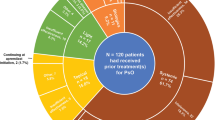
Abstract
Introduction
Real-world data on the needs of patients with psoriasis and patient-perceived benefits of apremilast are limited. We report such data from France.
Methods
The multicenter, observational REALIZE study was conducted in real-life clinical practice in France and enrolled patients with moderate-to-severe plaque psoriasis who had initiated apremilast per French reimbursement criteria in the 4 weeks preceding enrollment (September 2018–June 2020). Physician assessments and patient-reported outcomes (PROs) were collected at enrollment, 6 months, and 12 months. PROs included the Patient Benefit Index for skin diseases (PBI-S), Dermatology Life Quality Index (DLQI), and 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9). The primary outcome was PBI-S ≥ 1 (minimum clinically relevant benefit) at month 6.
Results
Of 379 enrolled patients who received ≥ 1 dose of apremilast, most [n = 270 (71.2%)] remained on apremilast at 6 months and over half [n = 200 (52.8%)] persisted at 12 months. Patients reported the following treatment goals as most important (≥ 70% reported goal as “very important” in the Patient Needs Questionnaire): get better skin quickly, regain disease control, be healed of skin alterations, and have confidence in the therapy. Most patients persisting on apremilast achieved a PBI-S ≥ 1 at months 6 and 12 (91.6% and 93.8%, respectively). Mean (SD) DLQI decreased from 11.75 (6.69) at enrollment to 5.17 (5.35) and 4.18 (4.39) at months 6 and 12, respectively. Most patients (72.3%) reported moderate-to-severe pruritus at enrollment and no/mild pruritus at months 6 and 12 (78.8% and 85.9%, respectively). Mean (SD) TSQM-9 Global Satisfaction scores were 68.4 (23.3) and 71.7 (21.5) at months 6 and 12, respectively. Apremilast was well tolerated; no new safety signals were identified.
Conclusions
REALIZE provides insights regarding the needs of patients with psoriasis and the patient-perceived benefits of apremilast. Patients who persisted on apremilast reported improvements in quality of life, high treatment satisfaction, and clinically relevant benefits.
Trial registration
NCT03757013.
Plain Language Summary
Psoriasis is a chronic disease and can have a large impact on patients’ quality of life. Patients often discontinue psoriasis treatments for a number of reasons, including side effects, ineffectiveness, and inconvenience. Apremilast (Otezla) is a twice-daily oral tablet for the treatment of moderate-to-severe plaque psoriasis. Data on the needs of patients with psoriasis and the patient-perceived benefits of psoriasis treatments, including apremilast, are limited. The REALIZE (Real Life Data for OTEZLA Evidence) study collected data from 379 patients with moderate-to-severe psoriasis receiving apremilast for up to 12 months in clinical practice across France. Patients completed questionnaires regarding their treatment goals, how well apremilast treatment met these goals, their quality of life, and their satisfaction with apremilast treatment. At the beginning of the study, patients reported getting better skin quickly, regaining control of their psoriasis, being healed of psoriatic lesions on their skin, and having confidence in their psoriasis treatment as their most important treatment goals. Over half of the patients continued apremilast for 12 months, with most reporting that apremilast successfully met their treatment needs. Patients also reported high satisfaction with apremilast and improved quality of life. The adverse events reported in the REALIZE study were similar to the known safety profile of apremilast. Our data show that apremilast is an effective, convenient, and well-tolerated treatment that improves the symptoms of psoriasis and meets patients’ needs and expectations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Real-world data on the needs of patients with psoriasis and the patient-perceived benefits of apremilast are limited. |
REALIZE assessed the needs of French patients with moderate-to-severe psoriasis treated with apremilast in clinical practice, and the patients’ perceived benefits of apremilast. |
What was learned from the study? |
Patients reported getting better skin quickly, regaining control of their disease, being healed of all skin alterations, and having confidence in their therapy as their most important treatment goals; most patients reported apremilast successfully met these treatment goals. |
Over half of the patients continued apremilast for 12 months, and patients reported high satisfaction and improved quality of life with apremilast. |
Our data show apremilast is an effective, convenient, and well-tolerated treatment option for psoriasis patients in France and that it fulfills patients’ needs. |
Introduction
Psoriasis is a chronic immunoinflammatory skin disease characterized by scaling, erythema, plaques, pruritus, and pain [1, 2]. Psoriasis can have a significant impact on patients’ quality of life, is often associated with comorbidities, and can be physically and emotionally disabling [1].
Patients with moderate-to-severe psoriasis are typically treated with long-term systemic therapies. Apremilast is an immunomodulating oral phosphodiesterase 4 inhibitor that was approved in Europe in 2015 and has been available in France since October 2016 [3]. It is indicated for the treatment of moderate-to-severe chronic plaque psoriasis in adult patients who failed to respond to, have a contraindication to, or are intolerant to other systemic therapies, including cyclosporine, methotrexate, and phototherapy [4].
The Real Life Data for OTEZLA Evidence (REALIZE) study evaluated the real-world use of apremilast in French patients with moderate-to-severe plaque psoriasis, including patient-reported needs and benefits, persistence rates, reasons for discontinuation, patient satisfaction, and safety. The primary goal of REALIZE was to assess treatment goals and expectations on the basis of patient-reported outcomes.
Methods
Study Design
REALIZE was a longitudinal, multicenter, observational study of French clinical practice. Eligible patients were identified by dermatologists in public hospitals, private clinics, or private practice and invited to enroll up to 4 weeks after initiating apremilast. The decision to initiate apremilast was independent of, and occurred before, study enrollment, and aligned with French reimbursement criteria [4]. Data were collected at enrollment, defined as baseline, and routine clinic visits scheduled 6 months and 12 months after apremilast initiation; there were no mandatory study visits. Physician assessments were performed by dermatologists according to their normal practice; patient assessments were conducted via questionnaires completed by patients during their dermatologist visits.
Patients
REALIZE enrolled patients ≥ 18 years old with stable, moderate-to-severe chronic plaque psoriasis who had failed, were intolerant to, or had a contraindication to other systemic therapy, including cyclosporine, methotrexate, or phototherapy, and who had initiated apremilast per French reimbursement criteria [4] at or in the 4 weeks preceding enrollment.
Study Objectives and Outcomes
Primary Objective and Outcome
The primary study objective was to assess patient-reported treatment benefits 6 months after apremilast initiation using the validated Patient Benefit Index for skin diseases (PBI-S) [5]. The PBI-S assesses patient-defined benefits of dermatological treatments and is divided into two parts: the Patient Needs Questionnaire (PNQ) and the Patient Benefit Questionnaire (PBQ) [5, 6]. The PNQ measures the relevance of 25 treatment goals using a 5-point Likert scale for each goal (0 “not important at all” to 4 “very important”). The PBQ evaluates the extent to which the treatment goals in the PNQ are fulfilled by treatment (from 0 “treatment did not help at all” to 4 “treatment helped a lot"). The PNQ and PBQ are converted to a preference-weighted global benefit score that ranges from 0 (no benefit) to 4 (maximal benefit). In REALIZE, patients completed the PNQ at baseline and the PBQ after 6 months and 12 months of apremilast treatment. The PBI-S score was calculated in patients completing at least one item in both the PNQ and the PBQ. The primary study outcome was achievement of a PBI-S ≥ 1 (minimum clinically relevant benefit) after 6 months of apremilast treatment. The primary endpoint was analyzed for the prespecified subgroups of patients who had previously been treated with ≥ 2 other systemic treatments and patients previously treated with biologics.
Secondary Objectives and Outcomes
Secondary objectives were to assess the characteristics of patients with psoriasis initiating apremilast in routine clinical practice in France, including disease severity, measured using body surface area (BSA), Psoriasis Area and Severity Index (PASI), and static Physician’s Global Assessment (sPGA); effect of apremilast on patient quality of life (QoL), assessed using the Dermatology Life Quality Index (DLQI); patient satisfaction with apremilast, measured using the 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9); effect of apremilast on disease severity, signs, and symptoms, from the perspectives of the patient and treating physician (BSA, PASI, sPGA); patient persistence on apremilast treatment; and apremilast tolerability.
BSA, PASI, and sPGA are physician-assessed measures of disease severity. BSA measures the extent of body coverage, PASI measures disease severity across four body regions, and sPGA measures induration, erythema, and scaling averaged over all lesions [7]. The DLQI is a validated questionnaire that measures patient QoL across various health dimensions to give an overall score ranging from 0 to 30, with lower values indicating better QoL [8]. The TSQM-9 is made up of scales for effectiveness, convenience, and global satisfaction, with each scale ranging from 0 to 100, with higher values indicating greater satisfaction [9]. Patients completed the DLQI at baseline and at 6 months and 12 months. Patients completed the TSQM-9 at baseline (if they had initiated apremilast in the preceding 4 weeks) and 6 months and 12 months.
Secondary and exploratory outcomes were PBI-S at 6 months and 12 months; PBI-S ≥ 1 at 12 months; BSA and PASI at baseline, 6 months, and 12 months (and changes from baseline); achievement of a ≥ 75% reduction in PASI score (PASI-75) at 6 months and 12 months; sPGA score of 0 (clear) or 1 (almost clear) at 6 months and 12 months; change from baseline sPGA at 6 months and 12 months; affected body locations, number of affected locations, and pruritus severity at baseline, 6 months, and 12 months; DLQI score and DLQI score ≤ 5 at baseline, 6 months, and 12 months; change from baseline in DLQI score, ≥ 5-point improvement from baseline DLQI score, and DLQI score of 0 or 1 at 6 months and 12 months; and TSQM-9 scores at baseline, 6 months, and 12 months.
Safety was assessed throughout the study. All adverse events (AEs; serious or non-serious) and adverse drug reactions (ADRs) were to be reported by the investigator. AEs included any events reported, no matter the causal relationship; ADRs included any AEs the investigator considered possibly related to apremilast treatment.
Statistical Analysis
All analyses were descriptive in nature and performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Baseline values at enrollment were analyzed using the full analysis set, which included all patients who had received ≥ 1 dose of apremilast and had ≥ 1 post-enrollment data point. Efficacy outcomes at 6 months and 12 months were analyzed for patients remaining on apremilast at the relevant timepoint. Data for non-persistent patients were collected as possible. Patients were excluded from analyses if they had discontinued apremilast > 61 days prior to the 6-month or 12-month assessment, if the date of discontinuation was missing or unknown, or if none of the items on a questionnaire were completed. Safety data were analyzed using the safety analysis set, which included all patients who had received ≥ 1 dose of apremilast.
Sensitivity analyses were performed for primary and secondary outcomes to account for missing data. For the primary endpoint, two sensitivity analyses were performed: (1) patients lost to follow-up at month 6 were considered to be without minimum clinically relevant benefit; and (2) patients lost to follow-up at month 6 were considered to have missing data. For secondary outcomes, patients with missing DLQI scores were considered to have no improvement, and patients with missing PASI scores and/or sPGA scores were considered to have no response.
Ethics
This study was conducted in accordance with Good Clinical Practice as described in International Council for Harmonisation Guideline E6. Oral informed consent was provided by all patients before any study-related assessments began. The independent ethics committee that approved this study was Comité de Protection des Personnes Ile de France X. The study was performed in accordance with the Declaration of Helsinki of 1964 and its later amendments.
Results
Patient Disposition
Between 25 September 2018 and 24 June 2020, 430 patients were enrolled across 87 sites in France (private practice, n = 229; hospital/clinic, n = 185; site information missing, n = 16). Patient disposition is displayed in Fig. 1.
The full analysis set included 365 patients, with 65 patients excluded because they did not receive apremilast treatment (n = 51) or they did not have any post-inclusion data (n = 14). The safety analysis set included 379 patients who had taken ≥ 1 dose of apremilast. More than half of the patients from the full analysis set [200/365 (54.8%)] remained on treatment and in the study at 12 months, while 165 patients (45.2%) withdrew before month 12. Of those who had withdrawn, 134/165 (81.2%) discontinued apremilast [reasons for discontinuation: adverse events, 71/165 (43.0%); lack of response, 46/165 (27.9%); other, 17/165 (10.3%)], 27/165 (16.4%) were lost to follow-up, 2/165 (1.2%) discontinued the study due to COVID-19, 1/165 (0.6%) withdrew consent, and 1/165 (0.6%) had a prescription renewal problem.
Patient Characteristics
Table 1 summarizes patient characteristics for the full analysis set. Mean patient age was 50 years and 55.3% of patients were men. The median body mass index was 25.8 kg/m2 and the majority of patients were either overweight (30.9%) or obese (27.3%). Over half [218/365 (59.7%)] reported current or historical comorbidities, the most common of which were vascular hypertensive disorders (18.4%), glucose metabolism disorders including diabetes mellitus (10.7%), and lipid metabolism disorders (9.9%).
Table 2 summarizes disease characteristics at enrollment for the full analysis set. The mean duration of psoriasis disease was 16 years. Psoriatic arthritis was reported in 7.4% (27/365) of patients; most [17/27 (63.0%)] had their diagnosis confirmed by a rheumatologist. Over half of patients (54.5%) had received one prior systemic treatment for plaque psoriasis and one-quarter (26.3%) had received ≥ 2 prior systemic treatments; 19.2% had not received any prior systemic treatment and 7.6% had a contraindication to other systemic treatments. The median (range) number of locations affected with plaque psoriasis was 6 (1–12). Figure 2 summarizes the locations of plaque psoriasis at enrollment.
Apremilast Persistence
At 6 months, 270 of 379 patients (71.2%) who had initiated apremilast remained on apremilast treatment. Over half [200/379 (52.8%)] remained on treatment at 12 months. Mean (SD) treatment duration was 8.9 (4.3) months. Following apremilast discontinuation, 76 patients switched to a subsequent treatment, the most common class being biologic therapies (39/76), especially interleukin inhibitors [33/76 (43.4%)] (Fig. 3). Approximately 21% switched to topicals.
Patient Needs and Benefits
Figure 4 summarizes the PNQ at baseline for the full analysis set (with all except one patient completing the PNQ). The treatment goals patients identified as being most important (reported “very much important” by ≥ 70% of patients) were to get better skin quickly, regain control of the disease, be healed of skin alterations, have confidence in the therapy, and be free of itching.
Figure 5 summarizes responses of “very much” in the PBQ at 6 months and 12 months. At 6 months, the most highly rated benefits (reported via the PBQ by > 40% of patients to have been “very much” helped by apremilast treatment) were getting better skin quickly, having confidence in their therapy, regaining control of their disease, needing less time for daily treatment, being free of itching, and finding a clear diagnosis and therapy. At 12 months, additional highly rated benefits were being able to lead a normal daily life and no longer having burning sensations on their skin.
Top Patient Benefits Questionnaire results: proportion of patients reporting apremilast helped them “very much” to achieve each treatment goal. Month 6 respondents ranged from n = 258 to n = 262. Month 12 respondents ranged from n = 178 to n = 181. At 12 months, 36.8% of patients also reported that apremilast had helped them “very much” to be able to engage in normal leisure activities
Figure 6 summarizes PBI-S at 6 months and 12 months for patients persisting on apremilast and completing at least one item in the PNQ and PBQ. At 6 months, 91.6% of patients achieved a PBI-S ≥ 1 (primary outcome, indicating a minimum clinically relevant benefit); 87.9% of patients who had been treated with ≥ 2 other systemic treatments achieved the primary outcome. Of four persistent patients previously treated with biologic therapy with PBQ data at 6 months, three reported a PBI-S ≥ 1. Comparatively, 85.9% (207/241) of biologic-naive patients had achieved PBI-S ≥ 1 at 6 months.
At 6 months, mean (SD) PBI-S was 2.7 (1.1), with 75.2% of patients achieving a PBI-S ≥ 2, 48.1% achieving a PBI-S ≥ 3, and 6.5% achieving a PBI-S = 4 (maximum benefit). At 12 months, mean (SD) PBI-S was 2.7 (0.9), with 93.8% of patients achieving a PBI-S ≥ 1, 78.5% achieving a PBI-S ≥ 2, 48.6% achieving a PBI-S ≥ 3, and 10.2% achieving a PBI-S = 4.
Physician Assessments of Disease Severity
At baseline, mean (SD) affected BSA(%) was 21.51 (18.91) and most patients with non-missing data [170/174 (97.7%)] had a BSA ≥ 3 (Table 2). At 6 months and 12 months, most patients persisting on apremilast had a BSA < 3 [44/104 (42.3%) and 30/63 (47.6%), respectively] or 3–10 [33/104 (31.7%) and 26/63 (41.3%), respectively]. Mean (SD) affected BSA had improved to 9.10 (15.53) at 6 months among the 104 persistent patients with BSA data; at 12 months, mean (SD) affected BSA had improved to 4.44 (6.36) among the 63 persistent patients with BSA data. Mean percent change from baseline at 6 months and 12 months is shown in Supplementary Fig. 1.
Mean (SD) baseline PASI score was 13.26 (8.50) (Table 1). At 6 months and 12 months, the majority of patients persisting on apremilast achieved a PASI score < 10 [72/88 (81.8%) and 57/63 (90.5%), respectively]. The mean (SD) PASI score had improved to 5.35 (5.92) at 6 months among the 88 persistent patients with PASI data; at 12 months, the mean (SD) PASI score was 3.82 (4.42) among the 63 persistent patients with PASI data (Supplementary Fig. 2). Mean percent change from baseline PASI score at 6 months and 12 months is shown in Supplementary Fig. 1. At 6 months, 41.0% (32/78) of patients persisting on apremilast had achieved PASI-75; by 12 months, 55.9% (33/59) of persistent patients had achieved PASI-75.
Most patients [328/365 (89.8%)] had an sPGA score of 3 or 4 at baseline (Table 1). At 6 months and 12 months, approximately half of all patients persisting on apremilast had an improvement from baseline sPGA score [126/268 (47.0%) and 105/198 (53.0%), respectively], and approximately half achieved an sPGA score of 0 or 1 [127/268 (47.4%) and 105/198 (53.0%), respectively] (Supplementary Fig. 3).
Patient-Reported Outcomes
Mean (SD) DLQI score improved from 11.75 (6.69) at baseline to 5.17 (5.35) and 4.18 (4.39) at 6 months and 12 months, respectively, with mean (SD) DLQI percent changes (reductions) of −49.9% (53.4) and −48.4% (79.3), respectively. Figure 7 summarizes DLQI scores at baseline, 6 months, and 12 months. Among patients persisting on apremilast, 61.3% and 67.0% achieved a DLQI ≤ 5 at 6 months and 12 months, respectively. Over half (56.2%) had a DLQI score improvement of ≥ 5 points at 6 months and 63.0% had an improvement of ≥ 5 points at 12 months; 30.3% had a DLQI score of 0 or 1 at 6 months and 39.6% had a score of 0 or 1 at 12 months.
DLQI total scores at baselinea, 6 monthsb, and 12 monthsb. aOn the basis of patients in the FAS who completed at least one item of the DLQI questionnaire. bOn the basis of persistent patients who completed at least one item of the DLQI questionnaire. DLQI Dermatology Life Quality Index, FAS full analysis set
Figure 8 summarizes mean TSQM-9 scores among persistent patients. Mean (SD) TSQM-9 global satisfaction score was 59.3 (20.8) at baseline, increasing to 68.4 (23.3) at month 6 and 71.7 (21.5) at month 12, with mean (SD) improvements from baseline of 28.5% (64.8) and 25.3% (48.1), respectively. Mean (SD) TSQM-9 effectiveness score was 63.9 (17.1) at baseline, increasing to 71.2 (19.5) at month 6 and 73.8 (17.8) at month 12, with mean (SD) improvements of 19.8% (41.9) and 22.8% (37.3), respectively. Mean (SD) TSQM-9 convenience score was 78.2 (17.8) at baseline, increasing to 80.5 (17.1) at month and 80.5 (17.0) at month 12, with mean (SD) improvements of 8.5% (38.8) and 5.2% (27.1), respectively.
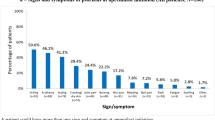
Figure 9 summarizes patient-reported pruritus symptoms. Approximately three-quarters [264/365 (72.3%)] of patients reported moderate-to-severe pruritus at baseline. Over half of patients persisting on apremilast reported they did not have any pruritus symptoms at 6 months or 12 months [158/269 (58.7%) and 123/199 (61.8%), respectively]. The median (range) number of psoriasis locations reported by patients decreased from 6 (1–12) at baseline to 2 (0–12) at 6 months and 2 (0–9) at 12 months. The locations with the most improvement reported were knees/lower legs/ankles, buttocks/thighs, and arms/axillae (Fig. 10).
Non-Persistent Patients
Data for non-persistent patients were limited at 6 months and 12 months but suggest that improvements were minimal compared with patients who continued apremilast treatment (data not shown).
Sensitivity Analyses
Results from sensitivity analyses were similar to those in primary analyses (data not shown).
Safety
In the safety analysis set, 37.5% (142/379) of patients reported at least one AE and 31.9% (121/379) of patients reported ADRs. The most commonly reported AEs were diarrhea (13.2% of patients), nausea (6.9%), and headache (7.4%). Serious AEs were reported in 2.1% (8/379) of patients [cardiovascular disorder (n = 1), myocardial infarction (n = 1), hemorrhoids (n = 2), depression (n = 1), suicidal depression (n = 1), type 1 diabetes mellitus (n = 1), and hernia repair (n = 1)], and 3 patients reported serious ADRs (n = 1 each of hemorrhoids, depression, and suicidal depression). AEs led to temporary or permanent discontinuation of apremilast in 21.6% (82/379) of patients. Despite reported AEs, 71 patients persisted on apremilast until month 12. No fatal AEs were reported.
Discussion
This real-world study of apremilast use in patients with moderate-to-severe plaque psoriasis in France reports patient-reported needs and treatment benefits, assessed using the PBI-S, which measures patient-relevant benefit and QoL impact [6]. Patients initiating apremilast placed the highest importance on getting better skin quickly, regaining control of their disease, being healed of skin alterations, having confidence in their treatment, and being free of itching. Over half of patients continued apremilast for ≥ 12 months, with most reporting that apremilast met their treatment needs (indicated by a PBI-S ≥ 1, the minimum clinically important treatment benefit).
The majority (60%) of patients enrolled in REALIZE had comorbidities, the most common being hypertensive disorders, glucose metabolism disorders, and lipid metabolism disorders. In contrast with reports that nearly a quarter of European patients with psoriasis have psoriatic arthritis [10], only 7.4% of patients in REALIZE reported this comorbidity. At baseline, approximately three-quarters of patients had psoriasis-affected BSA > 10%, with a mean BSA of 21.5% and a mean PASI score of 13.3. Although these values are lower than those observed at baseline in the pivotal ESTEEM 1 and 2 trials and the LIBERATE study [11,12,13], they reflect a population of patients with moderate-to-severe psoriasis, indicating that patients had a high level of disease involvement. Furthermore, the proportion of patients with a baseline sPGA score of 4 (indicating severe disease) in REALIZE was similar to or higher than in ESTEEM 1 and 2 or LIBERATE [11,12,13]. In addition, over half (56%) of all patients initiating apremilast had a baseline DLQI score > 10, indicating that psoriasis had a large detrimental impact on their QoL [8].
Disease severity, as assessed by the treating dermatologist, improved with apremilast treatment. Approximately half of persistent patients achieved clear or almost clear skin (as indicated by sPGA score of 0 or 1) at 6 months and 12 months (47% and 53%, respectively) and the proportion of patients with severe disease (as indicated by a BSA ≥ 10%) decreased, with approximately 40% and 50% of patients achieving a BSA < 3% at 6 months and 12 months, respectively. PASI scores also decreased, with more than half of persistent patients achieving a 75% reduction from their baseline score (PASI-75) at 12 months, a similar result to the LIBERATE study [13]. The majority (62%) of patients continuing apremilast reported they did not feel any pruritus at month 12. In addition, the majority of patients reported improved QoL, as indicated by decreased DLQI scores; most had DLQI scores ≤ 5 at 6 months and 12 months, indicating their psoriasis had little to no impact on their QoL [8]. Persistent patients were satisfied with apremilast treatment as indicated by high and stable TSQM-9 scores at months 6 and 12. In addition, high TSQM-9 convenience scores indicate apremilast treatment is highly convenient as an oral therapy. Patients previously treated with biologic therapy seemed to report proportionally lower treatment benefit; however, due to the small sample size of this subgroup, no conclusions can be drawn. No new safety signals were reported, and the safety profile was consistent with previous reports.
While the safety and efficacy of apremilast have been demonstrated across a number of randomized controlled trials [11,12,13,14,15], it is important to evaluate apremilast use in real-world clinical practice. The European, multi-country, cross-sectional APPRECIATE study assessed real-world outcomes in patients with chronic plaque psoriasis 5–7 months after apremilast initiation, with a mean treatment duration of 6 months [16, 17]. Patients continuing apremilast treatment reported a number of treatment benefits, including finding clear diagnosis and therapy, improved skin clearance, being free of itch, having confidence in therapy, and improved QoL [16, 17]. The prospective OTELO study assessed the real-world use and effectiveness of apremilast in Belgian patients with moderate-to-severe plaque psoriasis [18]. Over a mean follow-up of 8.7 months, apremilast was shown to improve patient QoL, disease activity, patient benefits, and treatment satisfaction [18]. REALIZE provides insights on the treatment needs of psoriasis patients in France and demonstrates the effectiveness and tolerability of apremilast in a large population with 12 months of follow-up, with results similar to those reported in OTELO and APPRECIATE [16,17,18]. REALIZE also highlights that outcome measures used in the clinical trial setting, such as PASI scores, are not used as frequently in real-world practice, perhaps due to their complexity or time-consuming nature. Results from REALIZE confirm the real-world applicability of the efficacy and tolerability of apremilast demonstrated in clinical trials [11,12,13,14,15].
Limitations of the REALIZE study include its observational, non-interventional design, and the proportion of patients who discontinued apremilast during the 12-month follow-up. In addition, French dermatologists do not routinely perform all the assessments included in the study and a large amount of data was missing for some scores (e.g., PASI scores were collected at baseline for < 40% of patients). However, completion rates were higher for patient-reported assessments and efficacy results were based on persistent patients, who were more likely to have non-missing data. The strengths of REALIZE include its broad inclusion criteria and real-life setting, which enrolled a population more representative of clinical practice than is included in randomized clinical trials.
Conclusions
In summary, REALIZE provides useful insight into the treatment goals of French patients with moderate-to-severe plaque psoriasis and demonstrates the clinical benefits that apremilast offers to these patients, including increased QoL, clearer skin, and less itch. Patients reported high levels of treatment satisfaction with apremilast and its convenient, twice-daily oral dosing regimen. REALIZE highlights the importance of patient-reported outcomes as measures of treatment efficacy.
Change history
08 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13555-023-00976-2
References
World Health Organization. Global report on psoriasis. Geneva: World Health Organization; 2016.
Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583–90.
Otezla [summary of product characteristics]. Breda, The Netherlands: Amgen Europe B.V.; July 21, 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf.
Otezla (apremilast), PDE4 inhibitor immunosuppressant. Brief summary of the transparency committee opinion: Haute Autorite de Sante; 2016 [15 Apr 2016]. Available from: https://www.has-sante.fr/jcms/c_2585408/en/otezla-apremilast-pde4-inhibitor-immunosuppressant.
Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304:433–41.
Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301:561–71.
Chow C, Simpson MJ, Luger TA, Chubb H, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (Part 1 of 2): change during therapy in Psoriasis Area and Severity Index, Static Physician’s Global Assessment and Lattice System Physician’s Global Assessment. J Eur Acad Dermatol Venereol. 2015;29:1406–14.
Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035.
Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-65.e19.
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73:37–49.
Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387–99.
Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31:507–17.
Crowley J, Thaci D, Joly P, Peris K, Papp KA, Goncalves J, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310–7.
Strober B, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, Chen R, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801–8.
Augustin M, Kleyn CE, Conrad C, Sator PG, Stahle M, Eyerich K, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2021;35:123–34.
Klein TM, Blome C, Kleyn CE, Conrad C, Sator PG, Ståhle M, et al. Real-world experience of patient-relevant benefits and treatment satisfaction with apremilast in patients with psoriasis: an analysis of the APPRECIATE study. Dermatol Ther (Heidelb). 2022;12:81–95.
Ghislain PD, Lambert J, Hoai XL, Hillary T, Roquet-Gravy PP, de la Brassinne M, et al. Real-life effectiveness of apremilast for the treatment of psoriasis in Belgium: results from the observational OTELO study. Adv Ther. 2022;39:1068–80.
Acknowledgements
We thank the patients and physicians who participated in the study.
Funding
This study was sponsored by Amgen Inc., Thousand Oaks, CA, USA. The sponsor is funding the Rapid Service Fee.
Medical Writing and Editorial Assistance
Writing support was funded by Amgen France and provided by Christina Mulvihill, PharmD, of Peloton Advantage, LLC, an OPEN Health company. Editorial support was provided by Claire Desborough, Amgen UK.
Author Contributions
Denis Jullien, Marie-Aleth Richard, Bruno Halioua, Christel Bessette, Christian Derancourt and Anne Bouloc contributed to the study concept and design; analysis of the data; and drafting and review of the manuscript.
Prior Presentation
Data presented in part in a poster presentation at the 31st EADV Congress in September 2022.
Disclosures
Denis Jullien: AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius Kabi, Janssen-Cilag, LEO Pharma, Lilly, MSD, MEDAC, Novartis, Pfizer, UCB, Sanofi – consulting fees or honoraria for lectures, advisory boards, and/or educational events. Marie-Aleth Richard: AbbVie, Almirall, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, GSK, Janssen-Cilag, LEO Pharma, Lilly, MSD, Medac Nordic, Novartis, Pfizer, Sanofi, UCB – consulting fees, payment or honoraria for lectures, advisory boards, and/or educational events. Bruno Halioua: AbbVie, Amgen, LEO Pharma, Lilly, Novartis, Pfizer – payment or honoraria. Christel Bessette & Anne Bouloc: Amgen – employees and stockholders. Christian Derancourt: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Galderma, Janssen-Cilag, NAOS, Novartis Pharma SAS, Pierre Fabre Dermo-Cosmétique, Préciphar, Sanofi Aventis France – payment or honoraria.
Compliance With Ethics Guidelines
This study was conducted in accordance with Good Clinical Practice as described in International Council for Harmonisation Guideline E6. Oral informed consent was provided by all patients before any study-related assessments began. The independent ethics committee that approved this study was Comité de Protection des Personnes Ile de France X. The study was performed in accordance with the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jullien, D., Richard, MA., Halioua, B. et al. The Needs of Patients with Psoriasis and Benefits of Apremilast in French Clinical Practice: Results from the Observational REALIZE Study. Dermatol Ther (Heidelb) 13, 1361–1376 (2023). https://doi.org/10.1007/s13555-023-00933-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00933-z