Abstract
Introduction
The aging process involves numerous biological mechanisms that have been characterized and proposed as the “hallmarks of aging.” Targeting the processes and pathways related to these hallmarks of aging that cause and promote skin aging could provide anti-aging benefits. A novel topical growth factor-based skin care serum (A+) was developed using human fibroblast conditioned media. This study aimed to assess the effects of A+ on four hallmarks of aging and its clinical efficacy in skin rejuvenation in subjects with moderate to severe overall facial photodamage.
Methods
Preclinical studies included immunohistochemistry in human ex vivo skin, and gene expression analysis in human 3D skin models. A 24-week, vehicle placebo-controlled study, including FaceQ patient-reported outcomes and skin biopsy analysis, was performed to assess clinical efficacy and tolerability.
Results
Treatment with A+ resulted in reduced expression of cell senescence biomarker H2A.J and upregulation of genes associated with proteasome, autophagy, stemness, and intercellular communication. Clinical assessments showed A+ provided significantly greater reductions in sagging, coarse lines/wrinkles, fine lines/wrinkles, overall photodamage, and overall hyperpigmentation compared with placebo. Subjects felt they appeared younger-looking, reporting a median decrease in self-perceived age of 6 years after 12 weeks of use. Decreased levels of H2A.J and increased expression of key dermal extracellular matrix and epidermal barrier components, including collagen and elastin, were observed in skin biopsy samples.
Conclusion
The present study shows for the first time the potential effects of a topical growth factor-based cosmeceutical on cellular processes related to four hallmarks of aging (cellular senescence, loss of proteostasis, stem cell exhaustion, and altered intercellular communication) to help delay the aging process and restore aged skin. A+ targets the biological mechanisms underlying the aging process itself and stimulates skin regeneration, resulting in rapid and significant clinical improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The “hallmarks of aging” describe the various biological mechanisms underlying the aging process itself. |
Targeting these processes and pathways that cause and promote aging could help delay the onset or progression of visible signs of skin aging. |
The aim of this study was to assess the efficacy of a topical growth factor-based skin care serum (A+) that targets several hallmarks of aging and supports skin regeneration to provide comprehensive anti-aging benefits. |
What was learned from the study? |
A+ targets four key hallmarks of aging (cellular senescence, loss of proteostasis, stem cell exhaustion, and altered intercellular communication), suggesting potential inhibition or reversal of cellular aging processes, in addition to stimulating extracellular matrix regeneration and skin barrier function. |
The result of leveraging this comprehensive approach to anti-aging skin care can be observed in the rapid and continued significant improvements achieved in the clinical study. |
Introduction
In the skin, keratinocytes, fibroblasts, melanocytes, and cells associated with the immune system all secrete a variety of growth factors that are associated with regulating skin structure and function [1, 2]. Growth factors interact with specific cell surface receptors and physiologically function as a synergistic cocktail of proteins to maintain skin health. Other specific factors, known as cytokines, modulate inflammation due to local injury and work with other growth factors to stimulate repair and regeneration [3, 4]. Skin aging is associated with reduced levels of growth factors that are critical to normal skin health and function. Since the 1980s, much of our understanding of the importance of growth factors and cytokines in the skin came from wound healing studies on acute and chronic wounds treated with cell-derived factors [5] or application of neonatal fibroblasts in three-dimensional structures [6,7,8]. The naturally secreted growth factors and cytokines in the media of in vitro neonatal human fibroblast cultures can upregulate genes associated with key dermal extracellular matrix (ECM) components including collagens and elastin [9]. Several clinical studies utilizing human fibroblast conditioned media in skin care formulations have demonstrated the ability of natural, physiologically balanced growth factors to reverse signs of intrinsic and extrinsic skin aging when topically applied to intact skin [2, 9,10,11,12,13]. Additional studies show that human fibroblast conditioned media can speed up healing and reduce inflammation when applied post-resurfacing [14, 15].
Next Generation Conditioned Media with Growth Factors
In the mid-1990s Adzick et al. made the discovery of scarless wound healing in the fetal stage of mammalian development. During wound healing, fetal fibroblasts show reduced production of transforming growth factor beta (TGF-β) and basic fibroblast growth factor (bFGF) as well as secretion of predominantly collagen types III and V instead of type I [16]. The intrauterine fetal environment is characterized by hypoxia (3–5% oxygen) rather than the normoxic 21%, and by growth of tissue in suspension. Neonatal human fibroblasts that were cultured in a bioreactor mimicking the fetal environment (a suspension cell culture on dextran beads in a constantly moving stir tank under hypoxic conditions) within 48 h showed the upregulation of over 5000 different genes, including those associated with pluripotent stem cells and secretion of stem cell markers [4]. Furthermore, the cell conditioned media created under hypoxic conditions (hCCM) shows differences in growth factor composition compared with normal culture conditioned media: hypoxia resulted in increased amounts of keratinocyte growth factor (KGF) and vascular endothelial growth factor (VEGF), while TGF-β was undetectable [15]. These findings suggest that hCCM created in a fetal-like environment offers potential as the next generation of growth factors that can be used in topical skin care products that promote skin rejuvenation by supporting stem cell proliferation and differentiation in addition to enhanced tissue function and regulation.
Targeting the Hallmarks of Aging
The traditional target of anti-aging skin treatments is to reverse the phenotype or “symptoms” of aging (e.g., wrinkles, dryness, etc.) by stimulating regeneration of the dermal ECM and epidermal barrier that are damaged and deteriorating over time through intrinsic and extrinsic factors. However, the aging process itself involves numerous biological mechanisms that have been characterized and proposed as the “hallmarks of aging,” and that are relevant in aging skin as well [17, 18]. These hallmarks of aging include genomic instability, Telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Recently, dysregulation of alternative RNA splicing is emerging as a tenth hallmark of aging [19, 20]. Targeting these processes and pathways that cause and promote skin aging could provide true anti-aging benefits and help delay or prevent the onset of visible signs of aging.
A novel topical growth factor-based skin care serum formulated with hCCM, botanicals, marine extracts, and peptides (Table 1) was assessed at the molecular level for its effects on processes related to four hallmarks of aging (cellular senescence, loss of proteostasis, stem cell exhaustion, and altered intercellular communication) as well as ECM regeneration and skin barrier function. A randomized, placebo-controlled clinical study was performed to assess the clinical efficacy and tolerability of this innovative serum.
Methods
Human Skin Explants
Skin explants of an average diameter of 12 mm (± 1 mm) were prepared from donated abdominoplasty skin from a 63-year-old Caucasian female. Explants were kept in a survival cell culture medium at 37 °C during the study. Test product (TNS Advanced + Serum (A+); SkinMedica, Allergan Aesthetics, an AbbVie Company, Irvine, CA) was applied (day 0, 1, 2, 3 and 4) on the basis of 2 µl per explant. The culture medium was half renewed (1 mL per well) on day 2 and 3. Samples were collected on day 6 for immunohistochemistry using antibodies against H2A.J (H2AFJ) (Origene, Rockville, MD).
Gene Expression in Human In Vitro Skin Model
Gene expression studies were performed using an in vitro human skin model validated against retinoic acid [21]. EpiDermFT 3D full thickness human skin model (EFT-400) was acquired from MatTek Corp (Ashland, MA). Tissues were cultured with EpiDermFT Assay Media (EFT-400-MM, MatTek Corp). EpiDermFT was irradiated with 200 mJ/cm2 ultraviolet (UV) light with UV-B filter UV lamp (Honle, Germany) to indicate an extrinsic aging model, followed by application of 15 µL of test product or dH2O (control), and incubated at 37 °C and 5% CO2 for 24 h. After incubation five tissues of each condition were placed into RNAlater solution (Thermo Fisher Scientific, Waltham, MA).
Quantitative Real-Time PCR
mRNA was extracted from whole tissues (Maxwell RSC simplyRNA Tissue Kit; Promega,) followed by cDNA synthesis (High-Capacity cDNA Reverse Transcription Kit; Thermo Fisher Scientific) and quantitative real-time PCR (TaqMan Fast Advanced Master Mix; Thermo Fisher Scientific). Gene expression analyses were performed using TaqMan Gene Expression Assays (Thermo Fisher Scientific) with real-time PCR System QuantStudio7 Flex (Thermo Fisher Scientific). The assay mix used for studies were (Cat#): GAPDH (Hs02758991_g1), COL1A1 (Hs001640004_m1), COL3A1 (Hs00943809_m1), COL4A1 (Hs00266237_m1), COL7A1 (Hs00164310_m1), ELN (Hs00355783_m1), FBN1 (Hs00171191_m1), TGFB1 (Hs00998133_m1), POMP (Hs01106088_m1), PSMB5 (Hs00605652_m1), PSMB6 (Hs00382586_m1), ATG5 (Hs00169468_m1), ATG7 (Hs00893766_m1), ATG12 (Hs04980076_s1), BECN1 (Hs01007018_m1), MAP1LC3A (Hs01076567_g1), ACTA2 (Hs00426835_g1), NES(Hs04187831_g1), CX37 also known as GJA4 (Hs00704917_s1), CX26 also known as GJB2 (Hs00269615_s1), CX30.3 GJB4 (Hs00920816_s1), CX31.1 also known as GJB5 (Hs01921450_s1), CX30 also known as GJB6 (Hs00922742_s1), IVL (Hs00846307_s1), UGCG (Hs00916612_m1), OCLN (Hs00170162_m1), TGM1 (Hs00165929_m1), TGM3 (Hs00162752_m1), SPRR1A (Hs00954595_m1), and SMPD1 (Hs03679346_g1). Gene expression levels were normalized to housekeeping gene GAPDH, and relative quantity (relative gene expression) was calculated using the delta-delta Ct method compared with non-UV irradiated, non-treated control samples (baseline gene expression, value set at 1). Comparisons were statistically analyzed using Student’s t-test; differences are considered statistically significant at the p < 0.05 level.
Clinical Study Design
A 24-week, double-blind, randomized, placebo-controlled, cross-over study was conducted to assess the efficacy and tolerability of A+. Criteria for study participation included female and male subjects in general good health between the ages of 35 and 70 years with Fitzpatrick skin types I–VI presenting with moderate to severe overall facial photodamage (baseline score between 4 and 9 as assessed on the overall photodamage scale). Subjects were not allowed to apply any other topical products (including skin brighteners, retinoids, alpha/beta/poly-hydroxy acids), undergo facial treatments, nor begin new cosmetic facial make-up throughout the duration of the study. Subjects were provided standard skin care products (facial cleanser and moisturizer with SPF 35 sunscreen) to use during the study. Subjects were instructed to avoid extended periods of sun exposure and the use of tanning beds.
Institutional Review Board approval (IntegReview, Austin, TX) was obtained prior to conducting any study procedures. The study was conducted following the ethical and regulatory principles from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. All subjects provided informed consent prior to study participation. All study subjects provided permission for use of their images in scientific publications in the consent form.
The study was divided into two phases: phase I (baseline through week 12) and phase II (week 12 through week 24). During phase I, all subjects were randomized to either the active group, which used A+ twice daily (once in the morning and night after cleansing), or to the placebo group, which used a vehicle placebo twice daily (once in the morning and night, after cleansing). Subjects were blinded to the treatment received. During phase II, starting at the week 12 visit, all placebo group subjects were crossed over to begin using A+ twice daily instead of the vehicle placebo. Subjects originally in the active group continued with their provided treatment. Standard skin care products (facial cleanser and moisturizer with SPF 35 sunscreen) were additionally provided to all subjects in the study. Subjects were instructed to remove all makeup and acclimate to ambient temperature and humidity conditions for at least 15 min prior to any study procedures at each visit.
Clinical Efficacy Assessments
Treatment-blinded investigator grading for the following efficacy parameters were performed at baseline, week 2, week 4, week 8, week 12, week 18, and week 24 using a modified Griffiths’ 10-point grading scale, where 0 is none (best possible condition), 1–3 is mild, 4–6 is moderate, and 7–9 is severe (worst condition possible), with half points allowed as necessary to differentiate degrees of severity. Efficacy parameters are listed below with description of grade 0 and 9 anchors:
-
Overall photodamage: 0 indicates none or minimal visual evidence of photodamaged skin, 9 indicates severe photodamaged skin
-
Sagging: 0 indicates skin is fully lifted with no sagging, 9 indicates severe sagging
-
Coarse Lines/wrinkles (periocular, perioral, forehead, and cheeks): 0 indicates none, 9 indicates numerous, deep coarse lines/wrinkles
-
Fine Lines/wrinkles (periocular, perioral, forehead, and cheeks): 0 indicates none, 9 indicates numerous, deep fine lines/wrinkles
-
Skin tone evenness: 0 indicates even, healthy skin color, no evidence of red/brown blotchiness, 9 indicates uneven, discolored appearance with severe red/brown blotchiness
-
Overall hyperpigmentation: 0 indicates even skin color, no areas of hyperpigmentation, 9 indicates significant (severe) hyperpigmented appearance, involving most of the face, with very strong intensity
-
Tactile roughness: 0 indicates smooth, even feeling skin texture, 9 indicates rough, uneven feeling skin texture
Tolerability Assessments
The investigator assessed each subject at baseline, and weeks 2, 4, 8, 12, 18, and 24 for erythema and scaling using a 4-point scale (0, none; 1, mild; 2, moderate; and 3, severe). Subjective parameters, including burning/stinging, itching, tightness, and tingling, were assessed by the subjects.
Standardized Digital Photography
Full face digital photographs were taken using the VISIA-CR photo system (Canfield Imaging Systems, Fairfield, NJ) with a Canon Mark II 5D digital SLR camera (Canon Inc., Tokyo, Japan) at baseline and weeks 2, 4, 8, 12, 18, and 24. Frontal as well as 45° lateral left and right images were taken using standard lighting 1, standard 2, standard 3, and cross-polarized lighting (brown and red channel light).
Statistical Analysis
Clinical grading scores at weeks 2, 4, 8, 12, 18, and 24 were compared with baseline scores using Wilcoxon signed-rank test. For phase I, active and placebo groups were compared using the Kruskal–Wallis one-way analysis of variance (ANOVA) followed by Wilcoxon rank-sum test. For phase II, weeks 8 and 24 were compared with week 12 (baseline for phase II) for the original placebo group to assess effects of the cross-over treatment. All differences are considered statistically significant at the p < 0.05 level.
Face-Q Appearance Appraisal Scales
A subset of the subjects assigned to the active group (n = 35) completed the validated patient reported outcome tool Face-Q [22], at baseline, week 4, week 12, and week 24. Subjects responded to questions regarding Satisfaction with Facial Appearance, Satisfaction with Skin, Aging Appearance Appraisal, and Age Appraisal Visual Analog Scale (VAS). Scores range from 0 to 100 with higher scores indicating higher satisfaction or appraisal.
Facial Skin Biopsy Samples
A subset of subjects from the active group (n = 20) and from the placebo group (n = 10) chose to have 2-mm skin biopsy samples from the temple area on the left side of the face, along the hairline at the extension of the corner of the eye. Biopsy samples were taken 1 cm apart from each other. Half of the subjects in each subset group received the biopsies at baseline and at week 12. The other half received biopsies at week 12 and week 24.
Histological Analysis
Samples were fixed with 10% neutral buffered formalin (NBF) and embedded into paraffin blocks. Samples were stained with hematoxylin and eosin (H&E), Gomori’s trichrome (Thermo Fisher Scientific), and immunohistochemistry using antibodies against H2A.J (H2AFJ) (Origene, Rockville, MD), collagen IV (Rockland, Limerick, PA), decorin (Abcam, Cambridge, MA), elastin (Elastin Products Company, Owensville, MO), claudin 1 (Abcam), filaggrin (Abcam), transglutaminase (Proteintech, Rosemont, IL), and perilipin 3 (R&D Systems, Minneapolis, MN). Immunohistochemistry was performed using Leica automated stainer BOND-III (Leica, Buffalo Grove, IL) followed by image capture with digital image scanner NanoZoomer (Hamamatsu, Bridgewater, NJ).
Bioinformatic Analysis of Staining Intensity
Analysis was performed using skin biopsies taken at baseline and week 12 from subjects assigned to the active group (n = 10). A watershed-based cell detection algorithm is used to detect H2A.J expressed nuclei in the epidermis for each slide. All nuclei are measured for mean expression intensity. Threshold selection is performed on the per subject level, and their ranges are selected by combining all nuclei mean expression intensities for each subject followed by determination of the nuclei expression intensity representing the 20th, 40th, 60th, and 80th percentile of intensities, and categorization into percentile positive groups by the intensity ranges 0–20% (no to weak positive expression) to 80–100% (strong positive expression).
Results
A+ Targets Multiple Hallmarks of Aging for Anti-Aging Benefits
The efficacy of A+ in targeting several hallmarks of aging was assessed through immunohistochemical analysis in ex vivo human skin (cellular senescence) and gene expression analysis in in vitro human 3D skin models (loss of proteostasis, stem cell exhaustion, altered intercellular communication) treated with A+.
Cellular Senescence
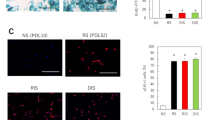
Both intrinsic and extrinsic aging result in epidermal and dermal cell senescence induced by DNA damage or other cellular stresses. Increased numbers of senescent cells can be observed in aging skin, which may contribute to age-related skin changes and pathologies [23]. Accumulation of histone variant H2A.J has been associated with skin cell senescence and promotes senescence-associated secretory phenotype (SASP) expression, which further impacts tissue health and function [24]. Immunohistochemical analysis of human skin explants treated with A+ for 5 days showed reduced expression of H2A.J compared with control, indicating that A+ may prevent cellular senescence of skin fibroblasts (Fig. 1a).
Efficacy of A+ ex vivo and in vitro: a Human skin explant (63-year-old female) treated with A+ once daily for 5 days shows reduced expression of cellular senescence biomarker H2A.J. Scale bar: 100 µm. b–g Quantitative PCR analysis of genes related to anti-aging and skin rejuvenation: human 3D skin models irradiated with UV light followed by application of A+ to the epidermal layer for 24 h show upregulation of several biomarkers for proteasome, autophagy, stemness, connexins (intercellular communication), ECM, and epidermal barrier. *Significant change versus UV irradiated, non-treated control (all p < 0.05)
Loss of Proteostasis
Cellular protein homeostasis, or proteostasis, is essential to proper cell function and survival [25]. Proteostasis not only requires stabilization and correct folding of proteins, but also efficient degradation and removal of damaged or misfolded proteins. The proteasome system and autophagy are both involved in maintaining proteostasis by degrading misfolded, damaged or aggregated proteins and cellular components, and their activities decline during aging, leading to cellular dysfunction and damage [26, 27]. For example, reduced autophagy activity in fibroblasts is linked to ECM deterioration [28]. Increased gene expression of biomarker panels for proteasome and autophagy in A+-treated human skin models suggests that A+ can induce proteasome and autophagy activity (Fig. 1b, c). Upregulated genes include proteasome maturation protein (POMP), which is essential for proteasome assembly, proteasome subunit beta type-5 and type-6 (PSMB5, PSMB6), which form part of the proteasome proteolytic chamber, and key autophagy-modulating genes (ATG5, ATG7, ATG12, BECN1, MAP1LC3A) .
Stem Cell Exhaustion
Stem cell exhaustion is a major factor in tissue aging, and studies have supported that stem cell rejuvenation may reverse aging [29]. Tissues lose their ability to effectively regenerate when stem cells lose their “stemness.” Studies have demonstrated that senescence and impaired proteostasis negatively affect stemness of adult human mesenchymal stem cells, but such loss of proliferation capacity and stemness could be reversed with proteasome activation [30]. Upregulation of ACTA2 and NES, genes that are only expressed within cells that have stem cell-like characteristics, suggests that A+ could support progenitor cell proliferation and function (Fig. 1d).
Altered Intercellular Communication
Diminished production of growth factors and cytokines results in subsequent decreases in intercellular communication and tissue regulation, contributing to deterioration and aging. A+ addresses altered intercellular communication through topical application of these signaling molecules as well as through upregulation of several connexins (Fig. 1e), which are key components of gap junctions that are important for direct cell–cell communications [31].
A+ Induces Expression of Genes That Support Skin Rejuvenation
The aging process eventually leads to loss of ECM and impaired skin barrier, resulting in visible signs of skin aging. Inducing the expression of ECM and epidermal barrier genes is a key factor within any skin rejuvenation strategy to improve the phenotype. Treatment of human skin models with A+ demonstrated increased expression of ECM genes, including collagens (COL1A1, COL3A1, COL4A1, COL7A1), elastic fiber proteins (ELN, FBN1), and transforming growth factor beta-1 (TGFB1), a growth factor that further supports ECM synthesis, as well as several genes involved in keratinocyte terminal differentiation (IVL, SPRR1A, TGM1, TGM3), ceramide synthesis (UGCG, SMPD1), and cell adhesion (OCLN) (Fig. 1f, g).
Clinical Efficacy and Tolerability of A+
A 24-week, double-blind, randomized, placebo-controlled, cross-over study was conducted from March through October 2018 to assess the clinical efficacy and tolerability of A+. A total of 68 female and male subjects aged 37–70 with Fitzpatrick Skin Types I–V were enrolled and 66 subjects completed the 24-week study (active: n = 45; placebo: n = 23). Subject demographics were Caucasian (79%), African American (13%), Asian (4%), Hispanic/Latino (9%), or mixed (3%). At baseline, subjects presented with mean overall photodamage scores of 5.64 (active) and 5.89 (placebo).
Investigator Assessments
Over the entire study period the active group (A+ treatment for 24 weeks) achieved statistically significant improvements compared with baseline as early as 2 weeks (global coarse lines/wrinkles, global fine lines/wrinkles, and tactile roughness), with significant improvements in all skin rejuvenation parameters including sagging after only 8 weeks, demonstrating the global effect of this intensive formulation with growth factors (Table 2; all p ≤ 0.031; Wilcoxon signed rank test). Furthermore, A+ provided significant improvements compared with vehicle placebo in global coarse lines/wrinkles at weeks 4, 8 and 12; in overall photodamage, global fine lines/wrinkles, and overall hyperpigmentation at weeks 8 and 12; and in sagging and skin tone evenness at week 12 (all p ≤ 0.035; Wilcoxon signed rank test). Representative graphs of global coarse lines/wrinkles and sagging are shown in Fig. 2. Figures 3, 4, and 5 show representative standardized digital photographs of subjects in the active group.
Investigator assessments: A+ provided significantly greater reductions compared with vehicle placebo in global coarse lines/wrinkles and sagging during phase I (baseline, n = 68; week 2, n = 68; week 4, n = 67; week 8, n = 67; week 12, n = 67). During phase II, placebo group subjects switched to using A+ twice daily, and significant improvements were observed for global coarse lines/wrinkles as well as sagging at weeks 18 and 24 compared with week 2, the phase II baseline visit (week 12, n = 67; week 18, n = 66; week 24, n = 66)
During phase II of the study (weeks 12–24), the original vehicle placebo group, having switched to A+ treatment, demonstrated significant improvements at weeks 18 and 24 in global coarse lines/wrinkles, global fine lines/wrinkles, overall photodamage, and sagging compared with the phase II baseline week 12 visit (Fig. 2; all p ≤ 0.008; Wilcoxon signed rank test), indicating the potency of the growth factors and other active ingredients in A+ to effectively address parameters such as coarse lines/wrinkles and sagging that are associated with deeper structural deficiencies in the skin during aging (e.g., loss of ECM).
Tolerability
Both active and vehicle placebo treatments were well-tolerated with mean tolerability scores remaining below mild throughout the study duration. Four subjects in the active group experienced mild skin-related adverse events during the 24-week study period: two subjects experienced contact dermatitis and acne, respectively, and discontinued from the study; one subject experienced folliculitis, edema, erythema (right cheek only), and dryness, and completed the study; one subject experienced inflammation right under the eye and completed the study.
Patient Reported Outcomes (PRO)
FaceQ is a validated PRO tool developed to provide clinically meaningful data and to effectively measure change in facial aesthetic patients, and it has been used in facial injectables studies [16, 17]. Subjects felt they appeared younger-looking in general, reporting median decreases in self-perceived age of 4 years at week 4, 6 years at week 12, and 5 years at week 24 when compared with baseline (Age Appraisal VAS, n = 35; Table 3). FaceQ Appearance Appraisal scores indicating subject satisfaction with A+ were significantly increased compared with baseline for Satisfaction with Facial Appearance (weeks 4, 12, and 24), Satisfaction with Skin (weeks 4, 12, and 24) and Aging Appraisal (weeks 12 and 24) (n = 35; all p ≤ 0.005; Student’s paired t-test).
Clinical Study Skin Biopsy Analysis
Cellular Senescence
Immunohistochemical analysis of facial skin biopsy samples obtained during the clinical study from subjects applying A+ twice daily for 12 weeks showed reduced expression of the skin cell senescence biomarker H2A.J compared with baseline. Bioinformatic analysis of staining intensity demonstrated a marked shift in intensity distribution with relatively fewer cells with high H2A.J levels at week 12 (Fig. 6).
Reduced expression of cellular senescence biomarker H2A.J in A+ treatment group: a Representative immunohistochemical staining (55-year-old female, Fitzpatrick Skin Type IV) b H2A.J staining intensity analysis in facial biopsy samples from subjects treated with A+ twice daily for 12 weeks (n = 10) shown as mean ± SD
ECM
Significant improvements in ECM biomarkers (Fig. 7) were observed by histological analysis of skin biopsy samples, demonstrating that A+ can help to reverse signs of skin aging. Gomori’s trichrome staining shows that twice-daily treatment with A+ for 12 weeks resulted in a strong increase of dermal collagen fibers (turquoise color). Specifically, increased expression of collagen type IV, a major component of basement membranes, can be observed at the dermal–epidermal junction (DEJ) and blood vessels. Decorin is a proteoglycan that regulates collagen fibril diameter and fibril orientation, and its expression was induced as well. Immunohistochemical analysis for elastin prior to A+ treatment shows amorphous expression at the reticular dermis suggesting elastosis, which is characterized by the accumulation of dystrophic elastotic material [32]; in contrast, the biopsy sample after 12 weeks of A+ treatment displays the natural fibrous structure of elastic fibers indicating removal of elastotic material and regeneration of functional elastic fibers in the dermis. Additionally, the thinner oxytalan fibers at the papillary dermis beneath the DEJ are observed after A+ treatment, whereas at baseline they are absent, which is another feature of chronically photodamaged skin [33, 34].
Histological analysis of biopsy samples from 60-year-old female (Fitzpatrick Skin Type III) in vehicle placebo group switching to A+ treatment during phase II (weeks 12–24) show improvements in ECM biomarkers. Gomori’s trichrome staining indicates collagen fibers (turquoise color). Scale bar: 100 µm
Epidermal Barrier
Skin biopsy analysis also demonstrated increased expression of epidermal biomarkers with A+ treatment (Fig. 8) including filaggrin, which plays an important role in skin barrier formation and moisture retention; transglutaminase 1, an enzyme that cross-links structural proteins during the cornification process; and claudin 1, which is a major component of tight junctions that provide cell–cell adhesion and serve as physical barrier sealing off intercellular spaces between epidermal cells. Furthermore, A+ treatment improved perilipin 3 expression; perilipins act as a protective coating from lipases and help to maintain lipid content in cells.
Discussion
Stimulating the synthesis of ECM components such as collagen and elastin is an established skin rejuvenation strategy in topical skin care. However, targeting the processes and pathways that cause and promote skin aging could provide further anti-aging benefits. Understanding the biological hallmarks of skin aging and the various processes involved enabled the development of a skin care serum that combines human fibroblast conditioned media created under hypoxic conditions (containing naturally secreted growth factors) with a unique blend of potent botanical, marine, and peptide ingredients. The result of leveraging this approach to comprehensively target multiple hallmarks of aging can be observed in the rapid and continued improvements achieved in the clinical study, which included investigator assessments, a validated PRO tool, and skin biopsy analysis. Furthermore, preclinical studies demonstrated the effects of A+ on four hallmarks of aging (cellular senescence, loss of proteostasis, stem cell exhaustion, and altered intercellular communication), suggesting potential inhibition or reversal of cellular aging processes, in addition to stimulating ECM regeneration and skin barrier function.
The 24-week clinical study demonstrated that A+ treatment provided significant improvements in skin rejuvenation parameters as early as week 2 (global coarse lines/wrinkles, global fine lines/wrinkles, and tactile roughness). These improvements appear faster compared with a similar topical skin care product combining human fibroblast conditioned media with a secondary blend of peptides and antioxidants that provided earliest significant results at week 4 (global coarse lines/wrinkles, global fine lines/wrinkles, overall photodamage, skin tone evenness, and tactile roughness) [9, 12]. The earlier onset of results with A+ could be attributed to hCCM and/or its secondary active ingredients, although it is not possible to pinpoint a specific active as the two cosmeceuticals vary greatly in formulation.
Investigator assessments showed that A+ provided significantly greater improvements compared with vehicle placebo control in multiple parameters, including global coarse lines/wrinkles (weeks 4, 8, and 12) as well as sagging (week 2). Moreover, the cross-over of vehicle placebo to active treatment at week 12 replicated the effects observed in the first 12 weeks of the active treatment group, as global coarse lines/wrinkles and sagging were similarly significantly improved at weeks 18 and 24 in the former vehicle placebo group. These results indicate the efficacy of the active ingredients in A+, as fundamental structural skin deficiencies need to be addressed to improve parameters such as global coarse lines/wrinkles and sagging. The improvement in sagging is particularly remarkable as most topical skin care is unable to address loose and lax skin. Histology showed that damaged, non-functional elastotic material was replaced with healthy, functional elastic fibers with A+ treatment, thereby restoring elasticity and firmness that was lost over time. While increased elastin expression is important, stimulation of other elastic fiber components, such as fibrillin-1, is also required for skin elasticity. The regeneration of the dermal elastic fibers and oxytalan fibers, as well as improvements in the DEJ demonstrated in both gene expression and biopsy analyses, could reduce skin sagging, since the elastic fibers and DEJ both play vital roles in maintaining skin elasticity and firmness [35, 36]. The comprehensive support of A+ to all skin layers (dermal ECM/elastic fibers, DEJ, and epidermis) may synergistically result in improvements in sagging skin. The effects of A+ related to mitigating the hallmarks of aging, such as reduced cellular senescence and support of progenitor cell proliferation and function, could provide overall skin health and rejuvenation benefits that further contribute to less sagging and lax skin.
The significant improvements compared with vehicle placebo control in multiple key skin aging parameters are especially noticeable considering the rigor of the study design. Unlike topical drug products, where efficacy must be established through rigorous preclinical and placebo-controlled clinical testing, topical cosmetic products are not required to undergo clinical efficacy testing nor require placebo-controlled study designs. Many clinical studies on cosmeceuticals are often performed in combination with a basic skin care regimen. However, basic skin care products used in a controlled manner, such as in a clinical study setting (e.g., daily cleanser, moisturizer, and sunscreen), or the vehicle of the active treatment product itself can provide measurable skin benefits and thus contribute to the observed efficacy attributed to the test product. These improvements may be observed in more superficial features, such as skin texture and fine lines, as these features are more easily impacted by moisturizer or a cosmetically elegant vehicle formulation [37]. Measurable improvements in the appearance of hyperpigmentation can be achieved with the addition of daily sunscreen [38, 39]. As a result, demonstrating significantly greater efficacy compared with control (basic skin care regimen and/or vehicle placebo) can be challenging for cosmetic clinical studies.
The skin aging process and associated visible signs of aging vary among race and ethnicity on the basis of structural and functional skin differences [40, 41]. Ideally, the efficacy of A+ in specific populations should be assessed; however, one of the clinical study’s limitations is the lack of subgroup analysis by race/ethnicity. While the clinical study included subjects from all backgrounds, most subjects were Caucasian, and the numbers of subjects identifying as either African American, Asian, Hispanic/Latino, or mixed (multiracial) were too low for statistical subgroup analysis. The study subject demographics do resemble the overall US population, which is 76% White/Caucasian, 13% Black/African American, 6% Asian, 19% Hispanic/Latino, and 3% mixed/multiracial [42] . Future studies with a focus on enrolling subjects with specific racial/ethnic backgrounds could be performed to assess product efficacy and tolerability in these populations. Another limitation is that the experiments regarding the hallmarks of aging are exploratory in nature. Further in-depth studies into each specific hallmark of aging are required to gain additional mechanistic insights. Still, the present study demonstrates for the first time the potential beneficial effects of a topical growth factor-based skin care product that targets four key hallmarks of aging (cellular senescence, loss of proteostasis, stem cell exhaustion, and altered intercellular communication) indicating A+ could inhibit and reverse cellular processes related to skin aging.
Designing topical skin care products that address the underlying mechanisms of skin aging to help delay the aging process, in addition to correcting the visible symptoms, has been the primary driver for this research endeavor. This approach toward anti-aging skin care not only results in skin rejuvenation, but would also fit the concept of prejuvenation: the prevention or delay of visible signs of aging, which is gaining widespread popularity among younger populations [43]. Long-term prospective studies would have to be performed to clinically demonstrate preventive effects. Since not all of the characterized hallmarks of aging are addressed by A+, other innovative skin care products could be developed in the future to complement A+ and together form a comprehensive topical skin care regimen that ultimately targets all hallmarks of aging to achieve optimal anti-aging outcomes.
Conclusions
Altogether, the preclinical and clinical data demonstrate the efficacy of a comprehensive topical growth factor-based skin care serum that targets the biological mechanisms underlying the aging process itself, and stimulates skin regeneration, resulting in rapid and significant clinical improvements in multiple skin quality parameters including coarse lines/wrinkles and sagging.
References
Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008.
Sundaram H, Mehta RC, Norine JA, et al. Topically applied physiologically balanced growth factors: a new paradigm of skin rejuvenation. J Drugs Dermatol. 2009;8s:4–13.
Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13(5):183–9.
Pinney E, Zimber M, Schenone A, Montes-Camacho M, Ziegler F, Naughton GK. Human embryonic-like ECM (hECM) stimulates proliferation and differentiation in stem cells while killing cancer cells. Int J Stem Cells. 2011;4(1):70–5.
Suresh DH, Suryanarayan S, Sarvajnamurthy S, Puvvadi S. Treatment of a non-healing diabetic foot ulcer with platelet-rich plasma. J Cutan Aesthet Surg. 2014;7(4):229–31.
Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab. 1999;1(5):265–79.
Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic ulcers. Artif Organs. 1997;21(11):1203–10.
Naughton GK, Tolbert WR, Grillot TM. Emerging developments in tissue engineering and cell technology. Tissue Eng. 1995;1(2):211–9.
Kadoya K, Makino ET, Jiang LI, et al. Upregulation of extracellular matrix genes corroborates clinical efficacy of human fibroblast-derived growth factors in skin rejuvenation. J Drugs Dermatol. 2017;16(12):1190–6.
Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003;5:25–34.
Mehta RC, Smith SR, Grove GL, et al. Reduction in facial photodamage by a topical growth factor product. J Drugs Dermatol. 2008;7:864–71.
Atkin DH, Ho E, Trookman NS, et al. Combination of physiologically balanced growth factors with antioxidants for reversal of facial photodamage. J Cosmet Laser Ther. 2010;12:14–20.
Draelos ZD, Karnik J, Naughton G. The antiaging effects of low oxygen tension generated multipotent growth factor containing serum. J Drugs Dermatol. 2017;16:30–4.
Kellar RS, Hubka M, Rheins LA, Fisher G, Naughton GK. Hypoxic conditioned culture medium from fibroblasts grown under embryonic-like conditions supports healing following post-laser resurfacing. J Cosmet Dermatol. 2009;8(3):190–6.
Zimber MP, Mansbridge JN, Taylor M, et al. Human cell-conditioned media produced under embryonic-like conditions results in improved healing time following laser resurfacing. Aesthet Plast Surg. 2012;36(2):431–7.
Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg. 1994;220(1):10–8.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Tigges J, Krutmann J, Fritsche E, et al. The hallmarks of fibroblast ageing. Mech Ageing Dev. 2014;138:26–44.
Bhadra M, Howell P, Dutta S, Heintz C, Mair WB. Alternative splicing in aging and longevity. Hum Genet. 2020;139(3):357–69.
Bramwell LR, Harries LW. Targeting alternative splicing for reversal of cellular senescence in the context of aesthetic aging. Plast Reconstr Surg. 2021;147(1S-2):25S-32S.
Vega VL, Mehta RC, Bachelor MA, Oldach J, Bolmarcich J, Armento AJ. Differential modulation of ECM and GFR by retinoic acid and a physiologically balanced GF formulation. J Invest Dermatol. 2013;133: s155.
Pachapakesan V, Klassen AF, Cano SJ, Scott AM, Pusic AL. Development and psychometric evaluation of the FACE-Q aging appraisal scale and patient-perceived age Visual Analog Scale. Aesthet Surg J. 2013;33(8):1099–109.
Toutfaire M, Bauwens E, Debacq-Chainiaux F. The impact of cellular senescence in skin ageing: a notion of mosaic and therapeutic strategies. Biochem Pharmacol. 2017;142:1–12.
Contrepois K, Coudereau C, Benayoun BA, et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat Commun. 2017;8:14995.
Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev. 2011;10(2):205–15.
Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2018;217(1):51–63.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41.
Tashiro K, Shishido M, Fujimoto K, et al. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem Biophys Res Comm. 2014;443:167–72.
Rando TA, Chang HY. Aging, rejuvenation and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148(1–2):46–57.
Kapetanou M, Chondrogianni N, Petrakis S, Koliakos G, Gonos ES. Proteasome activation enhances stemness and lifespan of human mesenchymal stem cells. Free Radic Biol Med. 2017;103:226–35.
Meşe G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2516–24.
Montagna W, Kirchner S, Carlisle K. Histology of sun-damaged human skin. J Am Acad Dermatol. 1989;21(5 Pt 1):907–18.
Kadoya K, Sasaki T, Kostka G, et al. Fibulin-5 deposition in human skin: decrease with ageing and ultraviolet B exposure and increase in solar elastosis. Br J Dermatol. 2005;153(3):607–12.
Lee JY, Kim YK, Seo JY, et al. Loss of elastic fibers causes skin wrinkles in sun-damaged human skin. J Dermatol Sci. 2008;50(2):99–107.
Adamo CS, Zuk AV, Sengle G. The fibrillin microfibril/elastic fibre network: a critical extracellular supramolecular scaffold to balance skin homoeostasis. Exp Dermatol. 2021;30(1):25–37.
Langton AK, Graham HK, McConnell JC, Sherratt MJ, Griffiths CEM, Watson REB. Organization of the dermal matrix impacts the biomechanical properties of skin. Br J Dermatol. 2017;177(3):818–27.
Draelos ZD. The science behind skin care: moisturizers. J Cosmet Dermatol. 2018;17(2):138–44.
Makino ET, Yano S, Cheng T, Mehta RC. Efficacy of a comprehensive serum in Japanese subjects with moderate to severe hyperpigmentation. J Drugs Dermatol. 2017;16(1):36–40.
Sarkar R, Ghunawat S, Narang I, Verma S, Garg VK, Dua R. Role of broad-spectrum sunscreen alone in the improvement of melasma area severity index (MASI) and Melasma Quality of Life Index in melasma. J Cosmet Dermatol. 2019;18(4):1066–73.
Alexis AF, Obioha JO. Ethnicity and aging skin. J Drugs Dermatol. 2017;16(6):s77–80.
Venkatesh S, Maymone MBC, Vashi NA. Aging in skin of color. Clin Dermatol. 2019;37(4):351–7.
United States Census Bureau. Population estimates July 1, 2019 (V2019). https://www.census.gov/quickfacts/fact/table/US/PST045219.
Hogan SR, Zachery CB, Arndt KA. Prejuvenation: definition of the term and evolution of the concept. Dermatol Surg. 2021;47(6):871–2.
Acknowledgements
Funding
This study, and the journal’s Rapid Service fees, were funded by Allergan Aesthetics, an AbbVie company.
Medical Writing, Editorial, and Other Assistance
The authors gratefully acknowledge Hieu Nguyen for technical support as well as John Pan (Pan Informatics LLC) for bioinformatic analysis. Technical support and bioinformatic analysis were funded by Allergan Aesthetics, an AbbVie company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Gail K. Naughton: Conceptualization, resources, writing (review and editing); Lily I. Jiang: Investigation, formal analysis, writing (review and editing); Elizabeth T. Makino: Conceptualization, resources, writing (review and editing); Robin Chung: Investigation, writing (review and editing); Audrey Nguyen: Investigation, writing (review and editing); Tsing Cheng: Conceptualization, visualization, writing (original draft preparation); Kuniko Kadoya: Conceptualization, methodology, resources, writing (review and editing); Rahul C. Mehta: Conceptualization, supervision, writing (review and editing).
Disclosures
Dr. Naughton is an employee and founder of Histogen, Inc. Dr. Jiang is an employee of SGS Stephens, Inc. Ms. Makino, Ms. Chung, Ms. Nguyen, Dr. Cheng, Dr. Kadoya, and Dr. Mehta are employees of AbbVie and may own stock in the company.
Compliance with Ethics Guidelines
Institutional Review Board approval (IntegReview, Austin, TX) was obtained prior to conduct of any study procedures. The study was conducted following the ethical and regulatory principles from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. All subjects provided informed consent as well as permission for use of their images in scientific publications prior to study participation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Naughton, G.K., Jiang, L.I., Makino, E.T. et al. Targeting Multiple Hallmarks of Skin Aging: Preclinical and Clinical Efficacy of a Novel Growth Factor-Based Skin Care Serum. Dermatol Ther (Heidelb) 13, 169–186 (2023). https://doi.org/10.1007/s13555-022-00839-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00839-2