Abstract
Introduction
Atopic dermatitis (AD) is heterogeneous in distribution pattern and clinical features. This analysis assessed the effect of dupilumab on the extent and severity of AD across various signs (erythema, edema/papulation, excoriation, lichenification) in different anatomical regions (head and neck, trunk, upper extremities, lower extremities) in patients aged 6 months to 5 years.
Methods
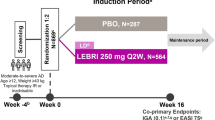
In LIBERTY AD PRESCHOOL, a double-blind, placebo-controlled, phase III clinical trial, children aged 6 months to 5 years with moderate-to-severe AD were randomized 1:1 to subcutaneous dupilumab or placebo with concomitant low-potency topical corticosteroids (TCS) every 4 weeks for 16 weeks. Changes in AD signs across anatomical regions were assessed using unweighted Eczema Area and Severity Index (EASI) body region scores.
Results
Overall, 162 patients were randomized to dupilumab (n = 83) or placebo (n = 79). A significant improvement in least squares mean EASI area score was seen by week 2 in all four anatomical regions (P < 0.0001 for dupilumab vs. placebo) and sustained throughout treatment. Least squares mean EASI sign scores in erythema, excoriations, and infiltration/papulation showed significant improvement by week 2 in all regions (P < 0.001), while lichenification showed significant improvement in all regions by week 4 (P < 0.001).
Conclusion
Dupilumab use with concomitant low-potency TCS treatment resulted in rapid and consistent improvement in AD signs in all anatomical regions, in patients aged 6 months to 5 years with moderate-to-severe AD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03346434 Part B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dupilumab Treatment Leads to Rapid and Consistent Improvement of Atopic Dermatitis in All Anatomical Regions in Patients Aged 6 Months to 5 Years

Why carry out this study? |
Infants and young children with atopic dermatitis (AD) often present lesions located on sensitive skin areas, such as the face, neck, and skin folds, which can severely impair quality of life and affect treatment options. |
This post hoc analysis assessed the effect of dupilumab on AD signs in different anatomical regions in pediatric patients aged 6 months to 5 years with moderate-to-severe AD. |
What was learned from the study? |
Dupilumab significantly improved AD signs in all anatomical regions by week 4 of treatment, with sustained improvement throughout the 16-week study. |
These results suggest that treatment with dupilumab can improve AD severity equally well in different anatomical regions, including sensitive skin areas, in infants and young children with moderate-to-severe AD. |
Digital Features
This article is published with digital features, including a video and graphical abstract to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.23182073.
Introduction
Atopic dermatitis (AD) is heterogeneous in distribution pattern and often affects distinct anatomical regions in different age groups [1]. In infants with AD, acute lesions are usually located on the face, neck, and trunk; from the age of 2 years onwards, eczema lesions become more localized and chronic, particularly in skin folds [1]. Thin and sensitive skin areas, such as the face, neck, and skin flexures, are more vulnerable to irritants and allergens [2]; thus, lesions in these areas can be exacerbated by irritant contact dermatitis [3]. Furthermore, lip licking, drooling, and the introduction of solid food in young children may initiate facial AD flares [4]. Facial AD may have a negative impact on patient quality of life by impairing self-confidence and social relationships as children reach school age. Topical corticosteroids (TCS) are the mainstay for treatment of AD, but can have adverse effects that occur particularly in sensitive areas [2, 5]. TCS use on the face can lead to perioral dermatitis and/or infantile acne, and prolonged use of TCS can cause skin atrophy, especially on the face, back of the hands, and intertriginous areas [5, 6].
Dupilumab is a fully human monoclonal antibody that inhibits signaling of the cytokines interleukin (IL)-4 and IL-13 [7], which are key drivers of type 2-mediated inflammation in several diseases [8, 9]. Phase III trials in infants [10], children [10, 11], and adolescents [12] with moderate-to-severe or severe AD showed that dupilumab use led to substantial improvement in disease severity, with an acceptable safety profile. In addition, phase III trials in adults and adolescents with moderate-to-severe AD and in children aged 6–11 years with severe AD showed that dupilumab significantly improved disease severity equally well in all anatomical regions, as assessed by the Eczema Area and Severity Index (EASI) [13, 14]. Improvements in adults were seen as early as week 4, with responses maintained up to week 52 [13]; in adolescents and children, improvements were also seen early, and maintained up to week 16 [14].
The aim of this analysis was to characterize the effect of dupilumab on the extent and severity of AD signs (erythema, edema/papulation, excoriation, lichenification) in different anatomical regions (head and neck, trunk, upper extremities, lower extremities) in patients aged 6 months to 5 years.
Methods
Study Design
We performed a post hoc analysis of data from a randomized, double-blind, placebo-controlled, phase III trial (NCT03346434 Part B) in patients aged 6 months to 5 years with moderate-to-severe AD inadequately controlled by topical medications. Details of the trial design are reported in the primary study [10]. The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients, or their parents/guardians, provided written informed consent before participating in the trial. Pediatric patients provided assent according to the Ethics Committee (Institutional Review Board/Independent Ethics Committee)-approved standard practice for pediatric patients at each participating center.
Patients were randomized 1:1 to placebo or subcutaneous dupilumab (200 mg if baseline weight ≥ 5 to < 15 kg, 300 mg if ≥ 15 to < 30 kg) every 4 weeks (q4w) during a 16-week treatment period. All patients received concomitant low-potency TCS (hydrocortisone acetate 1% cream), starting 2 weeks before baseline. Systemic immunomodulating treatments, medium- or high-potency TCS, and topical calcineurin inhibitors were prohibited, but could be used as rescue treatment at the discretion of the investigator after day 14.
Endpoints
Changes in AD signs across anatomical regions were assessed using unweighted EASI body region scores [15, 16]. Least squares (LS) mean of absolute value in unweighted EASI from baseline to week 16 and LS mean percentage change in EASI from baseline to week 16 were evaluated in the four anatomical regions (head and neck, trunk, upper extremities, lower extremities). In addition, LS mean of the absolute value in EASI signs (erythema, edema/papulation, excoriation, and lichenification) from baseline to week 16 and LS mean percentage change in EASI signs from baseline to week 16 were evaluated in the four anatomical regions. EASI sign scores were calculated as a composite of the intensity (0–3) and extent of involvement (0–6) of each sign. For each anatomical region, the intensity of signs was summated (0–12) and then multiplied by extent of involvement (0–6).
Statistical Analysis
All randomized patients were included in efficacy analyses. Endpoints were analyzed using an analysis of covariance (ANCOVA) model, with baseline measurements as covariate and treatment, randomization strata (baseline disease severity [Investigator’s Global Assessment {IGA} = 3 vs. IGA = 4]), geographical region (North America vs. Europe), and baseline weight group (5 to < 15 kg vs. 15–30 kg) as fixed factors. Values after first rescue treatment use were set to missing. Patients with missing values due to rescue treatment, withdrawn consent, adverse effects, and lack of efficacy were imputed by worst-observation-carried-forward (WOCF) or baseline value if there was no post-baseline value. Patients with missing values due to other reasons (including COVID-19) were imputed by multiple imputation (MI). All non-missing data before imputation of WOCF were used for MI.
Results
A total of 162 patients (mean age approx. 4 years; 61% male) were randomized to dupilumab + TCS (n = 83) or placebo + TCS (n = 79). At baseline, overall EASI score and regional scores were comparable between the dupilumab and placebo arms (Table 1).
In 16 weeks of treatment, dupilumab significantly improved regional EASI scores (Table 1; Fig. 1) and EASI signs (erythema, edema/papulation, excoriation, lichenification; Table 1; Figs. 2, 3, 4, 5) across the four anatomical regions compared with placebo. A significant improvement in regional EASI scores with dupilumab versus placebo was seen as early as week 2 in all four anatomical regions, with improvements sustained through week 16 (Fig. 1a). LS mean percentage change from baseline in unweighted EASI score was significantly different between dupilumab- and placebo-treated patients by week 2 in the head, upper extremities, and lower extremities, and by week 8 in the trunk (Fig. 1b).
Analysis of value in EASI body region scores from baseline to week 16. a LS mean of absolute value in unweighted EASI body region score. The right side of the body diagram represents patients treated with dupilumab, the left side represents patients treated with placebo. Dupilumab led to a significant improvement in EASI score in all anatomical regions by week 2 (P < 0.0001 in head, trunk, upper extremities, and lower extremities). b LS mean percentage change from baseline in EASI body region score. EASI Eczema Area and Severity Index, LS least squares, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Analysis of value in erythema EASI body region score from baseline to week 16. a LS mean of absolute value in erythema EASI sign score. The right side of the body diagram represents patients treated with dupilumab, the left side represents patients treated with placebo. Dupilumab led to a significant improvement in erythema EASI score in all anatomical regions by week 2 (P < 0.0001 in head and lower extremities; P = 0.0001 in upper extremities; P = 0.0014 in trunk). b LS mean percentage change from baseline in erythema EASI sign score. EASI Eczema Area and Severity Index, LS least squares, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Analysis of value in excoriations EASI body region score from baseline to week 16. a LS mean of absolute value in excoriations EASI sign score. The right side of the body diagram represents patients treated with dupilumab, the left side represents patients treated with placebo. Dupilumab led to a significant improvement in excoriations EASI score in all anatomical regions by week 2 (P < 0.0001 in head, upper extremities, and lower extremities; P = 0.0002 in trunk). b LS mean percentage change from baseline in excoriations EASI sign score. EASI Eczema Area and Severity Index, LS least squares, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Analysis of value in infiltration/papulation EASI body region score from baseline to week 16. a LS mean of absolute value in infiltration/papulation EASI sign score. The right side of the body represents patients treated with dupilumab, the left side represents patients treated with placebo. Dupilumab led to a significant improvement in infiltration/papulation EASI score in all anatomical regions by week 2 (P < 0.0001 in head, trunk, upper extremities, and lower extremities). b LS mean percentage change from baseline in infiltration/papulation EASI sign score. EASI Eczema Area and Severity Index, LS least squares, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Analysis of value in lichenification EASI body region score from baseline to week 16. a LS mean of absolute value in lichenification EASI sign score. The right side of the body represents patients treated with dupilumab, the left side represents patients treated with placebo. Dupilumab led to a significant improvement in lichenification EASI score by week 2 in the head (P = 0.0025) and lower extremities (P = 0.0081), and by week 4 in the trunk (P = 0.0006) and upper extremities (P = 0.0003). b LS mean percentage change from baseline in lichenification EASI sign score. EASI Eczema Area and Severity Index, LS least squares, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Significant improvements in excoriations, infiltration/papulation, and erythema were observed in all regions as early as week 2 (Figs. 2a, 3a, 4a), whereas a significant improvement in lichenification was seen in the head and lower extremities by week 2, and in the trunk and upper extremities by week 4 (Fig. 5a). LS mean percentage change from baseline in EASI sign score was significantly different between the two treatment arms by week 2 in all anatomical regions with erythema (Fig. 2b) and infiltration/papulation (Fig. 4b). With excoriations, a significant difference in LS mean percentage change from baseline between dupilumab and placebo was observed by week 2 in the head, and in the trunk, upper extremities, and lower extremities by week 4 (Fig. 3b), while a significant difference in LS mean percentage change from baseline in EASI lichenification score was observed by week 2 in the head and lower extremities, and by week 4 in the trunk and upper extremities (Fig. 5b).
Discussion
In this analysis of data from a randomized, placebo-controlled, phase III trial in children aged 6 months to 5 years with moderate-to-severe AD, dupilumab rapidly improved AD severity and the intensity of all AD signs evaluated with EASI (erythema, edema/papulation, excoriation, lichenification) equally well across all four anatomical regions (head and neck, trunk, upper extremities, lower extremities) within 16 weeks of treatment. Baseline disease burden in both the placebo and dupilumab groups was slightly higher in the upper and lower extremities compared with the head and trunk. Although infantile AD tends to favor the face, scalp, and trunk [1], this study population included both infants and young children up to 5 years old, which may explain the similar distribution across regions. From age 2 years onwards, AD lesions usually become more localized, typically affecting flexural folds [1].
At baseline, upper and lower extremities were equally affected across all four EASI signs, while head and trunk were slightly more affected by erythema than the other signs. Improvement across all four anatomical regions suggests that all signs and regions are responsive to dupilumab in this age group. It is noteworthy that, although head and neck dermatitis may be multifactorial and exacerbated by contact dermatitis [2, 3], as well as lip licking, drooling, and the introduction of solid food in infants [4], the effect of dupilumab in this region was similar to that in the other regions assessed. Lichenification, a sign of chronic disease and often resistant to therapy, improved significantly within 4 weeks in all regions in the treatment arm compared to the placebo arm, illustrating the benefit of type 2 inflammatory inhibition in both acute and chronic AD lesions.
Observed improvement in all AD signs across four anatomical regions is comparable to results seen in adults and adolescents with moderate-to-severe AD and in children aged 6–11 years with severe AD [13, 14]. As with adults, adolescents, and children older than 5 years, improvements in AD signs in infants and young children were seen by week 4 in all anatomical regions, and sustained throughout the duration of treatment. There have been concerns about the efficacy of dupilumab in the treatment of facial dermatitis [17]; this analysis showed a significant improvement in head and neck dermatitis with dupilumab, consistent with results in older age groups. Dupilumab was generally well tolerated, and overall safety was consistent with the known dupilumab safety profile [10].
A potential limitation of this study is that its analysis of EASI by anatomical region was not prespecified. In addition, EASI does not allow for further breakdown by anatomical regions: upper and lower extremities cannot be subdivided to assess hand or foot involvement and improvement, and head and neck cannot be subdivided to assess facial involvement and improvement. Other limitations are the short treatment duration (16 weeks) and the relatively small number of patients (n = 162).
Conclusion
In a randomized, placebo-controlled, phase III trial of dupilumab in infants and children aged 6 months to 5 years with moderate-to-severe AD, dupilumab treatment with concomitant low-potency TCS significantly improved AD severity in all anatomical regions. Dupilumab treatment showed equal efficacy in EASI sign scores across all anatomical regions. While individual sign scores of excoriation, infiltration/papulation, and erythema showed improvements as early as week 2, all signs were significantly improved by week 4, with sustained improvement through 16 weeks of treatment. Dupilumab was generally well tolerated with an acceptable safety profile.
References
Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–60.
Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin. 2008;24:985–94.
Tada J, Toi Y, Arata J. Atopic dermatitis with severe facial lesions exacerbated by contact dermatitis from topical medicaments. Contact Derm. 1994;31:261–3.
Siegfried EC, Herbert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884–917.
Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135–40.
Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717–44.
Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-14 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2019;75:1188–204.
Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–37.
Haddad E, Cyr SL, Arima K, et al. Current and emerging strategies to inhibit type 2 inflammation in atopic dermatitis. Dermatol Ther (Heidelb). 2022;12:1501–33.
Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908–19.
Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282–93.
Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis. A phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56.
Blauvelt A, Rosmarin D, Bieber T, et al. Improvement of atopic dermatitis with dupilumab occurs equally well across different anatomical regions: data from phase III clinical trials. Br J Dermatol. 2019;181:196–7.
Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643–56.
Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10:11–8.
Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10–22.
Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339–47.
Acknowledgements
Funding
This research and the Rapid service fee was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. (ClinicalTrials.gov identifier: NCT03346434). The study sponsors participated in the study design; collection, analysis, and interpretation of the data; writing of the report; and the decision to submit the article for publication.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance were provided by Alessandra Iannino, PhD, of Excerpta Medica, and were funded by Sanofi and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice Guideline. The authors would like to thank Tingting Tian of Regeneron Pharmaceuticals, Inc., for her excellent work on the body figures; and Adriana Mello of Sanofi and Linda Williams of Regeneron Pharmaceuticals, Inc., for their contributions. NIHR provided support to the Manchester Clinical Research Facility at Royal Manchester Children’s Hospital.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the accuracy and integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Elaine C. Siegfried, Ainara Rodríguez Marco, and Ashish Bansal contributed to the study concept and design. Elaine C. Siegfried, Eric L. Simpson, Michael J. Cork, Peter D. Arkwright, and Lara Wine Lee acquired data. Zhen Chen conducted the statistical analyses of the data. All authors interpreted the data and provided critical feedback on the manuscript.
Disclosures
Elaine C. Siegfried has served as a consultant, data safety monitoring board member, and/or principal investigator in clinical trials for Dermavant, Eli Lilly, GSK, Janssen, Leo Pharma, Novan, Pfizer, Regeneron Pharmaceuticals, Inc., Stiefel, and Verrica Pharmaceuticals. Eric L. Simpson has received personal fees from AbbVie, Advances in Cosmetic and Medical Dermatology Hawaii, Amgen, AOBiome, Arcutis Biotherapeutics, Arena Pharmaceuticals, Aslan Pharmaceuticals, Boehringer Ingelheim, Boston Consulting Group, BMS, Collective Acumen, CorEvitas, Dermira, Eli Lilly, Evelo Biosciences, Evidera, Excerpta Medica, Forté Biosciences, Fraunhofer, Galderma, GSK, Incyte, Janssen, Johnson & Johnson, Kyowa Kirin, LEO Pharma, Medscape, Merck, Maui Derm, MJH Life Sciences, MLG Operating, Pfizer, Physicians World, PRImE, Regeneron Pharmaceuticals, Inc., Revolutionizing Atopic Dermatitis, Roivant Sciences, Sanofi, Trevi Therapeutics, Valeant, Vindico Medical Education, and WebMD; and received grants from (or undertook a principal investigator role with) AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Creek Biosciences, CorEvitas, Dermavant, Dermira, Eli Lilly, Incyte, Kymab, Kyowa Kirin, National Jewish Health, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, and Target RWE. Michael J. Cork has been an investigator and/or consultant for AbbVie, Astellas Pharma, Boots, Dermavant, Galapagos, Galderma, Hyphens Pharma, Johnson & Johnson, LEO Pharma, L’Oréal, Menlo Therapeutics, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron Pharmaceuticals, Inc., and Sanofi. Peter D. Arkwright has acted as an investigator for Regeneron Pharmaceuticals, Inc., and has received grants from and acted as an advisor for Sanofi. Lara Wine Lee has acted as an advisory board member consultant, investigator, and/or speaker for AbbVie, Amgen, Amryt Pharma, Arcutis Biotherapeutics, Castle Creek Biosciences, Celgene, Eli Lilly, Galderma, Incyte, Krystal Biotech, Mayne Pharmaceuticals, Novartis, Pfizer, Pyramid Biosciences, Regeneron Pharmaceuticals, Inc., Sanofi, Target Pharma, Trevi Therapeutics, and UCB. Zhen Chen, Ashish Bansal, and Noah A. Levit are employees and shareholders of Regeneron Pharmaceuticals, Inc. Randy Prescilla and Ainara Rodríguez Marco are employees of and may hold stock and/or stock options in Sanofi.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. All patients, or their parents/guardians, provided written informed consent before participating in the trial. Pediatric patients provided assent according to the Ethics Committee (Institutional Review Board/Independent Ethics Committee)-approved standard practice for pediatric patients at each participating center.
Data Availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by a regulatory body, if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Siegfried, E.C., Simpson, E.L., Cork, M.J. et al. Dupilumab Treatment Leads to Rapid and Consistent Improvement of Atopic Dermatitis in All Anatomical Regions in Patients Aged 6 Months to 5 Years. Dermatol Ther (Heidelb) 13, 1987–2000 (2023). https://doi.org/10.1007/s13555-023-00960-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00960-w