Abstract
Introduction
Keloids are a fibroproliferative, multifactorial, cutaneous disorder whose pathophysiology is not completely understood. Various factors such as high blood pressure, pregnancy, female gender, mechanical tension of local sites, and prolonged wound healing are known to worsen keloids. Childhood-onset keloids are keloids that form before 10 years of age, before various factors in adulthood come into play, and thus studying childhood-onset keloids may provide additional insight into the underlying mechanisms that lead to keloid formation.
Methods
Retrospective chart review was performed on all patients with childhood-onset keloids who were evaluated at our plastic surgery clinic (one of the largest keloid referral centers in Japan) over a 1-year period.
Results
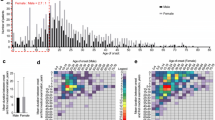
Of the 1443 patients with diagnosis of keloids, 131 patients had childhood-onset keloids. Of these, 106 patients (80.9%) were female, 38.9% of patients had family history of keloids, and 48.9% of patients had allergies or allergy-related conditions (asthma, atopic dermatitis, or allergic rhinitis). Vaccination (47.5%) and chickenpox (19.9%) were the most common triggers. Of vaccinations, BCG was the most common trigger. The majority of keloids from BCG were in female patients (92.9%). The most common location was the chest in male patients (30.0%) and the arm in female patients (41.1%).
Conclusion
To our knowledge, this is the largest report in the literature on childhood-onset keloids. There was overall female predominance in childhood-onset keloids, and even more significant female predominance in BCG-induced keloids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Over 90% of BCG vaccine-induced keloids developed in female patients. |
Vaccination and chickenpox were the most common triggers of childhood-onset keloids. |
Childhood-onset keloids were 4.2 times more common in female patients than in male patients. |
Allergy-related conditions (allergies, asthma, and atopic dermatitis) and family history of keloids were risk factors for childhood-onset keloids. |
Introduction
Keloids are a cutaneous, fibroproliferative disorder characterized by persistent inflammation in the wound and continuous deposition of collagen fibers. Despite being a relatively common skin lesion, many aspects such as pathogenesis are not fully understood.
Various triggers for keloid have been reported including operation and vaccinations such as bacillus Calmette–Guérin (BCG) vaccine (Fig. 1), as well as acne vulgaris, folliculitis, burn, ear piercing, trauma, insect bite, herpes zoster infection, and chickenpox [1]. The incidence rate of keloids varies widely among people of different ethnicities. The incidence of keloids derived from BCG has been reported to be 0.02–4.7% [2,3,4]. The incidence rate among Black and Hispanic individuals ranges from 4.5% to 16% [3]. Incidence varies among different countries, with Zaire at 16% [4], Taiwan at 0.15% [5], American Caucasians at approximately 0.1%, and the UK at 0.09% [6].
Typical BCG-induced keloids on Japanese patients. BCG-induced keloids on the upper arm typically have a dumbbell-like shape. This reflects distribution of skin tension around the keloids. Inflammation propagates in the longitudinal direction as tension continues to be applied in that direction as the tissue grows
Many lines of research suggest that keloids are driven by multiple factors, including a variety of local, systemic, and hereditary predisposition. The local factors are physical stimulation such as sustained tension, delayed wound healing, and the depth of wound; and common sites are the chest, back, abdomen, mandible, upper arms, and ears [1, 7]. Systemic factors include hormones: it is well known that keloids worsen with pregnancy [8], female sex [9], and hypertension [10,11,12]. Conditions that increase the blood levels of inflammatory cytokines such as interleukin (IL)-1α, IL-1β, and IL-6 may also promote the development of severe keloids [13]. Keloids occur in all age groups, from infants to the elderly, and they can go through exacerbations and remissions depending on the age and health status of patients. Furthermore, common causes, common sites, and relevant factors vary significantly depending on region, culture, living environment, and generation.
We have shown that keloids tend to be worse in patients with hypertension than in patients with normal blood pressure [10,11,12]. Additionally, endothelial dysfunction not only occurs in hypertension but is also associated with patients with keloids [14]. Because patients with childhood-onset keloids (before age 10) have involvement of such acquired factors, studying these patients can provide valuable insight into the complex pathophysiology of keloids. However, because childhood-onset keloids are a relatively small fraction of the entire keloid population, there are only few papers with relatively small numbers reporting data on childhood-onset keloids.
To address these gaps in knowledge, we conducted an extensive cross-sectional analysis of a large cohort of patients with childhood-onset keloid(s) (n = 131) who presented to our clinic for a medical consultation about their keloid(s) over a 1-year period. The aim of this study was to investigate the age at keloid onset, the predominant body sites, triggers, and how sex influenced these variables in the case of patients with childhood-onset keloid(s) in Japan.
Methods
Study Subjects
A retrospective chart review was carried out on all patients who were evaluated at the Department of Plastic, Reconstructive, and Aesthetic Surgery at the Nippon Medical School Hospital between April 1, 2014 and March 31, 2015 and diagnosed with keloids. Diagnosis was based on history and clinical examination by plastic surgeons. Patients whose charts did not have complete history were excluded. The study protocol was approved by the institutional review board of Nippon Medical School Hospital (approval no. 26-11-406), and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Study Variables
The following data were collected from all patients during their initial clinic visit: sex, race, age, body mass index, smoking habit, medical history (including hypertension, hyperlipidemia, diabetes mellitus), allergy-related conditions (allergies to food, medications, materials; allergic rhinitis, atopic dermatitis, or asthma), family history of keloids, age at onset of keloid, locations of keloids, and triggers for keloids.
Statistical Analyses
Continuous variables were reported as mode and/or mean ± standard deviation. Categorical variables were reported as numbers and percentages. Male and female patients were compared in terms of the variables described above by independent Student’s t test. All statistical analyses were performed using Microsoft Excel 97-2003 (Microsoft, USA). Two-tailed P values less than 0.05 were considered to be significant.
Results
Demographic and Medical History Characteristics of Total Cohort at Index Visit to the Clinic
A total of 1443 patients with keloids were evaluated at the hospital during the 1-year study period. All patients were Asian. Of the 1443 patients in the overall cohort, 302 were excluded because they did not know the age when their keloid(s) first arose. Of the remaining 1152 patients (79.8%), 131 patients (9.1%) reported that their keloid(s) appeared before 10 years of age. The distribution of age of keloid onset, which ranged from 0 to 79 years, is summarized in Table 1.
The demographics and patient characteristics are summarized in Table 2. A total of 106 patients (80.9%) were female. There were no significant differences between male and female patients in terms of age at onset, race, body mass index, hypertension, hyperlipidemia, diabetes, current smoking status, family history of keloids, or allergy-related conditions.
The prevalence of allergy-related conditions and that of family history of keloids were high (48.9% and 38.9%) in these early onset patients. The prevalence of allergy-related conditions was much lower in male (46.3%) and much higher in female (58.0%) patients who developed keloids after 10 years of age (data not shown). The prevalence of family history of keloids was much lower in male (26.2%) and female (31.8%) patients who developed keloids after 10 years of age (data not shown).
Triggers
All patients were asked what caused their keloid(s). Some patients did not recall any triggers, while some others reported multiple triggers. The results are summarized in Table 3. The most common trigger for both male and female patients was vaccination, but vaccination represented a much larger proportion of triggers in female than male patients (53.1% and 25%, respectively). Of the keloids triggered by vaccinations, BCG vaccine represented 52 out of 60 (86.7%) in female patients but only 4 out of 7 (57.1%) in male patients; 92.9% (52 out of 56) keloids triggered by BCG vaccination were in female patients. Chickenpox, or varicella zoster virus infection, was the second most common trigger in both male (17.9%) and female patients (20.4%).
Duration Between Age of Onset and Age at First Medical Examination for Keloid
Of the 56 patients with BCG vaccination-induced keloids, one was excluded because she did not know her age at the first medical examination for keloid. Of the remaining 55 patients, 51 and 4 were female and male. The female and male patients did not differ significantly in terms of mean ± SD age of duration between onset and the first examination (35.6 ± 16.7 vs. 39.0 ± 19.3 years).
Sites
Locations of keloids were evaluated and recorded in all patients. Some patients had keloids in multiple sites. The results are summarized in Table 4. The most common sites were the upper arm (34.3%), chest (19.5%), and shoulder (14.8%). When broken down by gender, the most common sites in male patients were the chest (30.0%), shoulder (20.0%), and back (15.0%). The most common sites in female patients were the upper arm (41.1%), chest (16.3%), and shoulder (13.2%).
Discussion
To our knowledge, this is the largest series reported on patients with childhood-onset keloids (onset before 10 years of age). There was a 4.2:1 female predominance in childhood-onset keloids, and 92.9% of keloids triggered by BCG vaccine were in female patients. Because BCG vaccination is a national requirement in Japan, there was no gender difference in vaccine administration. Our results suggest that female sex may be a significant risk factor for childhood-onset keloids.
Female Sex May be a Keloid Risk Factor in Childhood Onset
Previously, we reported that female patients with keloids were twice as prevalent as male patients with keloids at nearly all onset ages, and female patients predominated over male patients with a gender ratio of 2.7:1 in cases of onset before the age of 15 years [9].There may be confounding factors leading to women seeking medical care for keloids [15, 16] such as being more concerned about appearance and higher rate of piercings [17]. However, other reports have noted that there is a male predominate in medical consultations under the age of 15 in the USA [18,19,20,21]. At our plastic, reconstructive, and aesthetic surgery department, there were more outpatient visits from male than female patients in the age 0–9 group, as well as ages 10–19 and 80–89.
Previous studies on childhood-onset keloids report variable degrees of female predominance. A study from Taiwan found keloid incidence of 54 per 100,000 in female patients and 47 per 100,000 in male patients at ages 0–9 [5]. A study from China with 715 patients from all age groups had similar numbers of female and male patients (377 and 338, respectively), but in the 72 patients in the age 0–9 group, there were more female than male patients (43 and 29, respectively) [22]. Race is an important risk factor for keloids, but even among Asian countries, there are differences in cultures and medical systems which can introduce confounding factors. Ohura reported 101 patients (77 female, 24 male) with 211 keloids in all age groups in Japan [23] and found significant female predominance in childhood-onset keloids: 1 keloid in male and 28 keloids in female patients. The average number of keloids in this report was about 3 (2.96)/case, which suggests a female predominance in juvenile onset of keloids. Here, the data are specifically for juvenile keloid onset of 131 cases, which is the largest number among existing reports in Japan. Of the 1443 cases covered in this study, there were only four male BCG-derived keloids, which are extremely rare (cf. 56 female cases).
Sex Hormones May Play Key Roles in Childhood-Onset Keloids
Both genetics and hormonal levels may be contributing to the pathogenesis of childhood-onset keloids. Our results showed a high percentage of allergy-related conditions and family history of keloids (Table 2). It is conceivable that juvenile onset is strongly associated with genetic predisposition. However, it is conceivable that the reason for the female predominance is the strong influence of sex hormones during infancy. Estrogen levels change significantly over age; estrogen level is elevated during the first 6 months of life in girls but then remains low until puberty [24]. After puberty, estrogen level varies with menstrual cycle. It rises almost to three times the baseline during late pregnancy, normalizes after delivery, and remains low after menopause [25]. Keloids are known to worsen during late pregnancy [26], and improve after delivery or menopause [27, 28], and estrogen levels are therefore thought to influence keloid pathogenesis. The addition of tamoxifen, a non-steroidal anti-estrogen, usually used in breast cancer, to standard treatment may lead to improved keloids by decreasing the expression of transforming growth factor-β (TGFβ), with the consequent inhibitions of both fibroblast proliferation and collagen production [29]. In boys, testosterone levels are elevated during infancy. One study showed elevated levels of testosterone receptors in keloid tissue [30], but the relationship between testosterone levels and keloid formation has not been reported in the literature and remains unknown.
BCG and Inflammatory Reaction
The BCG vaccine is a vaccine provides some protection against tuberculosis (TB). As of 2020, 154 countries had a policy of providing BCG vaccination for the whole population, with 53 of those countries reporting coverage of at least 95% [31,32,33]. Currently, the most widely used strains for BCG vaccine production globally include French Pasteur strain (Pasteur 1173P2), Denmark 1331 strain (Danish 1331), Brazil strain (BCG Mearou RJ), Russian strain (Moscow-368), Bulgarian substrain (Sofia SL222), and the Japan 172 strain (Tokyo 172-1). The number of times and timing of vaccination vary depending on the regulations of each country. In Japan, the timing and method of vaccination have changed, but since 2014, once under the age of 1 year after birth, percutaneous inoculation is performed via the deltoid muscle of the brachii (intradermal inoculation is adopted in the stamp method) [34] using the Tokyo 172 strain. Keloids are mainly developed by the second injection and not the first (Fig. 2).
Difference in effect between 1st and 2nd vaccinations. The red circled areas are the scars from the first vaccination in infancy. It is thought that the inflammation did not last long and became a mature scar. On the other hand, upper scars caused by vaccination after entering elementary school became keloids
BCG, as a live bacterial vaccine, can inevitably disseminate beyond the vaccination site and regional lymph nodes to various parts of the body under certain conditions, and systemic adverse reactions have been reported as tuberculosis-like lesions (TBL), osteitis, periostitis, and abscess and keloids at the injection site [35]. After BCG vaccine is injected intracutaneously, monocytes, macrophages, and dendritic cells recognize the BCG at the vaccination site and form a strong immune response [36]. Compared to Danish 1331, Tokyo 172-1 has higher reported frequencies of BCG-specific interferon-7-producing CD4+ and CD8+ T cells in BCG-stimulated whole blood, greater secretion of the T helper 1-type cytokines interferon-7, tumor necrosis factor-α, and interleukin-2; significantly lower secretion of the T helper 2-type cytokine IL-4; and greater CD4+ and CD8+ T cell proliferation [37].
BCG vaccination in adults induced either a broad proinflammatory T cell response with local inflammatory reactogenicity or a predominant CD8+ regulatory T cell response with mild local inflammation, poor cytokine induction, and absent polyfunctional CD4+ T cells [38]. Normally, type 1 (Th1-mediated) and type 2 (Th2-mediated) immune responses are tightly regulated to maintain homeostasis [39]. Overactivation of either immune response may lead to disease states. Some studies have shown that keloids are driven by the Th2 immune response. Disproportionately increased activity of the Th2 cytokines IL-4 and IL-13 contributes to the pathogenesis of fibroproliferative disorders such as keloids and hypertrophic scar [40]. Wu et al. demonstrated that interferon-γ (IFNγ), the main Th1 cytokine, was significantly increased in keloid lesions and that the Th1 axis is also involved in keloid pathogenesis [41]. Furthermore, Fielding et al. also showed that highly upregulated IL-6 drives a chronic pro-fibrotic state via a Th1-mediated response [42]. It is also known that patients with Kawasaki disease have conspicuous redness in BCG scars [43]. BCG vaccination activates the immune system by causing inflammation in the skin, which may increase the risk of keloid formation.
Site of BCG Vaccination
As shown in Fig. 1, childhood-onset keloids were most common on the upper arm (34%), likely due to vaccinations being performed on arms. The upper arm is known to be a common site for keloids regardless of triggers. Because the BCG vaccine is a potent trigger for keloids, avoiding the upper arm may help reduce keloid incidence. In the case of infants, vaccination with BCG, which is a risk factor, on the lateral upper arm, which is a high-risk site for keloids, increases the risk of developing keloids [44, 45]. We hypothesize that the medial portion of the upper arm or the thigh would be a better injection site for BCG, as the skin is less taut there.
BCG Vaccine and COVID-19
Prior to the development of COVID-19 (SARS-CoV-2) vaccine, there was a surge in interest in the BCG vaccine owing to its potential protective effect against the virus. Many studies have looked at the relationship between COVID-19 and BCG vaccination [46]. Some previous data suggested the BCG vaccination may significantly reduce mortality associated with COVID-19; and the earlier a country established a BCG policy, the stronger the reduction in its number of deaths per million inhabitants [47]. The non-specific immune response induced was thought to be a factor, but data from ecological and analytical studies were heterogeneous possibly because of some confounding factors [48]. Other studies have failed to show a protective effect of BCG against COVID-19 [49,50,51]. A randomized phase II clinical trial to evaluate the efficacy and safety of BCG in preventing COVID-19 found that revaccination with BCG produced using Moscow strain was safe and resulted in a lower, but not statistically significant, incidence of COVID-19 positivity [52].
Study Limitations
The study has several limitations. All the keloid cases in the study were in Japanese patients. Race is a significant risk factor for keloids, and there may be cultural difference in patient behaviors. Additionally, vaccination policies vary significantly from one country to another. Therefore, the external validity of the study may be limited. Furthermore, the study does not include any histological, laboratory, or genetic data.
Conclusions
This is the largest report on childhood-onset keloids to date. Our results showed that childhood-onset keloids were 4.2 times more common in female than male patients. BCG vaccination was a common trigger for childhood-onset keloids, which causes keloids predominantly (> 90%) in female patients. Allergy-related conditions (allergies, asthma, and atopic dermatitis) and family history of keloids were risk factors for childhood-onset keloids.
Change history
05 April 2023
“Errors in article title updated”.
References
Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18:606.
Gonzalez O. Prevalence of hypertrophic and keloid scars according to the site of administration of intradermal BCG vaccine. Bol Oficina Sanit Panam. 1980;88:481–8.
Oluwasanmi JO. Keloids in the African. Clin Plast Surg. 1974;1:179–95.
Seifert O, Mrowietz U. Keloid scarring: bench and bedside. Arch Dermatol Res. 2009;301(4):259–72.
Sun AD, Wang KH, Lee YCG. Keloid incidence in Asian people and its comorbidity with other fibrosis-related diseases: a nationwide population-based study. Arch Dermatol Res. 2004;306(9):803–8.
Bloom D. Heredity of keloids; review of the literature and report of a family with multiple keloids in five generations. N Y State J Med. 1956;56(4):511–9.
Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60(4):445–51.
Moustafa MF, Abdel-Fattah MA, Abdel-Fattah DC. Presumptive evidence of the effect of pregnancy estrogens on keloid growth: case report. Plast Reconstr Surg. 1975;56(4):450–3.
Noishiki C, Hayasaka Y, Ogawa R. Sex differences in keloidogenesis: an analysis of 1659 keloid patients in Japan. Dermator Ther. 2019;9(4):747–54.
Arima J, Huang C, Rosner B, Akaishi S, Ogawa R. Hypertension: a systemic key to understanding local keloid severity. Wound Repair Regen. 2015;23:213–21.
Ogawa R, Arima J, Ono S, Hyakusoku H. Case report total management of a severe case of systemic keloids associated with high blood pressure (hypertension): clinical symptoms of keloids may be aggravated by hypertension. Eplasty. 2013;13: e25.
Ogawa R. High blood pressure (hypertension) may influence the results of clinical trials for scar and keloid treatments. Plast Reconstr Surg. 2013;132:1074e–1075e.
Quong WL, Kozai Y, Ogawa R. A case of keloids complicated by Castleman’s disease: interleukin-6 as a keloid risk factor. Plast Reconstr Surg Glob Open. 2017;5: e1336.
Noishiki C, Takagi G, Kubota T, Ogawa R. Endothelial dysfunction may promote keloid growth. Wound Repair Regen. 2017;25(6):976–83.
Bertakis KD, Azari R, Helmas LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147–52.
Wang Y, Hunt K, Nazareth I, Freemante N, Petersen I. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open. 2013;3:1–7.
Quaranta A, Napoli C, Fasano F, Montagna C, Caggiano G, Montagna MT. Body piercing and tattoos: a survey on young adults’ knowledge of the risks and practices in body art. BMC Public Health. 2011;11:774.
Ly N, McCaig LF. National hospital ambulatory medical care survey: 2000 outpatient department summary. Adv Data. 2002;327:1–27.
Hing E, Middleton K. National hospital ambulatory medical care survey: 2001 outpatient department summary. Adv Data. 2003;338:1–26.
Hing E, Middleton K. National hospital ambulatory medical care survey: 2002 outpatient department summary. Adv Data. 2004;345:1–36.
Middleton K, Hing E. National hospital ambulatory medical care survey: 2003 outpatient department summary. Adv Data. 2005;366:1–36.
Lu WS, Zheng XD, Yao XH, Zhang LF. Clinical and epidemiological analysis of keloids in Chinese patients. Arch Dermatol Res. 2015;307(2):109–14.
Ohura T. The treatment of keloids and hypertrophic scars. In: Ohura T, editor. Plastic surgery technique series: Tokyo: Kokuseido; 1994.
Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–22.
Catenaccio E, Mu W, Lipton ML. Estrogen- and progesteron-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct. 2016;221(8):3845–67.
Park TH, Chang CH. Keloid recurrence in pregnancy. Aesthetic Plast Surg. 2012;36(5):1271–2.
O’Sullivan ST, O’Shaughnessy M, O’Sonnor TP. Aetiology and management of hypertrophic scars and keloids. Ann R Coll Surg Engl. 1996;78(Part 1):168–75.
Qu M, Song N, Chai G, Wu X, Liu W. Pathological niche environment transforms dermal stem cells to keloid stem cells: a hypothesis of keloid formation and development. Med Hypotheses. 2013;81:807–12.
Gragnani A, Warde M, Furtado F, Ferreira LM. Topical tamoxifen therapy in hypertrophic scars or keloids in burns. Arch Dermatol Res. 2010;302(1):1–4.
Schierle HP, Scholz D, Lemperle G. Elevated levels of testosterone receptors in keloid tissue: an experimental investigation. Plast Reconstr Surg. 1997;100(2):390–5.
WHO. Global tuberculosis report 2021. Geneva: World Health Organization; 2021.
Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8(5):e1002507.
McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2782–9.
Shimao T. Peculiarity of national tuberculosis program, Japan–public-private mix from the very beginning, and provision of x-ray apparatus in most general practitioner’s clinics. Kekkaku. 2016;91(2):69–74.
Rermruay R, Thaveekarn W, Chokephaibulkit K. Clinical features and outcomes of Bacille Calmette-Guérin (BCG)-induced diseases following neonatal BCG Tokyo-172 strain immunization. Vaccine. 2019;37(26):3384.
Li J, Zhan L, Qin C. The double-sided effects of Mycobacterium bovis bacillus Calmette-Guérin vaccine. NPJ Vaccines. 2021;6(1):14.
Davids V, Hanekom WA, Mansoor N, et al. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006;193:531–6.
Boea MC, Prins C, Meijgaarden KEV, Diseel JTV, Ottenhoff THM, Jooste SA. Mycobacterium bovis BCG vaccination induces divergent proinflammatory or regulatory T cell responses in adults. Clin Vaccine Immunol. 2015;22(7):778–88.
Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9(4):532–62.
Nguyen JK, Austin E, Huang A, Mamalis A, Jagdeo J. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res. 2020;312:81–92.
Wu J, Del Duca E, Espino M, et al. RNA sequencing keloid transcriptome associates keloids With Th2, Th1, Th17/Th22, and JAK3-skewing. Front Immunol. 2020;11:597741.
Fielding CA, Jones W, McLoughlin RM, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40(1):40–50.
Araki T, Kodera A, Kitada K, et al. Analysis of factors associated with development of Bacille Calmette-Guérin inoculation site change in patients with Kawasaki disease. J Int Med Res. 2018;46(4):1640–8.
Boran C, Coban YK, Sasmaz S, Parmaksiz G. Huge keloid formation after BCG vaccination. Pediatr Dermatol. 2003;20:460–1.
Lotte A, Wasz-Höckert O, Poisson N, Dumitrescu N, Verron M, Couvet E. BCG complications: estimates of the risks among vaccinated subjects and statistical analysis of their characteristics. Adv Tuberc Res. 1984;21:107–93.
Gong W, Xie J, Li H, Aspatwar A. Research advances of tuberculosis vaccine and its implication on COVID-19. Front Immunol. 2023. https://doi.org/10.3389/fimmu.2023.1147704.
Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020;27:441.
Gong W, An H, Wang J, Chen P, Qi Y. The natural effect of BCG vaccination on COVID-19: the debate continues. Front Immunol. 2022;13:3715.
Faust L, Huddart S, MacLean E, Svadzian A. Universal BCG vaccination and protection against COVID-19: critique of an ecological study. 2020. https://microbiologycommunity.nature.com/posts/64892-universal-bcg-vaccination-and-protection-against-covid-19-critique-of-an-ecological-study. Accessed 5 Apr 2020.
Meena J, Yadav A, Kumar J. BCG vaccination policy and protection against COVID-19. Indian J Pediatr. 2020;87:749–749.
Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020. https://jamanetwork.com/journals/jama/article-abstract/2766182. Accessed 18 May 2020.
Dos Anjos LRB, Da Costa AC, Cardoso ADRO, et al. Efficacy and safety of BCG revaccination with M. bovis BCG Moscow to prevent COVID-19 infection in health care workers: a randomized phase II clinical trial. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2022.841868.
Acknowledgements
Funding
No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take as a whole, and have given their approval for this version to be published.
Author Contributions
C.N. conceived and designed the research; C.N. carried out the experiments; C.N., Y.H. and R.Y. analyzed the data; R.O. supported the study; C.N. wrote the manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Chikage Noishiki, Yoshiaki Hayasaka, Ryu Yoshida and Rei Ogawa have nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted at the Department of Plastic, Reconstructive, and Aesthetic Surgery of Nippon Medical School Hospital, Tokyo, Japan. The study protocol was approved by the institutional review board of Nippon Medical School Hospital (approval no. 26-11-406). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Data Availability
The data sets for the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Noishiki, C., Hayasaka, Y., Yoshida, R. et al. Over 90% of Childhood BCG Vaccine-Induced Keloids in Japan Occur in Women. Dermatol Ther (Heidelb) 13, 1137–1147 (2023). https://doi.org/10.1007/s13555-023-00916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00916-0