 patients, and 1 keratoacathoma type SCC in 1 patient. Only one SCC event was considered a SAE (Table 5). Of 16 patients who developed SCC, 4 (25%) had a history of skin neoplasm (3 with a history of SCC, 1 with a history of BCC), 2 (12.5%) patients had received prior PUVA phototherapy, 1 (6.3%) patient received UVB, and 9 (56.3%) were biologic experienced (including 4 TNFi; Table 4). At the time of the SCC event, PASI was ≤ 5 in 85.7% (12/14) and ≤ 2 in 57.1% (8/14) of patients with SCC, indicating low skin involvement in most patients with known PASI at time of SCC detection.
Melanoma Events
AEs of melanoma were recorded as preferred term “malignant melanoma.” In total, two melanoma events were noted, and neither case was considered a SAE. One event was reported in a 62-year-old white male patient in the USA in study IXORA-P. The investigator rated the event as mild. Event onset was 43 days after ixekizumab initiation. Previous therapies for psoriasis included topical only. The patient was discontinued from the study. Detailed information about melanoma thickness and/or specific therapy were not provided. The second event was reported as melanoma in situ in a 43-year-old white male patient from Australia, enrolled in UNCOVER-2, who had a 25-year history of psoriasis. Previous therapies included UVB phototherapy and one non-biologic systemic therapy. The melanoma event was rated as moderate in severity and did not lead to discontinuation. Event onset relative to ixekizumab initiation was 1726 days and the patient had reached a PASI90 response at time of melanoma identification. Both patients were reported to have recovered.
Other Malignant Neoplasms of the Skin
One other skin neoplasm was detected; a DFSP which occurred in a 47-year-old white female in the USA (study IXORA-P). Of note, the lesion, diagnosed as DFSP on the mons pubis 13 days after initiation of ixekizumab treatment, was reported to have been present for ≥ 1 year prior to enrollment and led to study drug discontinuation. Diagnosis was confirmed by immunohistochemical detection of CD34-positive spindle cells in the lesion, and surgery was planned at the most recent follow-up. The patient had received a previous therapy with brodalumab over 6 years.
Discussion
We assessed skin neoplasm events in 6892 patients with psoriasis treated with ixekizumab for up to 5 years with 18,025.7 total PYE. Incidence of skin cancer TEAEs was low, with 88 events in 58 patients. IRs remained stable or decreased over time. Most patients who had a history of skin cancer (n = 50), in line with the exclusion criteria, did not develop skin cancer in the ixekizumab trials. Most TEAEs were mild or moderate as reported by the study investigator and were eventually resolved. Two events of melanoma were noted, and neither were reported as SAEs. One DFSP, reported as serious, was also noted. Only two skin cancer events led to study drug discontinuation, one event each of melanoma and DFSP. Both cases were identified shortly after ixekizumab treatment initiation; 13 and 43 days for the DFSP and melanoma cases, respectively. Given that > 80% of patients in this analysis had a PASI ≤ 5 at the time of skin cancer detection, perhaps the ability of ixekizumab to clear skin in the study and the study setting may have favored identification of potentially preexisting conditions. Most TEAEs were NMSCs, all in white patients and predominantly located on body areas potentially exposed to sunlight. Patients with skin cancer TEAEs tended to be older than those without, an observation which is consistent with previous reports of increased skin cancer risk with increased age [29]. Overall, prior phototherapy use, a well-known risk factor for skin cancer, was similar between patients with versus without TEAE skin cancer events; however, patients with TEAE skin cancer events seemed to have higher prior biologic therapy use (Table 3). While recent evidence suggests the use of biologics in patients with a history of cancer appears to be safe [30,31,32], further research is warranted. In the general population the BCC:SCC ratio is believed to be around 4:1, with considerable variations according to age and ranging in the literature from 1:1 to 10:1 depending on population, ethnic group, and sex [33,34,35]. However, it is well known that immunocompromised patients have an increased risk of developing SCC leading to a reversal of the BCC:SCC ratio [36, 37]. In this study, although a small number of skin cancer events were identified, the BCC:SCC ratio was 2.8:1, in line with observations in the immunocompetent general population. Overall, these results corroborate previous descriptions for the safety profile of ixekizumab and support that longer exposure to this IL-17 inhibitor was not associated with increased risk of treatment-emergent skin cancer. [13,14,15,16]
IL-17, primarily produced by Th17 cells, is prevalent in the tumor microenvironment and is commonly found in various tumors including melanoma and NMSCs (BCC and SCC) [38,39,40]. Significantly higher levels of IL-17 expression have been detected by immunohistochemistry in melanomas than in melanocytic nevi, suggesting involvement of IL-17 in cutaneous melanoma development or progression [41]. IL-17 and Th17 cells have been described as having both pro- and anti-tumoral effects [42]. This dichotomy depends on tumor stage, tumor microenvironment, and level of IL-17 or number of Th17 cells as described elsewhere. [38, 43, 44]
In skin cancer specifically, IL-17 promotes tumor formation in mouse keratinocytes [45] and has a critical role in supporting cancer-associated inflammation in the tumor microenvironment of murine skin cancer [46]. In BCC and SCC in vitro, IL-17 and IL-22 promote tumor cell proliferation and migration [40]. Additionally, IL-17 upregulates anti-apoptotic and angiogenic genes, promoting tumor cell growth in mouse models of melanoma and NMSCs [40, 47, 48]. In support of a critical role for IL-17 in tumor progression, locally targeting the IL-17/IL-17RA axis via shRNA in a melanoma model suppressed tumor development [49]. In 2015 Nardinocchi et al. reported that their data encourage the administration of anti-IL-17 and anti-IL-22 biological drugs in patients with psoriasis at risk for development of skin cancer [40].
In contrast, tumor-inhibitory effects have also been attributed to IL-17 and Th17 cells. While IL-17 was reported to inhibit growth of hematopoietic tumors by enhancing cytotoxic T lymphocyte activity, Th17-polarized cells were shown to eliminate advanced B16 melanoma [38, 50, 51]. However, tumor elimination was primarily linked to large established tumors and was mainly driven by IFNγ.
Recently, other studies examined the incidence of skin cancer TEAEs in patients with psoriasis. An integrated safety analysis of 18 clinical trials in 3727 patients with psoriasis (5429.7 PYs) treated with the TNFi and adalimumab reported an IR/100PY of 0.6 and 0.2 for NMSC and melanoma, respectively [12]. Analysis of 1965 patients with psoriasis from seven studies who received etanercept treatment observed an IR of 0.46 and 0.59 for SCC and BCC, respectively, and no melanoma cases were observed [52]. A long-term safety analysis of treatment of 3117 patients (8998 PYs) with psoriasis treated with ustekinumab observed an IR of 0.52/100PY for NMSC events [53], while safety analysis of 2725 total PY of treatment with secukinumab over 52 weeks observed a NMSC event rate of 0.48/100PY [54]. Overall, observations of IRs for NMSC and melanoma for patients treated with ixekizumab in our study are comparable, if not lower than, rates reported in the literature for patients with psoriasis in general [4], those treated with biologics in real-world settings [4], or those treated for psoriasis in clinical trials with other biologics such as secukinumab [54] or ustekinumab [55].
While more research is needed to better understand the role of IL-17 in tumor biology, the current literature seems to support our findings that ixekizumab-mediated IL-17A blockade in patients with psoriasis did not lead to an increased risk for skin malignancies within the observation period.
Limitations
There were limitations to this analysis. Several of the ixekizumab trials excluded patients with skin cancer events within 5 years prior to baseline, therefore, the risk of developing skin cancer for patients receiving ixekizumab treatment with a recent history of skin cancer could not be fully determined here. The lack of placebo-controlled data beyond week 12 impaired the ability to compare IRs with a control group at later periods. The small number of events may mean that larger populations are required to confirm the risk of skin cancer events. High rates of prior treatment in the patient population limit interpretation of ixekizumab treatment alone. It should be noted that not all potential risk factors that may influence risk of skin cancer development, such as Fitzpatrick skin type and actinic damage, could be considered in the present analysis, and information on the cumulative dose of phototherapy received by patients was not collected. Finally, the limited observation period precludes risk assessment beyond 5 years. Nevertheless, these data contribute to growing evidence supporting the safety of ixekizumab treatment regarding skin cancer risk.
Conclusions
In summary, the incidence of skin cancer TEAEs was low and did not increase with longer exposure to ixekizumab in patients with psoriasis. Most patients had NMSCs, with a BCC:SCC ratio consistent with the general population. Most events were resolved and did not lead to ixekizumab discontinuation.
References
Wolinsky C, Lebwohl M. Biologic therapy and the risk of malignancy in psoriasis. Psoriasis Forum. 2011;17:238–53.
Wang X, Liu Q, Wu L, Nie Z, Mei Z. Risk of non-melanoma skin cancer in patients with psoriasis: an updated evidence from systematic review with meta-analysis. J Cancer. 2020;11:1047–55.
Pouplard C, Brenaut E, Horreau C, Barnetche T, Misery L, Richard MA, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):36–46.
Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421–9.
van Lumig PP, Menting SP, van den Reek JM, Spuls PI, van Riel PL, van de Kerkhof PC, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752–60.
Stern RS, Study PFU. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66:553–62.
Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet. 2001;358:1042–5.
Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy risk and recurrence with psoriasis and its treatments: a concise update. Am J Clin Dermatol. 2018;19:363–75.
Egeberg A, Thyssen JP, Gislason GH, Skov L. Skin cancer in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1349–53.
Asgari MM, Ray GT, Geier JL, Quesenberry CP. Malignancy rates in a large cohort of patients with systemically treated psoriasis in a managed care population. J Am Acad Dermatol. 2017;76:632–8.
Stern RS, Study PF. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44:755–61.
Leonardi C, Papp K, Strober B, Thaci D, Warren RB, Tyring S, et al. Comprehensive long-term safety of adalimumab from 18 clinical trials in adult patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2019;180:76–85.
Armstrong A, Paul C, Puig L, Boehncke WH, Freeman M, Torii H, et al. Safety of ixekizumab treatment for up to 5 years in adult patients with moderate-to-severe psoriasis: results from greater than 17,000 patient-years of exposure. Dermatol Ther (Heidelb). 2020;10:133–50.
Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76:432-40.e17.
Langley RG, Kimball AB, Nak H, Xu W, Pangallo B, Osuntokun OO, et al. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019;33:333–9.
Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–51.
Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–56.
Callis Duffin K, Bagel J, Bukhalo M, Mercado Clement IJ, Choi SL, Zhao F, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107–13.
Saeki H, Nakagawa H, Ishii T, Morisaki Y, Aoki T, Berclaz PY, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148–55.
Khattri S, Goldblum O, Solotkin K, Amir Y, Min MS, Ridenour T, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:33–7.
Reich K, Augustin M, Thaci D, Pinter A, Leutz A, Henneges C, et al. A 24-week multicentre, randomized, open-label, parallel-group study comparing the efficacy and safety of ixekizumab vs fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. Br J Dermatol. 2020;182:869–79.
Blauvelt A, Leonardi C, Elewski B, Crowley JJ, Guenther LC, Gooderham M, et al. A head-to-head comparison of ixekizumab vs guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047–58.
Morita A, Okubo Y, Morisaki Y, Torisu-Itakura H, Umezawa Y. Ixekizumab 80 mg every 2 weeks treatment beyond week 12 for Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis. Dermatol Ther (Heidelb). 2021;2:2.
Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–9.
Ryan C, Menter A, Guenther L, Blauvelt A, Bissonnette R, Meeuwis K, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179:844–52.
Langley RG, Papp K, Gooderham M, Zhang L, Mallinckrodt C, Agada N, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178:1315–23.
Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(145–54): e9.
Reich K, Pinter A, Lacour JP, Ferrandiz C, Micali G, French LE, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014–23.
Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184:6–10.
Pellegrini C, Esposito M, Rossi E, Gisondi P, Piaserico S, Dapavo P, et al. Secukinumab in patients with psoriasis and a personal history of malignancy: a multicenter real-life observational study. Dermatol Ther (Heidelb). 2022;12:2613–26.
Rusinol L, Camina-Conforto G, Puig L. Biologic treatment of psoriasis in oncologic patients. Expert Opin Biol Ther. 2022;22:1567–78.
Mastorino L, Dapavo P, Avallone G, Merli M, Cariti C, Rubatto M, et al. Biologic treatment for psoriasis in cancer patients: should they still be considered forbidden? J Dermatolog Treat. 2022;33:2495–502.
Chahal HS, Rieger KE, Sarin KY. Incidence ratio of basal cell carcinoma to squamous cell carcinoma equalizes with age. J Am Acad Dermatol. 2017;76:353–4.
Ciazynska M, Kaminska-Winciorek G, Lange D, Lewandowski B, Reich A, Slawinska M, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11:4337.
Jiyad Z, Marquart L, Green AC. A call to standardize the BCC:SCC ratio. Br J Dermatol. 2021;184:545.
Gonzalez JL, Reddy ND, Cunningham K, Silverman R, Madan E, Nguyen BM. Multiple cutaneous squamous cell carcinoma in immunosuppressed vs immunocompetent patients. JAMA Dermatol. 2019;155:625–7.
Tam S, Gross ND. Cutaneous squamous cell carcinoma in immunosuppressed patients. Curr Oncol Rep. 2019;21:82.
Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–75.
Chen C, Gao FH. Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front Immunol. 2019;10:187.
Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM, et al. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45:922–31.
Ganzetti G, Rubini C, Campanati A, Zizzi A, Molinelli E, Rosa L, et al. IL-17, IL-23, and p73 expression in cutaneous melanoma: a pilot study. Melanoma Res. 2015;25:232–8.
Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34–44.
Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7.
Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. Oncoimmunology. 2015;4: e984547.
Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med. 2015;212:1571–87.
Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–20.
Tang Q, Li J, Zhu H, Li P, Zou Z, Xiao Y. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediators Inflamm. 2013;2013: 713859.
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–64.
Chen YS, Huang TH, Liu CL, Chen HS, Lee MH, Chen HW, et al. Locally targeting the IL-17/IL-17RA axis reduced tumor growth in a murine B16F10 melanoma model. Hum Gene Ther. 2019;30:273–85.
Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73.
Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21.
Pariser DM, Leonardi CL, Gordon K, Gottlieb AB, Tyring S, Papp KA, et al. Integrated safety analysis: short- and long-term safety profiles of etanercept in patients with psoriasis. J Am Acad Dermatol. 2012;67:245–56.
Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844–54.
van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(83–98): e4.
Gordon KB, Papp KA, Langley RG, Ho V, Kimball AB, Guzzo C, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742–51.
Acknowledgements
The authoring team and Eli Lilly and Company would like to thank the clinical trial participants and their caregivers, without whom this work would not be possible. The authors would also like to acknowledge Julia Norman, PhD for her contributions to this manuscript.
Funding
This study and the journal’s Rapid Service Fee was funded by Eli Lilly and Company.
Medical Writing and Editorial Assistance
The authors wish to acknowledge Dwayne Byrne, PhD, a full-time employee of Eli Lilly and Company (Indianapolis, IN, USA), for project management support and scientific communication expertise.
Author Contributions
Susanne Grond, Wen Xu and Mark Lebwohl contributed to the study conceptualization and design. Saxon Smith, Alexandros Stratigos, Matthias Augustin, Jose Manuel Carrascosa, Susanne Grond, Elisabeth Riedl, Wen Xu, Himanshu Patel and Mark Lebwohl contributed to the acquisition, analysis or interpretation of the data. Susanne Grond and Himanshu Patel contributed to writing the original draft and all authors contributed to the review, editing and validation of the manuscript.
Disclosures
Saxon Smith has participated as a speaker and/or advisor for Pfizer, Sanofi Genzyme, Novartis, Abbvie, Eli Lilly and Company, Leo Pharma, Merck Sharpe & Dohme, Janssen Cilag, BMS, UCB and Johnson and Johnson. Saxon Smith is a principal investigator for clinical trials with Abbvie, Sanofi Aventis, Eli Lilly and Company, Novartis and BMS and an investigator for clinical trials with UCB and Regeneron. Alexandros Stratigos has participated as an advisor for Sanofi and Janssen CILAG. Alexandros Stratigos reports personal fees and/or research support from Novartis, Roche, BMS, Abbvie, Sanofi, Regeneron, Genesis Pharma outside the submitted work. Matthias Augustin has served as a consultant or paid speaker for AbbVie, Almirall, Amgen, Beiersdorf, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Galderma, GSK, Janssen-Cilag, Leo, Medac, Merck, MSD, Novartis, Pierre-Fabre, Pfizer, Sanofi, Trevi and UCB. Jose Manuel Carrascosa reports grants, personal fees and non-financial support from Janssen and Novartis, personal fees and non-financial support from Eli Lilly and Company, Abbvie, Almirall and Amgen and personal fees from IB, Sandoz and Leo Pharma. Susanne Grond, and Wen Xu are employees and stockholders with Eli Lilly and Company. Elisabeth Riedl and Himanshu Patel were employees and stockholders of Eli Lilly and Company during the time of study analysis and manuscript preparation, contributions to the manuscript were made as part of their roles at Eli Lilly and Company. Elisabeth Riedl reports subsequent consulting/speaking fees for Eli Lilly and Company. Mark Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara therapeutics, Dermavant Sciences, Eli Lilly and Company, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc., Mark Lebwohl is also a consultant for Aditum Bio, Almirall, AltruBio Inc., AnaptysBio, Arcutis, Inc., Aristea Therapeutics, Avotres Therapeutics, Brickell Biotech, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Celltrion, Corevitas, Dermavant Sciences, Dr. Reddy, EPI, Evommune, Inc., Facilitatation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Galderma, Helsinn, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, Strata, Trevi, and Verrica.
Compliance with Ethics Guidelines
Protocols for all studies included in this analysis were approved by the Institutional Review Board or Ethics Committee at each participating site. All studies included in this analysis were conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all eligible patients before undergoing study-related procedures.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Smith, S.D., Stratigos, A., Augustin, M. et al. Integrated Safety Analysis on Skin Cancers among Patients with Psoriasis Receiving Ixekizumab in Clinical Trials. Dermatol Ther (Heidelb) 13, 1773–1787 (2023). https://doi.org/10.1007/s13555-023-00966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00966-4