Abstract
Introduction
To date, there have been no head-to-head clinical studies comparing calcipotriol 0.005% plus betamethasone dipropionate 0.064% (Cal/BD) aerosol foam and halobetasol propionate 0.01% plus tazarotene 0.045% (HP/Taz) lotion for the treatment of plaque psoriasis. However, the efficacy of 4 weeks of Cal/BD foam and 8 weeks of HP/Taz lotion has been compared using a matching-adjusted indirect comparison (MAIC) approach. Here, we compare the efficacy and safety of Cal/BD foam and HP/Taz lotion for up to 52 weeks.
Methods
An unanchored MAIC was conducted using individual patient data from the PSO-LONG Cal/BD foam trial and a 52-week, open-label phase 3 study of HP/Taz lotion (NCT02462083). Key outcomes of interest were Physician’s Global Assessment (PGA) success (PGA 0/1 with ≥ 2-point improvement) after 4 or 8 weeks of open-label therapy; the proportion of patients who had body surface area affected (BSA) ≤ 3 after open-label therapy who maintained BSA ≤ 3 to week 52; and adverse events (AEs).
Results
After matching, patients were statistically significantly more likely to have PGA success after 4 weeks of Cal/BD foam than after 8 weeks of HP/Taz lotion (84.5% versus 54.4%; p < 0.01). At week 52, 92.5% and 92.4% of patients receiving proactive and reactive Cal/BD foam, respectively, maintained BSA ≤ 3, compared with 49.3% of those treated with HP/Taz lotion (both p < 0.01). Treatment-related AEs, AEs leading to withdrawal, and AEs associated with drug application (dermatitis, application site pain, and pruritus) were significantly rarer with Cal/BD foam than with HP/Taz lotion (all p < 0.01).
Conclusions
Cal/BD aerosol foam demonstrated significantly greater efficacy than HP/Taz lotion, and had a more favorable safety profile, compared with HP/Taz lotion, for up to 52 weeks. Proactive Cal/BD foam maintenance therapy and reactive use of Cal/BD foam following relapse both had significant advantages over HP/Taz lotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Comparisons among therapies are important for determining which treatments are the most efficacious in improving patients’ symptoms. |
Calcipotriol 0.005% plus betamethasone dipropionate 0.064% (Cal/BD) aerosol foam and halobetasol propionate 0.01% plus tazarotene 0.045% (HP/Taz) lotion are both used to treat plaque psoriasis, but have not been compared in head-to-head studies. |
Here, we use a matching-adjusted indirect comparison approach to compare the efficacy and safety of Cal/BD foam and HP/Taz lotion for up to 52 weeks. |
What was learned from the study? |
Patients treated with Cal/BD foam were more likely to have a response to their therapy, and more likely to maintain their initial improvements, than those receiving HP/Taz lotion. |
Cal/BD aerosol foam was shown to have a more favorable safety profile, compared with HP/Taz lotion, for up to 52 weeks. |
Proactive Cal/BD foam maintenance therapy and reactive use of Cal/BD foam following relapse both had significant advantages over HP/Taz lotion. |
Introduction
Plaque psoriasis, the most common type of psoriasis, is a chronic, inflammatory skin disorder with symptoms including scaling, itching, redness, tightness of the skin, bleeding, and burning [1,2,3]. These symptoms can affect patients’ sleep and physical functioning [3, 4]. In addition, plaque psoriasis can have a substantial impact on patients’ daily lives and on their health-related quality of life [3, 5,6,7].
Multiple topical agents are available for the treatment of plaque psoriasis, including combination therapies comprising a corticosteroid and a vitamin D derivative or a retinoid [8]. Calcipotriol 0.005% plus betamethasone dipropionate 0.064% (Cal/BD), used in the treatment of mild-to-severe plaque psoriasis, is available in several formulations, including an aerosol foam that has been shown in randomized controlled trials (RCTs) to have significantly greater efficacy than gel and ointment formulations [9, 10]. A further combination therapy—halobetasol propionate 0.01% plus tazarotene 0.045% (HP/Taz) lotion—has been investigated for the treatment of moderate-to-severe plaque psoriasis in multiple RCTs [11,12,13]. However, no head-to-head studies of Cal/BD foam and HP/Taz lotion have been conducted.
Comparisons among therapies are important for determining which treatments are the most efficacious in improving patients’ symptoms. In the absence of head-to-head data, indirect comparison methods adjusting for cross-trial differences can be used to compare therapies [14]. Matching-adjusted indirect comparison (MAIC) is one approach that can be used to compare therapies when no common comparator is available [14, 15]. MAIC analyses can be conducted either when two therapies have been studied in RCTs with the same comparator arm (“anchored”) or when no common comparator is available (“unanchored”) [14]. MAIC analyses are based on individual patient data (IPD) from clinical trials of one intervention and publicly available aggregate data from trials of the second [14, 15]. IPD from the first intervention are weighted to match the mean characteristics—including both potential treatment effect modifiers and, in the case of unanchored analyses, potential prognostic variables—of the population in which the second was studied [14, 15]. The matching process gives participants who are similar to the mean of the second trial population a higher weight than those whose characteristics are different from the mean participant in the second trial [14, 15].
A previous MAIC analysis compared the short-term efficacy of Cal/BD foam and HP/Taz lotion. Use of Cal/BD foam for 4 weeks was found to be associated with a statistically significantly greater likelihood of treatment success, compared with 8 weeks of treatment with HP/Taz lotion [16].
Here, we use MAIC to compare the efficacy and safety of Cal/BD foam and HP/Taz lotion for up to 52 weeks. The objectives of this study are to compare the efficacy of Cal/BD foam and HP/Taz lotion maintenance treatment, as determined by achievement of Physician’s Global Assessment (PGA) success and by maintenance of reductions in the affected body surface area (BSA), and to compare safety outcomes among patients treated with Cal/BD foam and HP/Taz lotion for up to 52 weeks.
Methods
Matching-Adjusted Indirect Comparison Methods and Source Data
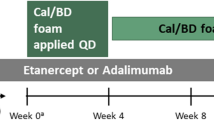
An unanchored MAIC analysis was conducted as described by Signorovitch et al. [15, 17]. The clinical trials included in the analysis are summarized in Fig. 1.
Design of included RCTs. In PSO-LONG, treatment success was defined as PGA 0/1 with a ≥ 2-point improvement. In the HP/Taz trial, treatment success was defined as PGA 0/1 and improvement was defined as a ≥ 1-point improvement in PGA. BD betamethasone dipropionate, Cal calcipotriol, HP halobetasol proprionate, PGA Physician’s Global Assessment, Taz tazarotene
IPD were taken from patients treated with Cal/BD foam in the PSO-LONG trial (NCT02899962) [18]. In PSO-LONG, patients were treated with 4 weeks of open-label, once-daily Cal/BD foam, after which patients with PGA success (defined as PGA 0/1 [clear/almost clear] with a ≥ 2-point improvement) were randomized to 52 weeks of proactive or reactive treatment with Cal/BD foam. Patients in the proactive management group were treated with Cal/BD foam twice weekly, while the reactive management group used foam vehicle. Upon relapse (PGA score ≥ 2), patients in both treatment groups received rescue treatment with once-daily Cal/BD foam for 4 weeks. If PGA 0/1 was regained after 4 weeks, patients continued with maintenance treatment; otherwise, patients were withdrawn from the trial.
The Cal/BD foam arms were compared with a 52-week, open-label phase 3 study of HP/Taz lotion (NCT02462083) [11]. In the HP/Taz trial, patients were initially treated with 8 weeks of once-daily HP/Taz lotion. Patients with PGA 0/1 at week 8 stopped treatment for 4 weeks, while those with PGA ≥ 2 continued with daily HP/Taz lotion for a further 4 weeks. Patients with an improvement (≥ 1-point improvement in PGA) continued to receive HP/Taz lotion in 4-week cycles, depending on whether they had PGA 0/1 after each cycle. Data from the HP/Taz lotion trial were rounded to the nearest integer number of patients.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Study Outcomes
The primary efficacy comparison for daily treatment was between Cal/BD foam results at 4 weeks and HP/Taz lotion at 8 weeks, reflecting the length of the initial treatment period in the relevant trials; an additional analysis compared results for both treatments at 4 weeks.
The efficacy outcome for daily treatment was the proportion of patients with PGA success (defined as PGA 0/1 with a ≥ 2-point improvement from baseline) after 4 or 8 weeks of open-label therapy. For maintenance treatment, the efficacy outcomes were the proportions of those patients who had BSA ≤ 3 or ≤ 5 after 4 or 8 weeks of open-label therapy who maintained BSA ≤ 3 or ≤ 5 to weeks 26 and 52.
Because the PSO-LONG trial required patients to have PGA success at week 4 in order to enter the maintenance phase—in contrast to the HP/Taz trial in which only a ≥ 1-point improvement in PGA at week 12 was needed—PGA success was not assessed as a maintenance phase outcome.
Safety outcomes were the proportion of patients with all treatment-emergent adverse events (AEs), AEs by severity, treatment-related AEs, serious AEs, AEs leading to withdrawal from the study, and AEs of special interest (dermatitis, application site pain, and pruritus). Safety was compared among matched patients over the entire study period.
Results are reported as percentages and odds ratios (efficacy outcomes) or risk differences (safety outcomes).
Matching Trial Populations
IPD from the Cal/BD foam trial were selected by applying the inclusion criteria from the HP/Taz trial. For analysis of outcomes in the open-label phase, IPD from the overall PSO-LONG trial were adjusted by weighting to match the baseline characteristics of patients treated with open-label HP/Taz lotion. For the maintenance phase analysis, IPD from the proactive and reactive maintenance therapy arms were matched to patients treated with HP/Taz maintenance therapy. Matching was conducted separately for the efficacy and safety populations.
The baseline characteristics matched were age, sex, ethnicity, race, BSA, and PGA; the prognostic relevance of these characteristics was confirmed by clinical input. Both ethnicity and race were included to match the reported characteristics in the HP/Taz trial; while race is described on the basis of physical traits, ethnicity is typically defined according to cultural identity, with only partial overlap between racial and ethnic categories.
Results
Matching Populations
A total of 555 patients treated with Cal/BD foam in the PSO-LONG open-label phase were compared with 550 patients treated with HP/Taz lotion. Of these, 470 PSO-LONG patients were included in the maintenance phase efficacy analysis, and 478 in the maintenance phase safety analysis. After matching, the effective sample sizes were 40.9–45.7% of the original Cal/BD foam populations. The baseline characteristics of the matched Cal/BD foam arms were well balanced with the HP/Taz arms (Tables 1 and 2; Supplementary Material).
Open-Label Phase
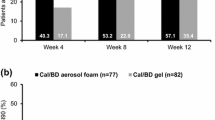
After weighting, 84.5% of patients using Cal/BD foam had PGA success at week 4, compared with 37.5% and 54.4% of patients treated with HP/Taz lotion after 4 weeks and 8 weeks, respectively (both p < 0.01; Fig. 2).
Maintenance Phase
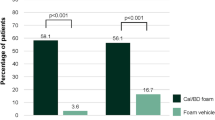
At week 52, 92.5% and 92.4% of patients receiving proactive and reactive Cal/BD foam, respectively, maintained BSA ≤ 3, compared with 49.3% of those treated with HP/Taz lotion (both p < 0.01; Fig. 2). At week 26, the corresponding proportions were 90.7% and 91.5% in the respective Cal/BD foam arms, and 39.7% in the HP/Taz arm (both p < 0.01; Fig. 2).
BSA ≤ 5 was maintained by 97.2% and 97.1% of patients receiving proactive and reactive Cal/BD foam, respectively, at week 52, compared with 77.5% of those treated with HP/Taz lotion (both p < 0.01; Fig. 2). The week 26 results were similar.
Safety
Compared with HP/Taz lotion, the rate of all treatment-emergent AEs over 1 year was lower in the proactive Cal/BD foam arm and similar in the reactive Cal/BD foam arm (Fig. 3). AEs possibly or probably related to treatment and AEs leading to withdrawal were more likely in the HP/Taz lotion arms and rare in the Cal/BD foam arm (all p < 0.01 versus HP/Taz lotion; Fig. 3). There were no statistically significant differences between Cal/BD foam and HP/Taz lotion in the proportion of patients with serious AEs; numerically, these were less common in the reactive Cal/BD foam arm than in the proactive treatment group (Fig. 3).
Patients treated with HP/Taz lotion were more likely to have severe AEs than those receiving Cal/BD foam, and patients treated with Cal/BD foam were more likely to have mild or moderate AEs than those treated with HP/Taz lotion (Fig. 3).
Dermatitis, application site pain, and pruritus were all rare among patients treated with Cal/BD foam, with statistically significant differences between both the Cal/BD foam groups and the HP/Taz population (all p < 0.01; Fig. 3).
Discussion
This study used a MAIC approach to compare the efficacy and safety of Cal/BD aerosol foam and HP/Taz lotion for up to 52 weeks. Patients treated with Cal/BD foam were more likely to have a response to their therapy, and more likely to maintain their initial improvements, than those receiving HP/Taz lotion.
This is the first analysis to compare the efficacy of Cal/BD aerosol foam and HP/Taz lotion for the long-term maintenance of treatment responses. This study provides evidence that can be used to optimize the long-term management of plaque psoriasis for individual patients, expanding on the previous MAIC of Cal/BD foam versus HP/Taz lotion [8] by investigating outcomes up to 52 weeks and by assessing comparative safety across the whole study period.
In the PSO-LONG RCT, proactive maintenance therapy with Cal/BD foam was associated with a statistically significantly higher proportion of days in remission (PGA 0/1), compared with reactive therapy [18]. The present analysis shows that both Cal/BD foam maintenance regimens are statistically significantly more likely to lead to patients’ maintaining treatment responses, assessed as BSA ≤ 3 or ≤ 5, compared with HP/Taz lotion. The efficacy of Cal/BD foam maintenance therapy was similar whether patients were treated proactively or reactively.
Compared with HP/Taz lotion, Cal/BD foam was associated with lower rates of treatment-related AEs, AEs leading to withdrawal, and AEs of special interest. In particular, AEs related to treatment application (dermatitis, application site pain, and pruritus) were rare with Cal/BD foam, with statistically significantly higher rates of these AEs seen with HP/Taz lotion.
There were several differences between the designs of PSO-LONG and the HP/Taz trial. First, the initial treatment period was 4 weeks in PSO-LONG, versus 8 weeks in the HP/Taz trial. However, significant differences in the proportion of patients with PGA success were found in analyses comparing 4-week data for Cal/BD foam with both HP/Taz timepoints. In addition, the results of this analysis are consistent with the previous MAIC comparing the short-term efficacy of Cal/BD foam with HP/Taz lotion, which was based on different RCTs [16]. Second, the HP/Taz trial included only one maintenance regimen, with intermittent treatment as needed depending on whether patients had PGA 0/1 at 4-weekly study visits. The MAIC results demonstrate that reactive therapy with Cal/BD foam is more efficacious than intermittent HP/Taz maintenance treatment. In addition, proactive therapy with Cal/BD foam is associated with a greater likelihood of retaining treatment responses, compared with HP/Taz lotion. Third, in the unanchored analyses, the reduction in effective sample size when data for the Cal/BD foam treatment arms were adjusted to match the HP/Taz trial was more than 50%, suggesting that the original populations of the two trials were substantially different. Use of the MAIC approach allowed for the selection of a subpopulation of patients from the PSO-LONG trial that matched the characteristics of the HP/Taz trial, thereby allowing a relatively unbiased comparison between the two studies. Despite the reduction in effective sample size, the proportion of matched patients with PGA success at week 4 was similar to that in the original PSO-LONG trial population (84.5% versus 80.2%) [16], suggesting that results for the cohort of patients included in the MAIC are not atypical of the overall population.
The MAIC methodology has been used to compare therapies for psoriasis since 2010 [17], and is now a widely used tool for the study of comparative efficacy across multiple therapy areas [14]. One strength of the MAIC approach is that, as in the present study, it can be used to compare therapies for which no common comparator is available by aligning the mean characteristics of the populations [14, 15]. In addition to the previous analysis comparing 4 weeks of Cal/BD foam therapy with 8 weeks of HP/Taz lotion [16], MAIC approaches have recently been used to compare Cal/BD foam to nonbiological systemic treatments for plaque psoriasis [19], as well as to compare the biological therapies brodalumab and guselkumab [20].
The results of this analysis have some limitations. First, as with all indirect comparisons, there may be some bias due to unobserved differences across the trials, for which it was not possible to adjust. Second, the relatively small effective sample size after matching reduces the statistical power of the analysis. Third, the open-label nature of the trials may introduce some bias with regard to efficacy outcomes, as compared with RCT results; however, this effect applies to both of the trials in the MAIC and is unlikely to substantially influence the overall findings of the analyses. Fourth, the open-label phase comparison between Cal/BD foam and HP/Taz lotion could potentially be affected by investigator bias because—unlike the HP/Taz trial—PGA success at week 4 in PSO-LONG was required for ongoing continuation in the study. This limitation is unlikely to affect the overall study findings: the proportion of patients with PGA success was higher at week 4 in PSO-LONG than at week 12 in the HP/Taz trial (84.5% versus 69.8%; the results of the HP/Taz trial week 12 visit also determined ongoing participation in the study) [11, 18].
Conclusions
In this MAIC analysis, Cal/BD aerosol foam demonstrated greater efficacy than HP/Taz lotion. Patients had a significantly higher rate of PGA success after 4 weeks of treatment with Cal/BD aerosol foam, compared with 4 or 8 weeks of HP/Taz lotion, and were significantly more likely to maintain BSA improvements for up to 52 weeks of treatment. In addition, Cal/BD aerosol foam was shown to have a more favorable safety profile, compared with HP/Taz lotion, for up to 52 weeks. Reactive use of Cal/BD foam following relapse and proactive Cal/BD foam maintenance therapy, which has been shown to have greater benefits to patients than reactive maintenance therapy, were found to have similar advantages over HP/Taz lotion.
References
Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64:ii18–23.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50.
Ljosaa TM, Rustoen T, Mörk C, et al. Skin pain and discomfort in psoriasis: an exploratory study of symptom prevalence and characteristics. Acta Derm Venereol. 2010;90:39–45.
Gowda S, Goldblum OM, McCall WV, Feldman SR. Factors affecting sleep quality in patients with psoriasis. J Am Acad Dermatol. 2010;63:114–23.
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(871–81):e1-30.
Lynde CW, Poulin Y, Guenther L, Jackson C. The burden of psoriasis in Canada: insights from the pSoriasis Knowledge IN Canada (SKIN) survey. J Cutan Med Surg. 2009;13:235–52.
Anstey A, McAteer H, Kamath N, Percival F. Extending psychosocial assessment of patients with psoriasis in the UK, using a self-rated, web-based survey. Clin Exp Dermatol. 2012;37:735–40.
Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–70.
Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized phase II study. J Dermatol Treat. 2016;27:120–7.
Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31:119–26.
Lebwohl MG, Stein Gold L, Papp K, et al. Long-term safety and efficacy of a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis: phase 3 open-label study. J Eur Acad Dermatol Venereol. 2021;35:1152–60.
Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855–61.
Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287–93.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38:200–11.
Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–7.
Wu JJ, Hansen JB, Patel DS, Nyholm N, Veverka KA, Swensen AR. Effectiveness comparison and incremental cost-per-responder analysis of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam vs halobetasol 0.01%/tazarotene 0.045% lotion for plaque psoriasis: a matching-adjusted indirect comparative analysis. J Med Econ. 2020;23:641–9.
Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935–45.
Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269–77.
Bewley AP, Shear NH, Calzavara-Pinton PG, Hansen JB, Nyeland ME, Signorovitch J. Calcipotriol plus betamethasone dipropionate aerosol foam vs apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: a matching-adjusted indirect comparison. J Eur Acad Dermatol Venereol. 2019;33:1107–15.
Hampton P, Borg E, Hansen JB, Augustin M. Efficacy of brodalumab and guselkumab in patients with moderate-to-severe plaque psoriasis who are inadequate responders to ustekinumab: a matching adjusted indirect comparison. Psoriasis (Auckl). 2021;11:123–31.
Acknowledgements
Funding
This study and the journal’s rapid service were funded by LEO Pharma.
Medical Writing and Editorial Assistance
Medical writing and editorial support in the preparation of this article was provided by Symmetron Ltd (London, UK) and by Dr. Paul Overton of Beacon Medical Communications Ltd (Brighton, UK) in accordance with Good Publication Practice (GPP3) guidelines, and was funded by LEO Pharma (Ballerup, Denmark).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. The MAIC analysis was conducted by Henrik Thoning. All authors interpreted the data and critically revised each version of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Prior Presentation
This analysis was presented as a poster at the European Academy of Dermatology and Venereology Spring Symposium in May 2022.
Disclosures
David Adam has been an investigator, speaker, or advisory board member for LEO Pharma Inc, AbbVie, Amgen, Actelion, Arcutis, Bausch Health, Boehringer Ingelheim, BMS, Celgene, Coherus, Dermira, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Merck, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma and UCB. Jashin Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, DermTech, Dr. Reddy's Laboratories, Eli Lilly, EPI Health, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health. Marie Jablonski Bernasconi and Henrik Thoning are employees of LEO Pharma.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Adam, D.N., Jablonski Bernasconi, M.Y., Thoning, H. et al. Matching-Adjusted Indirect Comparison of Long-Term Efficacy and Safety Outcomes for Calcipotriol Plus Betamethasone Dipropionate Foam Versus Halobetasol Proprionate Plus Tazarotene Lotion in the Treatment of Plaque Psoriasis. Dermatol Ther (Heidelb) 12, 2589–2600 (2022). https://doi.org/10.1007/s13555-022-00824-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00824-9