Abstract
Introduction
The purpose of this study was to assess the efficacy and safety of fractional CO2 laser combined with halometasone cream in patients with moderate-to-severe chronic hand eczema (CHE).
Methods
A prospective, single-center, parallel-group, open-label randomized trial including 67 patients with moderate-to-severe CHE was carried out. Patients were randomly assigned to group A (n = 33, fractional CO2 laser once every 4 weeks 1–2 times and halometasone cream twice daily for 8 weeks) or group B (n = 34, halometasone cream alone twice daily for 8 weeks). The primary endpoint was the proportion of patients achieving treatment success at week 12 in each group. Secondary endpoints included differences between groups in the change of hand eczema severity index (HECSI), patient global assessment (PaGA), dermatology life quality index (DLQI), and quality of life in hand eczema questionnaire (QOLHEQ) from baseline to week 12. Relapse rate and adverse effects were also recorded.
Results
A total of 29 patients in each group completed the trial. At week 12, the treatment success rate was 62.1% (18/29) in group A and 27.6% (8/29) in group B (p = 0.009). At week 12, HECSI, PaGA, DLQI, and QOLHEQ all decreased compared with baseline in both groups (p < 0.05). HECSI, DLQI, and QOLHEQ decreased more in group A than group B (p = 0.014, 0.010, and 0.014, respectively), but there was no significant difference in change of PaGA between the two groups (1.0 versus 3.0, p = 0.419). Among patients achieving treatment success, 11.1% (2/18) patients in group A and 50.0% (4/8) patients in group B relapsed at week 24 (p = 0.011). Skin pigmentation was the most common adverse effect.
Conclusions
For patients with moderate-to-severe CHE, fractional CO2 laser combined with halometasone cream is more effective than halometasone cream alone, with few adverse effects.
Trial Registration Number
ChiCTR2100051948.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Moderate-to-severe CHE seriously affects patients’ life and causes significant problems and burdens to society. However, approximately 2–4% of patients with severe CHE are refractory to topical treatment. Fractional CO2 laser can increase the delivery of topical drugs. |
This study aimed to explore whether the fractional CO2 laser is better than conventional therapy for those patients. |
What was learned from the study? |
For patients with moderate-to-severe CHE, fractional CO2 laser combined with halometasone cream is more effective than halometasone cream alone, with few adverse effects. |
Introduction
Hand eczema (HE) is a common disease, with a 1-year prevalence of up to 10% in the general population [1]. Clinically, HE is characterized by signs of erythema, vesicles, papules, scaling, fissures, hyperkeratosis, and symptoms of itch and pain. Usually, the condition is recurrent. About 5–7% of patients with HE developed chronic severe HE [2]. Chronic hand eczema (CHE) affects physical, material, social, and psychological aspects of patients, thereby impairing health-related quality of life [3]. It also limits patients’ choices in choosing some occupations because of symptoms such as itching, blisters, and painful fissures. Up to 8% of patients with HE had been forced to change jobs because of the disease [4]. Meanwhile, CHE causes significant problems and burdens to society. The direct and indirect cost of illness in work-related CHE in Germany was comparable with the costs incurred for severe psoriasis or atopic eczema [2].
Currently, topical corticosteroids are the most common treatment for patients with CHE. However, approximately 2–4% of patients with severe CHE are refractory to topical treatment [1]. For these patients, effective treatments are limited. Phototherapy can be useful but not convenient. Oral corticosteroids or other oral immunosuppressants may provide temporary relief, but patients often cannot tolerate long-term applications due to their side effects [5]. Therefore, new treatment methods are urgently needed for patients with CHE.
Transepidermal drug delivery is a novel method of drug delivery in dermatology. Fractional CO2 laser, which creates microscopic ablation channels in the epidermis, can increase the delivery of topical drugs [6]. To date, the fractional CO2 laser is widely used in skin diseases such as melasma, scarring, keloid, vitiligo, alopecia areata, and androgenetic alopecia [7,8,9,10,11,12]. In this randomized trial, we compared the efficacy and safety between fractional CO2 laser combined with halometasone cream and halometasone cream alone in the treatment of patients with moderate-to-severe CHE. We aimed to explore whether the fractional CO2 laser is better than conventional therapy for those patients.
Methods
This study was performed in accordance with the Declaration of Helsinki and all applicable amendments, and the clinical trial registry number is ChiCTR2100051948 (http://www.chictr.org.cn/showproj.aspx?proj=135065). All the patients agreed to participate in the trial and signed the informed consent forms. The study protocol was approved by the Ethical Review Committee of Beijing Friendship Hospital (2021-P2-284).
Patient Selection
We conducted a prospective, single-center, parallel-group, randomized trial in the Department of Dermatology at Beijing Friendship Hospital, Capital Medical University in China. Consecutive patients were enrolled from September 2021 to December 2021. Patients aged 18–70 years and diagnosed with moderate-to-severe CHE of at least 6-month duration were eligible for enrollment in this study. Severity was defined according to the Physician Global Assessment (PGA) [13].
Exclusion criteria included: (1) active bacterial, fungal, or viral infection of the hands; (2) allergy to halometasone cream and drugs with similar chemical structure; (3) presence of severe cardiac, hepatic, and renal damage and immunocompromise; (4) presence of other diseases on hands such as psoriasis and lichen planus; (5) use of UVB phototherapy, psoralen, and ultraviolet A radiation (PUVA) or X-ray radiation, or systemic corticosteroids, retinoids, or immunosuppressants within the previous 8 weeks; (6) use of local corticosteroids or retinoid within the previous 2 weeks; and (7) pregnancy or lactation.
The sample size required for this trial was calculated by PASS 15.0 software (NCSS, LLC, Kaysville, Utah, USA). Referring to our previous study, the treatment success rate of fractional CO2 laser therapy and halometasone cream alone therapies were 70.0% and 30.0%, respectively. We set that α = 0.05 and β = 0.20, and 21 samples for each group were required for statistical analyses. Considering the perhaps 20% loss rate of follow-up, the sample size for each group is 26.
Study Design
The participants were randomly assigned to group A or group B by using a computer-generated randomization table.
Patients in group A received fractional CO2 laser once every 4 weeks for 1–2 times according to therapeutic response combined with halometasone cream (Aoneng, Bright Future Pharmaceutical Lab. Ltd.) twice daily for 8 weeks. Patients in group B were treated with local halometasone cream alone twice daily for 8 weeks. In addition, all patients were required to use moisturizer (Baume Hydratant, CeraVe, France) every day and avoid contact with irritants or allergic materials during the whole study period. Patients were not allowed to use other effective medications for CHE.
Before fractional CO2 laser treatment, local anesthesia was performed using lidocaine cream for 1 h, and hands were then cleansed with 75% medical alcohol. The lesions on the hands of patients in group A were treated by a fractional CO2 laser treatment system (PIXEL CO2, Alma Lasers, Israel). Treatment parameters were set according to patients’ severity and tolerance. The pulse time was 1.4–1.6 ms and the energy density was 150–200 mJ/pixel with 1–2 pulse and a coverage of 60%. In most cases, the lesions were scanned 1–2 times. After fractional CO2 laser treatment, local halometasone cream was immediately applied and then 3% boric acid solution wet dressing was performed on the treated area for 15 min to reduce adverse effects. Patients were not allowed to have contact with water with treated hands within 3 days. In addition, all patients were required to use hand cream every day and avoid contact with irritants or allergic materials during the entire study period. Patients were not allowed to use any other treatment expected to be effective for CHE. All patients were allowed to drop out of the trial according to their request or if they suffered from a severe or life-threatening side effect.
Study visits were scheduled for week 4, week 8, and week 12. Patients achieving treatment success were followed up to week 24 to assess relapse, and no treatment likely to be effective for CHE was allowed during this time. Treatment success was defined as achieving “clear” or “almost clear” skin with at least a 2-point improvement in PGA from baseline [14]. Relapse is defined according to two standard; one as a Hand Eczema Severity Index (HECSI) score ≥ 75% of the baseline score [15] and another as a PGA rating of “mild,” “moderate,” or “severe” [16]. At baseline, we collected all patients’ information including age, gender, body mass index (BMI), and disease duration. Global photographs were taken with a digital camera (M100, Canon, Japan) at baseline and each visit. The posture and position of patients’ hands, the angle and distance of the camera, and the lighting conditions were consistent. According to the photographs, three board-certified dermatologists blinded to the study completed PGA and HECSI scores. Patient assessments including Patient Global Assessment (PaGA), Dermatology Life Quality Index (DLQI), and Quality of Life in Hand Eczema Questionnaire (QOLHEQ) were also collected.
The primary endpoint was the proportion of patients achieving treatment success at week 12 in each group. Secondary endpoints included differences between groups in the change of HECSI, PaGA, DLQI, and QOLHEQ from baseline to week 12. The relapse rate was also observed at week 24 in each group. For safety assessment, adverse effects were recorded at each visit.
Statistical Methods
Continuous variables in normal distribution were recorded as means ± standard deviations. Continuous variables in non-normal distribution and ordinal variables were recorded as median (interquartile range, IQR). Categorical variables were recorded as frequency and percentage. The comparison of continuous variables in normal distribution between groups was analyzed with an independent-sample t-test. The comparison of continuous variables in abnormal distribution and ordinal variable between groups were analyzed with the Wilcoxon rank sum test. The comparison of continuous variables between baseline and follow-ups was analyzed with paired t-test or Wilcoxon rank sum test. The comparison of the ordinal variables between baseline and follow-ups was analyzed with the Wilcoxon rank sum test. Quantitative data were analyzed with the chi-squared test, and p < 0.05 was considered to be statistically significant. All p-values were two-sided. All data were analyzed using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). GraphPad Prism 8 (GraphPad Software Ltd., USA) was used to draw all the figures in this study.
Results
Baseline Characteristics
The flow chart of the study is shown in Fig. 1. A total of 67 patients were enrolled in the study, including 34 patients in group A and 33 patients in group B. In total, 9 patients dropped out of the study. There were no statistical differences in demographic and clinical characteristics between the two groups at baseline. Details are presented in Table 1.
Treatment Success on the Basis of Physician’s Global Assessment
At week 12, 18 (62.1%) patients in group A and 8 (27.6%) patients in group B achieved treatment success (χ2 = 6.971, p = 0.008). Among them, 16 patients in group A and 6 patients in group B achieved “almost clear,” while 2 patients in each group achieved “clear” (Fig. 2). The representative clinical images before and after treatments are shown in Fig. 3.
Hand Eczema Severity Index
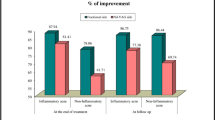
In general, HECSI scores showed an overall declining trend (Fig. 4). Significant decreases were observed in both groups at week 12 compared with baseline (12.0 versus 4.0, p = 0.014). At week 12, the HECSI score decreased more in group A than in group B (p = 0.014).
Patient Assessment
Table 2 presented the changes in PaGA, DLQI, and QOLHEQ. They all significantly decreased in both groups at week 12 compared with baseline (p < 0.05). At week 12, DLQI and QOLHEQ decreased more in group A than group B (p = 0.010 and 0.014, respectively), but there was no significant difference between the two groups in PaGA. (p = 0.419).
Adverse Effects
Adverse effects were recorded in all 67 patients. The main adverse effects are listed in Table 3. Skin pigmentation was the most common adverse effect, affecting 14 patients (42.4%) in group A and 10 patients (29.4%) in group B. Image b of Fig. 3 showed the pigmentation after combination therapy of fractional CO2 laser and halometasone cream. Pruritus and xerosis cutis were also seen in several patients in both groups. There were no significant differences between the two groups (p = 0.270, 0.535, and 0.186, respectively).
Relapse
The relapsed patients were same according to the two kinds of relapse standards. Among patients achieving treatment success, 11.1% (2/18) patients in group A and 50.0% (4/8) patients in group B relapsed at week 24 (χ2 = 11.200, p = 0.011).
Discussion
CHE is a common and burdensome inflammatory skin disease associated with functional impairment. The treatment of CHE is challenging for dermatologist until now. Topical therapies including corticosteroids, immune modulators, and calcipotriol are acceptable treatment but have limited efficacy. Systemic therapies such as corticosteroids, alitretinoin, acitretin, cyclosporine, azathioprine, and methotrexate are effective but have serious side effects. In recent years, the emerging therapeutic options for chronic and recurrent hand eczema are recommended with biologics and Janus kinase (JAK) inhibitors, however, the cost of these medications is very high and patients may need long-term treatment [17]. The aim of our study is to explore an innovative therapy for patients with CHE. On the basis of our successful experience in treating androgenetic alopecia [11], the efficacy of halometasone cream delivered by fractional CO2 laser on CHE may work well.
To date, topical corticosteroids such as halometasone are the first-line treatment for CHE [18]. There are still many patients who are resistant to topical corticosteroids or who suffer from relapses [17, 19]. Up to now, phototherapy or laser therapy are new attempts in treating chronic eczema and dermatitis. The efficacy of 308 nm excimer laser for chronic hand and foot eczema was excellent and sustained, with an average of 13 sessions of treatment, which dramatically reduced the compliance in patients with CHE [20]. A total of 8 patients with CHE treated by self-administered daylight-activated photodynamic therapy improved by 2.7 points in Investigator Global Assessment (IGA) scores and by 6.6 points in DLQI scores, respectively. Nevertheless, it is infeasible for patients with CHE to expose their hands to outdoor sunlight for 2.5 h 4 times at 2-week intervals [21]. In a word, it is not convenient to select 308 nm excimer laser or photodynamic therapy for patients with CHE. Taking into consideration the transdermal delivery by the channel of fractional CO2 laser, our trial paid attention to the efficacy and safety of fractional CO2 laser combined with halometasone cream in treating CHE.
The results from our study show that fractional CO2 laser combined with halometasone cream was an efficacious and well-tolerated treatment for patients with moderate-to-severe CHE. It had an obvious advantage over halometasone cream alone in our study. In fractional CO2 laser group, 62.1% of patients achieved treatment success at week 12, while 27.6% did in halometasone cream alone group. Therefore, the fractional CO2 laser group shortened duration of treatment and increased efficiency. Moreover, the enrolled patients with CHE showed great compliance on the condition that no oral medication was taken and only one to two sessions laser treatment were necessary. By contrast, alitretinoin is the only approved systemic treatment licensed specifically for hand eczema. In hand eczema guidelines, it is recommended for severe CHE that does not respond, or responds inadequately, to topical corticosteroids [17, 22]. Only 48% of patients with severe CHE treated with alitretinoin 30 mg daily responded after 24 weeks of treatment [15], and headache was the most common adverse event leading to discontinuation. Additionally, fractional CO2 laser combined with halometasone cream therapy obtained significantly better improvement in HECSI, DLQI, and QOLHEQ than halometasone cream alone therapy. Therefore, fractional CO2 laser combined with halometasone cream therapy not only improved clinical conditions, but also the life quality of patients.
Definitively, fractional CO2 laser is widely and successfully used for the treatment of scars, actinic keratosis, melasma, vitiligo, and other cutaneous lesions and disorders [9, 12, 23,24,25,26]. Why is the role of fractional CO2 laser superior to other lasers? There are several factors included below.
On the one hand, the fractional CO2 laser is numerous narrowed microscopic columns of laser light. It leads to microscopic columns of thermally damaged tissue (microthermal zones) in the target skin, leaving intervening areas of skin unaffected. The punch-like damaged area at the treatment site can act as a channel for drug delivery. Thereby, fractional CO2 laser facilitates penetration and distribution of topically applied drugs [27, 28]. The low treatment success rate of halometasone cream alone mainly may result from the thickened epidermis of hands, which may reduce the transdermal absorption in moderate-to-severe CHE (especially keratinized HE). Another reason may be that the risk for skin atrophy limits the prolonged use of topical corticosteroids in this chronic recurrent disease. In this study, the halometasone creams were only used for 8 weeks in both groups. Above all, topical halometasone cream alone cannot meet the demand for the treatment of patients with moderate-to-severe CHE.
On the other hand, the fractional CO2 laser can promote wound healing and collagen remodeling in hypertrophic scars [29]. The energy density used in this study was similar to that of routine hypertrophic scar treatment protocol. Thus, our proposed hypothesis is that fractional CO2 laser increases the abortion of halometasone cream in lichenified and thickened HE lesions and promotes the regeneration of normal skin. They are the possible two reasons why combined therapy of fractional CO2 laser and halometasone cream was superior to halometasone cream alone.
Skin pigmentation was the most common adverse effect. However, pigmentation had a similar occurrence rate in both groups, and largely resolved by itself at week 12. This suggests that fractional CO2 laser combined with halometasone cream merely increases the probability of pigmentation and only results in transient skin pigmentation. Pruritus and xerosis cutis were also found in both groups, but these may not be associated with the treatments since these were common in patients with CHE before treatment. Infection was not observed during the study period.
Recurrence is very common in patients with CHE [30]. In this study, the relapse rate in the combined therapy group was significantly lower than that in the halometasone cream alone group at week 24 (11.1% versus 50.0%). Furthermore, the recurrent lesions in combined therapy were not as severe as the baseline. As shown in Fig. 4, the HECSI scores significantly decrease in both groups at week 4, which may result from the quick treatment effect of halometasone cream. The HECSI scores in group B began to increase at week 12, which indicate subsequent relapse. However, the HECSI scores in group A were stable within 12 weeks, which was consistent with the low relapse rate in group A. These results indicate that fractional CO2 laser combined with halometasone cream treatment improved the long-term prognosis of moderate-to-severe CHE. It may result in skin regeneration after fractional CO2 laser treatment.
To the best of our knowledge, this research is the first randomized controlled trial demonstrating the efficacy and safety of the combination of fractional CO2 laser and halometasone cream for the treatment of moderate-to-severe CHE. However, this study also has some limitations. Firstly, potential bias from unblinded treatment between the two groups existed due to the small size of this study cohort, thus the conclusions of this study need to be further verified in future clinical work. Secondly, the combined therapy could be further improved. For example, halometasone cream could be replaced with corticosteroid solutions that have better permeability. Thirdly, it would be better to add another group treated by topical halometasone cream with occlusion since occlusion could also enhance drug absorption. Fourthly, boric acid dressing after laser should be carried out in both groups to ensure consistency between groups. Lastly, the use of high-potency corticosteroids with 8 consecutive weeks may have potential side effects. We will further improve the treatment project in subsequent studies.
Conclusions
For patients with moderate-to-severe CHE, the treatment regimen of fractional CO2 laser combined with halometasone cream is an effective and safe option, and also has a lower relapse rate.
References
Diepgen TL, Agner T, Aberer W, Berth-Jones J, Cambazard F, Elsner P, et al. Management of chronic hand eczema. Contact Dermatitis. 2007;57(4):203–10.
Bissonnette R, Diepgen TL, Elsner P, English J, Graham-Brown R, Homey B, et al. Redefining treatment options in chronic hand eczema (CHE). J Eur Acad Dermatol Venereol. 2010;24(Suppl 3):1–20.
Kouris A, Armyra K, Christodoulou C, Katoulis A, Potouridou I, Tsatovidou R, et al. Quality of life, anxiety, depression and obsessive-compulsive tendencies in patients with chronic hand eczema. Contact Dermatitis. 2015;72(6):367–70.
Meding B. Epidemiology of hand eczema in an industrial city. Acta Derm Venereol Suppl (Stockh). 1990;153:1–43.
Christoffers WA, Politiek K, Coenraads PJ, van der Schaft J, de Bruin-Weller MS, Schuttelaar ML. Drug survival of cyclosporine in the treatment of hand eczema: a multicentre, daily use study. J Eur Acad Dermatol Venereol. 2016;30(1):63–6.
Waibel JS, Rudnick A, Shagalov DR, Nicolazzo DM. Update of ablative fractionated lasers to enhance cutaneous topical drug delivery. Adv Ther. 2017;34(8):1840–9.
Chen R, Liu Z, Zheng H, Wang M, Wang B. Efficacy of combining erbium:YAG and fractional CO2 laser for the treatment of facial scarring. Eur J Dermatol. 2022;32(6):770–80.
Soliman M, Etman Y, AbdElhameed A, Elsharaby R, Tawfik A. Comparative study between Nd-YAG laser, fractional CO2 laser, and combined Nd-YAG with fractional CO2 laser in the management of keloid: clinical and molecular study. J Cosmet Dermatol. 2021;20(4):1124–32.
Afify AA, Zuelfakkar NM, Eshafi MA. Fractional CO2 laser, platelet rich plasma and narrow band ultraviolet B in the treatment of vitiligo (A randomized clinical trial). Lasers Med Sci. 2021;36(7):1479–86.
Nouh AH, Kadah AS, Said M. Comparative study of the use of fractional CO(2) laser versus the use of liquid nitrogen cryotherapy in the treatment of alopecia areata in a sample of the Egyptian population. Dermatol Ther. 2022;35(4): e15358.
Huang Y, Zhuo F, Li L. Enhancing hair growth in male androgenetic alopecia by a combination of fractional CO(2) laser therapy and hair growth factors. Lasers Med Sci. 2017;32(8):1711–8.
Qu Y, Wang F, Liu J, Xia X. Clinical observation and dermoscopy evaluation of fractional CO(2) laser combined with topical tranexamic acid in melasma treatments. J Cosmet Dermatol. 2021;20(4):1110–6.
Coenraads PJ, Van Der Walle H, Thestrup-Pedersen K, Ruzicka T, Dreno B, De La Loge C, et al. Construction and validation of a photographic guide for assessing severity of chronic hand dermatitis. Br J Dermatol. 2005;152(2):296–301.
Worm M, Bauer A, Elsner P, Mahler V, Molin S, Nielsen TSS. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182(5):1103–10.
Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol. 2008;158(4):808–17.
Lee JS, Park HS, Yoon HS, Cho S. Efficacy and safety of oral alitretinoin in hand eczema and palmoplantar pustulosis in Korean patients. Ann Dermatol. 2019;31(2):139–45.
Elsner P, Agner T. Hand eczema: treatment. J Eur Acad Dermatol Venereol. 2020;34(Suppl 1):13–21.
Kim HJ, Bang CH, Kim HO, Lee DH, Ko JY, Park EJ, et al. 2020 Korean Consensus Guidelines for diagnosis and treatment of chronic hand eczema. Ann Dermatol. 2021;33(4):351–60.
Dubin C, Del Duca E, Guttman-Yassky E. Drugs for the treatment of chronic hand eczema: successes and key challenges. Ther Clin Risk Manag. 2020;16:1319–32.
Shroff A, Malajian D, Czarnowicki T, Rose S, Bernstein DM, Singer GK, et al. Use of 308 nm excimer laser for the treatment of chronic hand and foot eczema. Int J Dermatol. 2016;55(8):e447-453.
Kremer N, Sherman S, Lapidoth M, Enk CD, Leshem YA, Mimouni T, et al. Self-administered daylight-activated photodynamic therapy for the treatment of hand eczema: a prospective proof-of-concept study. Dermatol Ther. 2020;33(6): e14329.
Diepgen TL, Andersen KE, Chosidow O, Coenraads PJ, Elsner P, English J, et al. Guidelines for diagnosis, prevention and treatment of hand eczema–short version. J Dtsch Dermatol Ges. 2015;13(1):77–85.
Zaouak A, Chamli A, Hammami H, Fenniche S. Efficacy of ablative fractional CO2 laser in the treatment of a cutaneous leishmaniasis scar. Skinmed. 2021;19(6):475–80.
Alexander S, Girisha BS, Sripathi H, Noronha TM, Alva AC. Efficacy of fractional CO(2) laser with intralesional steroid compared with intralesional steroid alone in the treatment of keloids and hypertrophic scars. J Cosmet Dermatol. 2019;18(6):1648–56.
Kose O. Carbon dioxide ablative laser treatment of acquired junctional melanocytic nevi. J Cosmet Dermatol. 2021;20(2):491–6.
Helsing P, Togsverd-Bo K, Veierod MB, Mork G, Haedersdal M. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169(5):1087–92.
Meesters AA, Bakker MM, de Rie MA, Wolkerstorfer A. Fractional CO2 laser assisted delivery of topical anesthetics: a randomized controlled pilot study. Lasers Surg Med. 2016;48(2):208–11.
Sobhi RM, Sharaoui I, El Nabarawy EA, El Nemr Esmail RS, Hegazy RA, Aref DHF. Comparative study of fractional CO(2) laser and fractional CO(2) laser-assisted drug delivery of topical steroid and topical vitamin C in macular amyloidosis. Lasers Med Sci. 2018;33(4):909–16.
DeBruler DM, Blackstone BN, Baumann ME, McFarland KL, Wulff BC, Wilgus TA, et al. Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med. 2017;49(7):675–85.
Lee GR, Maarouf M, Hendricks AK, Lee DE, Shi VY. Current and emerging therapies for hand eczema. Dermatol Ther. 2019;32(3): e12840.
Acknowledgements
Funding
This research was supported by the Beijing Natural Science Foundation (No.7222040) and National Natural Science Foundation of China (No.82273555). The materials fee was funded by the foundation (No.7222040). The Rapid Service Fee was funded by the National Natural Science Foundation of China (No.82273555). It is free laser therapy for the enrolled CHE patients.
Authorship
All named authors meet the International Committee of Medical Journal. Editor’s criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
This article did not receive any medical writing support or editorial assistance.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Gongfeng Tang, Yuan Chang, Haixuan Wu, Xuelei Liang, Yi Liu. Data analysis were performed by Gongfeng Tang and Yuan Chang. The first draft of the manuscript was written by Gongfeng Tang and Yuan Chang. Fenglin Zhuo revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Gongfeng Tang, Yuan Chang, Haixuan Wu, Xuelei Liang, Yi Liu, and Fenglin Zhuo declare that they have no competing interests.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of the Beijing Friendship Hospital (2021-P2-284). Written informed consent was obtained for the publication and the use of all patients’ images before their enrollment in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tang, G., Chang, Y., Wu, H. et al. Efficacy and Safety of Fractional CO2 Laser Combined with Halometasone Cream for Treatment of Moderate-to-Severe Chronic Hand Eczema: A Prospective, Single-Center, Parallel-Group, Open-Label Randomized Trial. Dermatol Ther (Heidelb) 13, 1789–1799 (2023). https://doi.org/10.1007/s13555-023-00944-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00944-w