Abstract
Introduction
Thirty-percent supramolecular salicylic acid (SSA), a modified salicylic acid preparation, is a safe and effective treatment for moderate-to-severe acne vulgaris (AV). However, its mechanism of action remains unclear. We aimed to analyze the role of 30% SSA peels on skin microbiota and inflammation in patients with moderate-to-severe AV.
Methods
A total of 28 patients were enrolled and received 30% SSA peels biweekly for 2 months. The Global Acne Grading System (GAGS) score, skin water content, transepidermal water loss (TEWL), pH, and sebum levels were assessed. Skin microbial samples and perilesional skin biopsies were obtained at the onset and 2 weeks after treatment completion. Samples were characterized using a high-throughput sequencing approach targeting a portion of the bacterial 16S ribosomal RNA gene.
Results
After treatment, patients showed a significant improvement in their GAGS score and skin barrier indicators (P < 0.05). The GAGS score was positively associated with both the sebum concentration (R = 0.3, P = 0.027) and pH (R = 0.39, P = 0.003). Increased expression of caveolin-1 and decreased expression of interleukin (IL)-1a, IL-6, IL-17, transforming growth factor beta, and toll-like receptor 2 were observed in the skin tissue after treatment. The richness and evenness of the cutaneous microbiome decreased after treatment and the Staphylococcus proportion decreased significantly (P < 0.05), whereas the Propionibacterium proportion tended to decrease (P = 0.066).
Conclusions
On the basis of analyses of the skin barrier and microbiota, we speculate that the 30% SSA peel may have a therapeutic effect in patients with moderate-to-severe AV by improving the skin microenvironment and modulating the skin microbiome, thus reducing local inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While 30% supramolecular salicylic acid (SSA) is known to be a safe and effective treatment for acne vulgaris (AV), its mechanism of action is unknown. |
This study aimed to clarify the effects of 30% SSA on the skin microbiota and inflammation in patients with AV. |
What was learned from the study? |
Species diversity and proportions were affected with 30% SSA treatment; Staphylococcus species decreased significantly, and Propionibacterium species tended to decrease |
Caveolin-1 expression increased and transforming growth factor beta, toll-like receptor 2, and interleukin (IL)-1a, IL-6, and IL-17 expression decreased significantly in the skin tissue after treatment. |
Introduction
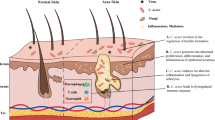
Acne vulgaris (AV) is a chronic inflammatory disorder of the pilosebaceous unit that affects approximately 85% of adolescents and young adults [1,2,3]. Moderate and severe AVs are characterized by papules, pustules, cysts, and nodules, which can appear as disfiguring scars and need a relatively long treatment course. The etiopathogenesis of AV is multifactorial and involves follicular hyperproliferation, increased sebum production, inflammation, and microbial overgrowth [4]. Recently, studies have confirmed that patients with acne harbor an altered skin microbiome, and a more significant dysbiosis is found in patients with severe acne [5,6,7]. The loss of skin microbial diversity and the activation of innate immunity may lead to chronic inflammation [8]. However, this role and its level of contribution remain unclear [9].
Patients with moderate-to-severe acne are often treated with topical and/or systemic isotretinoin and antibiotics. Combination therapy comprising chemical peels, phototherapy, and lasers, together with traditional therapy, can reduce the side effects such as skin irritation and redness, antibiotic resistance, and photosensitivity [10,11,12].
Salicylic acid (SA) is an o-hydroxybenzoic acid that acts against both non-inflammatory and inflammatory lesions in AV. Therefore, SA peels are widely used in the treatment of active AV [13,14,15]. However, SA has low water solubility. Supramolecular SA (SSA) is a new formulation that increases its solubility and reduces the side effects on the skin [16,17,18].
In a previous study, we found that 30% SSA was a safe and effective treatment for AV and could reduce sebum production [19]. However, very few studies have explored the role of SSA in the treatment of skin microbiota and inflammation in patients with moderate-to-severe AV. Therefore, we conducted a clinical trial to clarify the effects of 30% SSA on the skin microbiota composition and inflammation in patients with moderate-to-severe acne.
Methods
Patients
Overall, 28 patients diagnosed with moderate-to-severe AV and 13 healthy controls with no skin problems were recruited from Chongqing, China, between September 2020 and April 2022.
The inclusion criteria were as follows: (i) patients aged > 18 years with AV; (ii) patients with a minimum of 30 acne lesions (comedones, inflammatory papules, or pustules); and (iii) patients presenting with Pillsbury II–IV facial AV.
The exclusion criteria were as follows: (i) patients with a history of any topical or oral medication use within 3 months of the baseline study; (ii) patients with other types of dermatoses, such as atopic dermatitis, rosacea, melasma, or contact dermatitis; and (iii) patients who were breastfeeding or pregnant. This is a before–after case series, and the study was approved by the ethical review board of the First Affiliated Hospital of Chongqing Medical University, China. Written informed consent for participation in the study and publication of photographs was obtained from each participant before enrollment, adhering to the principles of the Declaration of Helsinki.
Clinical Evaluation
Investigator Evaluation
The severity of AV was evaluated objectively using the Global Acne Grading System (GAGS) [20] before administration (V0) and 8 weeks (V56) after the start of treatment. Side effects in the targeted areas were evaluated by investigators at each visit.
Patients’ Self-Assessment
At the fifth visit, patients completed a questionnaire that included questions on their acne improvement. The patients ranked their acne improvement as 0 (no improvement or worse), 1 (mild improvement), 2 (moderate improvement), and 3 (obvious improvement).
Evaluation of the Epidermal Barrier Function
Facial images and red areas were measured at each visit using the VISIA‐CR imaging system (Canfield Scientific, Fairfield, NY). The VISIA skin analysis system assesses the severity of red areas and provides a score ranging from 0% to 100%, with a higher score indicative of fewer red areas. The epidermal barrier function was evaluated at the first and fifth visits. Skin hydration was assessed using the Corneometer CM 825 (Courage + Khazaka Electronic GmbH, Cologne, Germany), transepidermal water loss (TEWL) was measured using the Tewameter TM 300 (Courage + Khazaka Electronic GmbH), and the skin pH and casual sebum level were measured using the Skin‐pH‐Meter PH 905 and the Sebumeter 815 (both Courage + Khazaka Electronic GmbH), respectively. All parameters of the epidermal barrier function were measured in the forehead, nose, left cheek, right cheek, and chin. Finally, the average values of the five sites were used in this study.
Skin Microbial Sample Collection
Skin samples of patients and healthy controls were collected before treatment administration (V0) and at 8 weeks (V56). All participants were asked to avoid washing their face and applying any topical agent 24 h prior to the examination. Samples were collected from a 9-cm2 acne area on the cheek of each patient by rubbing the skin with a sterile swab in horizontal and vertical directions 50 times (lasting approximately 30 s). The swabs were immediately stored at −80 °C for subsequent DNA extraction. Each 9-cm2 sampled area was identified using standardized photography to ensure that the same area was sampled at each follow-up visit. Microbiota sampling was conducted by the same investigator (XYS) during all visits.
Skin Biopsy Sample Collection
Perilesional skin biopsy samples were taken at V0 and V56 using a 2 mm punch biopsy. We chose a unified biopsy skin position in patients before and after treatment. The biopsied skin tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned (4-μm-thick sections). Immunocytochemical staining was performed as previously described [21, 22]. The expressions of interleukin (IL)-1a, IL-6, IL-17, transforming growth factor beta (TGF-β), toll-like receptor-2 (TLR-2), and caveolin-1 were examined using immunohistochemistry. The sections were then observed and imaged under a microscope.
30% SSA Treatment
Patients received a 30% SSA (Broda; Borenda Biochemical Technology, Shanghai, China) peel at an interval of 2 weeks for a total of 8 weeks (a total of four peeling sessions were performed). Sensitive areas of the face, such as the lateral and medial canthi, oral commissures, lips, and alar nasi grooves, were protected by applying a thin layer of petrolatum. Using a cotton tip applicator, a coat of the 30% SSA peeling agent was applied to patients’ faces, moving from the forehead and advancing to the cheeks, chin, glabella, nose, and perioral area. Once white crystallization appeared, patients were asked to wash their faces with water and pat their faces dry, immediately followed by the topical use of a mask (Broda; Borenda Biochemical Technology).
DNA Extraction and 16S Ribosomal RNA Gene Polymerase Chain Reaction Amplification and Sequencing
Total genomic DNAs from swab samples were extracted using the OMEGA Soil DNA Kit (M5635-02; Omega Bio-Tek, Norcross, GA, USA), following the manufacturer’s instructions. The V3–V4 region of the bacterial 16S ribosomal RNA (rRNA) genes was amplified using polymerase chain reaction (PCR) (initial denaturation at 98 °C for 5 min; 25 cycles of denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 45 s; final extension of 5 min at 72 °C) using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT‐3′). PCR reactions were performed in 24 μL solution containing 5 μL of buffer (5×), 0.25 μL of fast Pyrococcus furiosus DNA polymerase (5 U/μL), 2 μL (2.5 mM) of deoxyribonucleoside triphosphates, 1 μL (10 µM) of each primer, 1 μL of DNA template, and distilled and deionized water. PCR amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA). After the individual quantification step, amplicons were pooled in equal amounts, and paired-end 250-bp sequencing was performed using the Illumina MiSeq platform with MiSeq Reagent Kit v3 (Illumina, San Diego, CA) at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
Sequence-Based Microbiota Analysis
Sequencing data were mainly analyzed using QIIME2 and R packages (v3.2.0; R Foundation, Vienna, Austria). Observed relative abundances were estimated by dividing the observed number of 16S rRNA amplicon reads by the total number of reads per sample. Microbiota alpha diversity, representing microbial diversity within an individual sample, such as the Chao1 richness estimator, Shannon diversity index, Simpson index, and Pielou’s evenness, were calculated using the operational taxonomic units (OTU) table in QIIME2. Beta diversity analysis was performed to investigate the structural variation of microbial communities across samples using UniFrac distance metrics and visualized via principal coordinate analysis. Taxa abundances at the OTU levels were statistically compared among samples or groups using MetagenomeSeq analysis. Pearson’s correlation analysis was performed to assess the correlation between the skin microbes and the skin barrier parameters.
Statistical Analysis
The statistical analysis of the clinical and sequencing data was performed using R 4.1.1 (R Foundation for Statistical Computing). Data are statistically described in terms of mean ± standard deviation (mean ± SD), median and range, or frequencies and percentages. Comparisons were performed using Wilcoxon’s signed-rank test for paired samples. Bacterial populations at different taxonomical levels (genus and phylum) were compared before and after the 30% SSA treatment using Wilcoxon’s rank-sum test for paired observations. Statistical analysis of skin microbial differences before and after treatment was performed using R 4.1.1 (R Foundation for Statistical Computing). Pearson’s correlation analysis was performed to assess the correlation between skin microbes and the skin barrier parameters. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients and Healthy Controls
A total of 30 patients were enrolled, and 28 completed the trial. Two patients were excluded owing to noncompliance with the treatment schedule. The skin samples and clinical characteristics of 13 controls were collected at baseline. The baseline characteristics of the patients and healthy controls are shown in Table 1.
Treatment with 30% SSA Peel Decreased the GAGS Score
VISIA‐CR (Canfield Scientific Inc.) testing revealed a good improvement in acne, and acne lesions were significantly alleviated after 56 days of treatment on both sides (Supplementary Fig. S1). At the end of the eighth week of therapy, there was a significant decrease in the GAGS score (P < 0.001) (Fig. 1a).
The peel was well tolerated by all patients. Post-peel burning and a stinging sensation were the most common adverse effects noted in 25 patients. Post-peel erythema occurred in three patients. Post-inflammatory hyperpigmentation, blistering, crusting, scaling, hypertrophic scarring, or keloid formation were not observed in any patients. The results of patients’ self-evaluation showed that 42.9% of patients’ scores were 2 (moderate improvement) and 57.1% of patients’ scores were 3 (obvious improvement) (Table 2). All patients were satisfied with the improvement in their acne lesions.
Treatment with 30% SSA Peel Improved Skin Barrier Function
After treatment with 30% SSA peels, we observed an important difference in the indicators of the skin barrier function (Fig. 1b–f). The pH, sebum, TEWL, skin water content, and red areas were significantly improved post-treatment compared with pre-treatment (P < 0.001).
Treatment with 30% SSA Peel Decreased the Alpha Diversity of the Skin Microbiome
We analyzed the diversity in the skin microbiomes of patients with AV and healthy participants. The alpha diversity methods determined that the skin bacterial communities diverged significantly between the samples from the faces of healthy participants and those with AV (Simpson diversity index: P = 0.016, Chao1 diversity index: P = 0.019).
The alpha diversities of the skin microbiomes of the 56 samples before and after the 30% SSA peel treatment were analyzed on the basis of the estimated (Chao1) richness value and the Shannon and Simpson diversity values. The three analyses resulted in similar outcomes, revealing that the skin microbiome prior to the 30% SSA treatment had the highest alpha diversity, followed by the skin microbiome after treatment (Fig. 2a–d). The Shannon and Simpson indices were higher before the treatment than after (P < 0.05). We also used unweighted UniFrac distance metrics to perform a principal coordinate analysis of the skin samples. This analysis did not reveal any significant differences before and after the 30% SSA peel treatment.
a–d Boxplots of the alpha diversity of the skin microbiome before and after 30% SSA peel treatment. e–f Boxplots of Staphylococcus and Propionibacterium on the skin surface before and after treatment. g Analysis of Staphylococcus and Propionibacterium. h Correlation between Staphylococcus, Propionibacterium, and the skin biophysical parameters (pH, skin water content, sebum, red area, TEWL, GAGS score). SSA supramolecular salicylic acid, GAGS Global Acne Grading System, TEWL transepidermal water loss
Treatment with 30% SSA Peel Decreased the Abundance of Staphylococcus and Propionibacterium
The results of sequencing showed that the skin microbiomes of both patients with AV and the healthy controls were dominated by four bacterial phyla (Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes) and five genera (Sphingomonas, Propionibacterium, Pseudomonas, Halomonas, and Staphylococcus). However, the relative abundance of Staphylococcus had an increasing trend in patients with AV (P = 0.012).
Meanwhile, we found the ten most important bacterial families in relative abundance; the dominant phyla were Proteobacteria, Actinobacteria, Firmicutes, Bacteroidetes, and Chloroflexi, accounting for > 97% of bacteria. The relative abundance of Staphylococcus was significantly decreased after treatment in samples collected from the face (P < 0.05, Fig. 2e). Additionally, there was a trend toward a decrease in the relative abundance of Propionibacterium after 30% SSA treatment, but there was no significant difference in the abundance before and after 30% SSA treatment (P = 0.066, Fig. 2f).
Moreover, the results of the correlation matrix (Supplementary Fig. S2) of the major bacterial genera showed that the abundance of Staphylococcus was positively correlated with that of other genera and significantly positively correlated with that of Propionibacterium (R = 0.47) (Fig. 2g).
Treatment with 30% SSA Peel Reduced Tissue Expression of Inflammatory Factors
There was a significant difference before and after the 30% SSA peel treatment in terms of changes in the skin tissue with respect to IL-1a, IL-6, IL-17, TGF-β, and TLR-2 expression levels, all of which had a significantly lower staining intensity after the treatment (Fig. 3a–e). However, an increasing trend in staining intensity in the skin tissue was observed for caveolin-1 after treatment (Fig. 3f).
a1–a2 IL-1a, IL-6, IL-17, TGF-β, TLR-2 expression with intense staining before 30% SSA treatment; IL-1a with weaker staining after 30% SSA treatment (IHC, ×200; IHC, ×400). b1–b2 IL-6 expression with intense staining before 30% SSA treatment; IL-6 with weaker staining after 30% SSA treatment (IHC, ×200; IHC, ×400). c1–c2 IL-17 expression with intense staining before 30% SSA treatment; IL-17 with weaker staining after 30% SSA treatment (IHC, ×200; IHC, ×400). d1–d2 TGF-β expression with intense staining before 30% SSA treatment; TGF-β with weaker staining after 30% SSA treatment (IHC, ×200; IHC, ×400). e1–e2 TLR-2 expression with intense staining before 30% SSA treatment; TLR-2 with weaker staining after 30% SSA treatment (IHC, ×200; IHC, ×400). f1–f2 Caveolin-1 expression with weaker staining before 30% SSA treatment; caveolin-1 with intense staining after 30% SSA treatment (IHC, ×200; IHC, ×400). IHC immunohistochemistry, SSA supramolecular salicylic acid, IL interleukin, TGF- β transforming growth factor beta, TLR-2 toll-like receptor 2
Treatment with 30% SSA Peel Regulated Skin Microbiome by Improving the Epidermal Barrier
We analyzed the correlation between the diversity of the microbiome and the epidermal barrier functions and showed a positive correlation between the Simpson diversity value and TEWL (R = 0.27, P = 0.047) (Fig. 4a). The correlations between Staphylococcus, Propionibacterium, and the skin biophysical parameters (skin pH, skin water content, sebum, red area, TEWL, and the GAGS score) were further investigated (Fig. 2h). The abundance of Staphylococcus was negatively correlated with the skin water content (R = − 0.44, P = 0.018). Sebum secretion was positively correlated with the GAGS score (R = 0.3, P = 0.027) and skin pH (R = 0.38, P = 0.004). In addition, the GAGS score was positively associated with the skin pH (R = 0.39, P = 0.003) (Fig. 4b–e). Other parameters showed no significant correlations (P > 0.05).
a Correlation between the Simpson index and TEWL (R = 0.27, P = 0.047). b Correlation between the GAGS score and sebum concentration. c Correlation between pH and sebum concentration. d Correlation between the GAGS score and pH. e Correlation between the skin water content and abundance of Staphylococcus. GAGS Global Acne Grading System, TEWL transepidermal water loss
Discussion
SA peeling is an established, safe, and easy treatment option for AV. Its mechanism for acne treatment includes suppressing the activated protein kinase/sterol regulatory element-binding transcription factor 1 pathway in the sebocytes to reduce sebum production and inhibiting the activity of the nuclear factor kappa B (NF-κB) to control inflammation [23]. Traditional synthetic SA has poor solubility in water. To increase its solubility, it is often dissolved in an organic solvent (i.e., ethanol). However, alcoholic solutions cause skin irritation, which may lead to poor compliance in patients. SSA is a water-soluble complex that delivers SA without alcohol or other solvents, is stable in water, and reduces irritation to the skin. Meanwhile, the effects of a 30% SSA peel are better than those of other concentrations of SSA peels in vitro [24, 25]. In this study, patients with moderate-to-severe AV who were treated with 30% SSA had significantly fewer lesions and lower GAGS scores, suggesting that the treatment was effective. Nevertheless, the mechanism of action of 30% SSA peel treatment remains unclear.
The skin is a physical barrier between the body and the environment, preventing the colonization of pathogens [26]. Changes in the skin microenvironment are closely related to the occurrence and development of many skin diseases [27]. A previous study suggested that the secretion of sebum and TEWL were increased in patients with AV more than in healthy controls [26]. However, the correlation between the skin surface pH and the severity of AV remains controversial [28,29,30]. In the present study, pH and sebum and TEWL concentrations were significantly decreased, indicating improvement in the skin barrier. Further, Pearson’s correlation analysis suggested that the sebum concentration and skin pH were positively correlated with the GAGS score (R = 0.3, P = 0.027; R = 0.38, P = 0.0041). This might have resulted from the slow release properties and high permeability of SSA [18]. SSA achieves maximum efficacy in low pH and reduces irritation of the skin. Additionally, the bidirectional regulation of keratinocytes by SSA may play a significant role in the improvement of skin barrier function. At high concentrations of SSA, the upper layer of the stratum corneum is exfoliated owing to the dissolution of desmosomes and a decrease in corneocyte adhesions. However, SSA can increase epidermal thickness at lower concentrations by activating basal keratinocytes [31, 32]. The results suggested that 30% SSA treatment improved moderate-to-severe AV by improving the skin microenvironment.
Human skin is colonized by a wide variety of microbes, which play an important role in skin homeostasis. The dysbiosis of the skin microbiome is implicated in the protection and pathogenesis of various diseases [33, 34]. There is evidence to suggest that Propionibacterium and Staphylococcus contribute significantly to the pathogenesis of AV [35,36,37]. The over-colonization of Propionibacterium acne triggers the immune response in sebocytes, keratinocytes, and monocytes [38]. In addition, the proportion of Staphylococcus increases with the severity of AV [38,39,40,41]. In this study, we found that patients with moderate-to-severe AV showed increased amounts of Staphylococcus and Propionibacterium compared with healthy controls (P = 0.013 and P = 0.0015, respectively), suggesting that the two genera could be possible biomarkers of moderate-to-severe AV and that their overgrowth may be related to the pathogenesis of acne. A recent study suggests that Propionibacterium and Staphylococcus are associated with disease flares; P. acne may produce a factor or provide an environment that promotes Staphylococcus biofilm formation, and an unbalanced equilibrium between P. acne and Staphylococcus epidermidis contributes to the development of AV [38]. Our study found that the abundance of Staphylococcus decreased significantly and that of Propionibacterium tended to decrease after SSA treatment. Moreover, the abundance of Propionibacterium decreased with the reduction in the abundance of Staphylococcus (R = 0.27, P = 0.047). Our findings add further evidence to corroborate that propionibacteria and staphylococci interact with each other and suggest that 30% SSA may play a therapeutic role by regulating the skin microbiome in patients with moderate-to-severe AV.
Inflammation plays an important role in the onset, development, and resolution of AV [42]. Inflammation may be associated with changes in the skin surface pH and disturbance of the stratum corneum, allowing microorganisms to stimulate the production of pro-inflammatory cytokines. With the reduction in skin bacteria, the production of pro-inflammatory cytokines is also reduced, thereby improving inflammation and acne severity. Virulent P. acnes is one of the most important factors that induce an inflammatory response in acne and activates TLR-2 and TLR-4 in keratinocytes and sebocytes, leading to the activation of signaling cascades and the production of pro-inflammatory cytokines. Subsequently, IL-1, IL-6, IL-17, caveolin-1, and TGF-β cytokines induce innate immunity [43,44,45,46,47,48,49,50]. By analyzing the immunohistochemistry results of the perilesional skin biopsy in patients with moderate-to-severe AV, we found that the levels of inflammatory biomarkers, such as IL-1a, IL-6, IL-17, TGF-β, and TLR-2, were significantly decreased after 30% SSA peel treatment. In addition, the secretion of caveolin-1 in the same area was upregulated after treatment. Thus, 30% SSA treatment significantly reduces the production of pro-inflammatory cytokines in the skin of patients with moderate-to-severe acne and improves local skin inflammation.
Different microenvironments may play a role in the growth or inhibition of microorganisms [26, 30, 51]. In addition, an increase in the skin surface pH leads to impaired barrier function, disturbances in the skin microbiome, and inflammation [34]. Hyperseborrhea favors P. acnes overgrowth and biofilm formation, promotes subsequent inflammation, disturbs follicular barrier function, and induces comedogenesis [52]. The sudden changes, together with the activation of innate immunity, might lead to chronic inflammation [53, 54]. In the above studies, 30% SSA affected the skin microenvironment and skin microbiota in patients with moderate-to-severe acne. In addition, it may alleviate the disease by reducing the level of local skin tissue inflammation. Combined with previous studies, we speculated that 30% SSA may improve the skin microenvironment in patients with moderate-to-severe AV, inhibit the colonization of Propionibacterium and Staphylococcus, regulate the skin microbiota, reduce the production of local pro-inflammatory cytokines, and treat moderate-to-severe AV. A potential limitation of our study is the small sample size and that no metagenomic sequencing was performed.
Conclusions
Our study demonstrated that 30% SSA treatment can improve the GAGS score of patients with moderate-to-severe AV. On the basis of further analysis of the skin barrier and microbiota, we speculate that 30% SSA may exert its therapeutic effect by improving the skin microenvironment and modulating the skin microbiome, thereby improving local inflammation. Our findings provide a novel insight into the therapeutic rationale of 30% SSA treatment for moderate-to-severe AV.
References
Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672–6. https://doi.org/10.1046/j.1365-2133.1999.02768.x.
Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1. https://doi.org/10.5070/D34HP8N68P.
Tanghetti EA, Kawata AK, Daniels SR, Yeomans K, Burk CT, Callender VD. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22–30.
Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl 2):5–14. https://doi.org/10.1111/jdv.15043.
Dagnelie MA, Montassier E, Khammari A, Mounier C, Corvec S, Dréno B. Inflammatory skin is associated with changes in the skin microbiota composition on the back of severe acne patients. Exp Dermatol. 2019;28:961–7. https://doi.org/10.1111/exd.13988.
Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:39491. https://doi.org/10.1038/srep39491.
Ramasamy S, Barnard E, Dawson TL Jr, Li H. The role of the skin microbiota in acne pathophysiology. Br J Dermatol. 2019;181:691–9. https://doi.org/10.1111/bjd.18230.
Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–60. https://doi.org/10.1038/jid.2013.21.
Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222–8. https://doi.org/10.1016/j.jaad.2019.08.078.
Cooper AJ, Harris VR. Modern management of acne. Med J Aust. 2017;206:41–5. https://doi.org/10.5694/mja16.00516.
Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335–44. https://doi.org/10.1007/s40257-018-00417-3.
Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73.e33. https://doi.org/10.1016/j.jaad.2015.12.037.
Dayal S, Kalra KD, Sahu P. Comparative study of efficacy and safety of 45% mandelic acid versus 30% salicylic acid peels in mild-to-moderate acne vulgaris. J Cosmet Dermatol. 2020;19:393–9. https://doi.org/10.1111/jocd.13168.
Lin AN, Nakatsui T. Salicylic acid revisited. Int J Dermatol. 1998;37:335–42. https://doi.org/10.1046/j.1365-4362.1998.00452.x.
Zeichner JA. The use of Lipohydroxy acid in skin care and acne treatment. J Clin Aesthet Dermatol. 2016;9:40–3.
Zheng Y, Yin S, Xia Y, Chen J, Ye C, Zeng Q, et al. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: a randomized, split-face, open-label, single-center study. Cutan Ocul Toxicol. 2019;38:48–54. https://doi.org/10.1080/15569527.2018.1518329.
Abounassif MA, Mian MS, Mian NAA. Salicylic acid. In: Anal profiles drug subst excipients. CA: Academic Press; 1994. p. 421–70. https://doi.org/10.1016/S0099-5428(08)60609-7.
Cao S, Fu X, Wang N, Wang H, Yang Y. Release behavior of salicylic acid in supramolecular hydrogels formed by l-phenylalanine derivatives as hydrogelator. Int J Pharm. 2008;357:95–9. https://doi.org/10.1016/j.ijpharm.2008.01.054.
Zhang L, Shao X, Chen Y, Wang J, Ariyawati A, Zhang Y, et al. 30% supramolecular salicylic acid peels effectively treats acne vulgaris and reduces facial sebum. J Cosmet Dermatol. 2022;21:3398–405. https://doi.org/10.1111/jocd.14799.
Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–8. https://doi.org/10.1046/j.1365-4362.1997.00099.x.
Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/β-catenin signaling. Genes Dis. 2021;8:677–88. https://doi.org/10.1016/j.gendis.2020.06.003.
Chen Y, Zou D, Wang N, Tan T, Liu Y, Zhao Q, et al. SFRP5 inhibits the migration and invasion of melanoma cells through Wnt signaling pathway. Onco Targets Ther. 2018;11:8761–72. https://doi.org/10.2147/OTT.S181146.
Lu J, Cong T, Wen X, Li X, Du D, He G, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28:786–94. https://doi.org/10.1111/exd.13934.
Scherrer M, Knüsel F. Weirich EG [Antimicrobial activity of broad-spectrum antimicrobial agents, with special reference to salicylic acid]. Mykosen. 1971;7:323–34.
Knüsel F, Weirich EG. Microbiological evaluation of salicyclic acid and other broad spectrum antimicrobials. Dermatologica. 1972;145:233–44. https://doi.org/10.1159/000252050.
Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018;310:181–5. https://doi.org/10.1007/s00403-017-1795-3.
Yang G, Seok JK, Kang HC, Cho YY, Lee HS, Lee JY. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int J Mol Sci. 2020;21:2867. https://doi.org/10.3390/ijms21082867.
Prakash C, Bhargava P, Tiwari S, Majumdar B, Bhargava RK. Skin surface pH in acne vulgaris: insights from an observational study and review of the literature. J Clin Aesthet Dermatol. 2017;10:33–9 (Epub 2017 Jul 1).
Youn SH, Choi CW, Choi JW, Youn SW. The skin surface pH and its different influence on the development of acne lesion according to gender and age. Skin Res Technol. 2013;19:131–6. https://doi.org/10.1111/srt.12023.
Kim MK, Choi SY, Byun HJ, Huh CH, Park KC, Patel RA, et al. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch Dermatol Res. 2006;298:113–9. https://doi.org/10.1007/s00403-006-0666-0.
Imayama S, Ueda S, Isoda M. Histologic changes in the skin of hairless mice following peeling with salicylic acid. Arch Dermatol. 2000;136:1390–5. https://doi.org/10.1001/archderm.136.11.1390.
Ye D, Xue H, Huang S, He S, Li Y, Liu J, et al. A prospective, randomized, split-face study of concomitant administration of low-dose oral isotretinoin with 30% salicylic acid chemical peeling for the treatment of acne vulgaris in Asian population. Int J Dermatol. 2022;61:698–706. https://doi.org/10.1111/ijd.16127.
Yamazaki Y, Nakamura Y, Núñez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017;66:539–44. https://doi.org/10.1016/j.alit.2017.08.004.
Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–55. https://doi.org/10.1038/nrmicro.2017.157.
Meisel JS, Sfyroera G, Bartow-McKenney C, Gimblet C, Bugayev J, Horwinski J, et al. Commensal microbiota modulate gene expression in the skin. Microbiome. 2018;6:20. https://doi.org/10.1186/s40168-018-0404-9.
Suh DH, Kwon HH. What’s new in the physiopathology of acne? Br J Dermatol. 2015;172(Suppl 1):13–9. https://doi.org/10.1111/bjd.13634.
Coughlin CC, Swink SM, Horwinski J, Sfyroera G, Bugayev J, Grice EA, et al. The preadolescent acne microbiome: a prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatr Dermatol. 2017;34:661–4. https://doi.org/10.1111/pde.13261.
Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seité S. Skin microbiome and acne vulgaris: staphylococcus, a new actor in acne. Exp Dermatol. 2017;26:798–803. https://doi.org/10.1111/exd.13296.
Claudel JP, Auffret N, Leccia MT, Poli F, Corvec S, Dréno B. Staphylococcus epidermidis: a potential new player in the physiopathology of acne? Dermatology. 2019;235:287–94. https://doi.org/10.1159/000499858.
Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98:411–24. https://doi.org/10.1007/s00253-013-5394-8.
Nakase K, Yoshida A, Saita H, Hayashi N, Nishijima S, Nakaminami H, et al. Relationship between quinolone use and resistance of Staphylococcus epidermidis in patients with acne vulgaris. J Dermatol. 2019;46:782–6. https://doi.org/10.1111/1346-8138.15000.
Cong TX, Hao D, Wen X, Li XH, He G, Jiang X. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337–49. https://doi.org/10.1007/s00403-019-01908-x.
Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150:421–8. https://doi.org/10.1046/j.1365-2133.2004.05762.x.
Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–13. https://doi.org/10.1111/j.1365-2133.2005.06933.x.
Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8. https://doi.org/10.1111/j.0022-202X.2005.23705.x.
Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371–88. https://doi.org/10.2147/CCID.S69135.
Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110–8. https://doi.org/10.1038/jid.2014.290.
Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol. 2009;129:375–82. https://doi.org/10.1038/jid.2008.237.
Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, et al. Caveolin-1 deficiency dampens toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 2010;176:2344–51. https://doi.org/10.2353/ajpath.2010.091088.
Kruglikov IL, Scherer PE. Caveolin-1 as a possible target in the treatment for acne. Exp Dermatol. 2020;29:177–83. https://doi.org/10.1111/exd.14063.
Korting HC, Lukacs A, Vogt N, Urban J, Ehret W, Ruckdeschel G. Influence of the pH-value on the growth of Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes in continuous culture. Zentralbl Hyg Umweltmed. 1992;193:78–90.
Schürer N. pH and acne. Curr Probl Dermatol. 2018;54:115–22. https://doi.org/10.1159/000489525.
Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. 2012;167:50–8. https://doi.org/10.1111/j.1365-2133.2012.10897.x.
Szegedi A, Dajnoki Z, Bíró T, Kemény L, Törőcsik D. Acne: transient arrest in the homeostatic host-microbiota dialog? Trends Immunol. 2019;40:873–6. https://doi.org/10.1016/j.it.2019.08.006.
Acknowledgements
The authors thank Broda (Shanghai Rui Zhi Medicine Technology, Shanghai, China) for providing the 30% SSA chemical peel for this study.
Funding
This study was supported by the National Natural Science Foundation of China (n82073462). The journal’s Rapid Service Fee was funded by the authors.
Author Contributions
Xinyi Shao, Yangmei Chen, and Jin Chen contributed to the study’s conception and design. Xinyi Shao and Yangmei Chen contributed to the acquisition of data. Xinyi Shao, Lingzhao Zhang, Jin Chen, Yihuan Pu, and Yuxin Li performed the statistical analyses and interpretation of data. Yujie Zhang, Lin Liu, and Tingqiao Chen provided editing support. All authors contributed to the interpretation and analysis of the literature, as well as careful and critical revision and approval of the final manuscript.
Disclosures
Xinyi Shao, Yangmei Chen, Lingzhao Zhang, Yujie Zhang, Asoka Ariyawati, Tingqiao Chen, Jiayi Chen, Lin Liu, Yihuan Pu, Yuxin Li and Jin Chen have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of the First Affiliated Hospital, Chongqing Medical University (Ethical code number: 2022-066). Written informed consent was obtained from each participant before enrollment, and this study adhered to the Principle of the Declaration of Helsinki. Photography consent was also obtained from each participant before enrollment.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shao, X., Chen, Y., Zhang, L. et al. Effect of 30% Supramolecular Salicylic Acid Peel on Skin Microbiota and Inflammation in Patients with Moderate-to-Severe Acne Vulgaris. Dermatol Ther (Heidelb) 13, 155–168 (2023). https://doi.org/10.1007/s13555-022-00844-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00844-5