Abstract
Metabolic syndrome (MetS) is well recognized as a frequent comorbidity of psoriasis with important implications for efficacy and safety of psoriasis treatment. The presence of concomitant MetS is associated with decreased efficacy response to biologic treatment for psoriasis in observational studies. In post hoc analyses of clinical trial data, the anti–IL-23p19 antibody tildrakizumab appears to maintain efficacy in patients compared to those without MetS; no published subgroup analyses by MetS status are yet available for other biologics. However, there is some evidence that obese patients have decreased psoriasis treatment efficacy with biologics with certain mechanisms of action relative to overweight patients. This confounds interpretation of the effect of MetS due to the association between MetS and body weight. Because of the association between MetS and cardiovascular risk, treatment of psoriasis in patients with concomitant MetS requires special consideration for cardiovascular safety and attention to potential for exacerbation of MetS and related conditions, including nonalcoholic fatty liver disease. Additional studies are needed to clarify the risks for treatment failure and cardiovascular safety concerns in patients with psoriasis and concomitant MetS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metabolic syndrome is a common comorbidity of psoriasis with implications for both the safety and efficacy of psoriasis treatments |
Metabolic syndrome is associated with decreased effectiveness of some psoriasis treatments, but it is unclear whether this effect is independent of body weight |
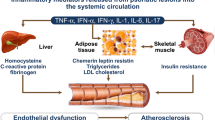
The exacerbation of metabolic syndrome may affect patients’ body weight, cholesterol, hyperglycemia/insulin resistance, and liver function |
Treatment selection in patients with psoriasis and concomitant metabolic syndrome requires special consideration of both potential treatment failure and cardiovascular concerns |
Biologic therapeutics appear to be safe for patients with both psoriasis and metabolic syndrome and may be preferable to methotrexate and other conventional systemic therapies |
Introduction
Metabolic syndrome (MetS) is a frequent comorbidity of psoriasis, affecting 34% of patients in a 2012 population-based study in the UK [1]. MetS is a combination of risk factors, including hypertension, dyslipidemia, elevated blood glucose, and central obesity—possibly linked by insulin resistance [2]—that confers significant risk for developing cardiovascular disease (CVD) or diabetes [3]. The number of MetS risk factors increases the risk of disease progression [2]. The National Cholesterol Education Program Adult Treatment Panel III defines MetS as present when patients meet ≥ 3 of the following 5 criteria: abdominal obesity (waist circumference > 102 cm in men and > 88 cm in women), triglycerides ≥ 150 mg/dl (≥ 1.8 mmol/l), high-density lipoprotein (HDL) cholesterol < 40 mg/dl in men and < 50 mg/dl in women, blood pressure ≥ 130/85 mmHg, and fasting glucose ≥ 110 mg/dl [4]. MetS is associated with twofold increased risk for CVD, a fivefold increased risk for diabetes, and increased risk of stroke and fatty liver disease relative to individuals without MetS [2, 5, 6].
Psoriasis is associated with cardiovascular comorbidities of MetS in a “dose response” manner; prevalence of cardiovascular conditions is increased in patients with severe psoriasis relative to those with less-severe disease [1, 3, 7, 8]. Additionally, patients diagnosed with psoriasis have increased risk of developing insulin resistance or diabetes mellitus [9]. There is evidence for a shared genetic basis for psoriasis and MetS, with psoriasis-associated genes also implicated in the development of MetS, type II diabetes, and cardiovascular disease (recently reviewed in detail [10]). The American Heart Association (AHA) and the American College of Cardiology (ACC) now classify psoriasis (among other inflammatory diseases) as a risk factor for CVD [11] because of accumulating evidence that adults diagnosed with chronic inflammatory disorders have increased rates of multiple cardiovascular conditions [11, 12]. Current psoriasis treatment guidelines recommend screening patients for MetS to enable early intervention and appropriate treatment escalation but offer little guidance on psoriasis treatment selection in these patients [3, 12]. This review summarizes the clinical evidence for efficacy and cardiovascular safety of biologic psoriasis therapies for patients with concomitant MetS. It is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Efficacy of Available Biologic Agents for Treatment of Psoriasis in Patients with Metabolic Syndrome
Clinical Efficacy of Biologic Treatment of Psoriasis in Patients With vs Without Metabolic Syndrome
MetS and its components, especially obesity, may decrease efficacy of biologic treatment for psoriasis. In the retrospective noninterventional Outcome of Psoriatic Patients Switched to Adalimumab (OPPSA) study, absence of MetS was associated with greater probability of achieving Psoriasis Area and Severity Index (PASI) responses after 3, 6, and 12 months of psoriasis treatment with adalimumab relative to patients with MetS in a multivariate analysis; neither body weight nor body mass index (BMI) was included as a dependent variable [13]. In the prospective, multicenter, non-interventional CorEvitas Psoriasis Registry, including 2924 patients diagnosed with psoriasis who initiated biologic therapy, more frequent treatment failure was observed in obese relative to nonobese patients and patients with vs without diabetes mellitus [14]. Additionally, in a real-world study to assess the impact of secukinumab treatment on MetS parameters in patients diagnosed with psoriasis, PASI ≥ 90 responders more frequently had lower BMI when compared to PASI ≤ 90 responders [15]. Tildrakizumab demonstrated comparable efficacy based on PASI response rates and median PASI scores, with no apparent difference in discontinuation rates, in patients with or without MetS through up to 5 years of treatment in post hoc analyses of clinical trial data [16,17,18]. However, the subgroup of patients with MetS was not large enough to examine the effect of body weight. In a post hoc analysis of pooled data from phase 3 trials of secukinumab for treatment of moderate-to-severe plaque psoriasis, prevalence of MetS was higher among patients with lower responses after 16 weeks of treatment with secukinumab relative to those with better responses (63.2% in patients who did not achieve PASI 50 vs 26.6% in those who achieved PASI 100); similar but less consistent trends were apparent among patients in the ustekinumab and etanercept active-controlled arms [16, 19]. Effects of MetS on the efficacy of biologics for psoriasis treatment are summarized in Table 1.
Treatment persistence or drug survival is often used as a proxy for effectiveness for psoriasis treatment. Among a group of patients treated with adalimumab, efalizumab, etanercept, infliximab, and/or ustekinumab, components of MetS including arterial hypertension, diabetes mellitus, dyslipidemia, and/or obesity were present in 55.2% of patients and were associated with significantly shorter drug survival time relative to patients without these comorbidities (P = 0.033) [20]. Similarly, in an analysis of real-world patterns of biologic and apremilast treatment for psoriasis, patients with metabolic conditions (diabetes, hyperlipidemia, hypertension, metabolic syndrome, or obesity) had significantly higher rates of discontinuation and switching on adalimumab and ustekinumab and numerically higher rates on secukinumab and etanercept relative to patients without these conditions [21]. However, in a retrospective study of 907 patients with psoriasis treated in Israel between 2002 and 2015, metabolic syndrome was a significant positive predictor for biologic drug survival [22]. The effect of metabolic syndrome on persistence of biologics in treatment of psoriasis was explored in a 2019 meta-analysis, but the authors concluded there were insufficient data for pooled analysis [23].
Body Weight as a Confounder in Randomized Controlled Trials and Observational Studies
Although the above results suggest MetS may decrease efficacy of some biologics for psoriasis treatment, the question cannot be properly addressed without controlling for the effects of obesity. Obesity is a component of the cluster of risk factors in MetS; increased body weight is also a potential confounder for the efficacious treatment of psoriasis; studies on the effects of body weight and obesity on the efficacy of biologics for psoriasis treatment are summarized in Table 2. The IL-12/IL-23p40 inhibitor ustekinumab is dose-adjusted by weight because of decreased efficacy for treatment of psoriasis in patients weighing > 100 kg vs patients weighing ≤ 100 kg within the clinical trial population [24, 25], and increased ustekinumab concentrations had a direct effect on the clinical efficacy in overweight patients [26]. A retrospective subgroup analysis of the etanercept clinical development program showed a tendency toward greater efficacy in patients with body weight less than the population mean (89 kg) relative to obese (BMI ≥ 30) patients; however, the effect of body weight was not consistently apparent in observational studies [27, 28]. Additionally, higher body weight and BMI are associated with decreased rates of ≥ 75% improvement from baseline PASI score (PASI 75 response) in patients with psoriasis treated with adalimumab [29]. A 2018 meta-analysis found that obese patients had 60% higher odds for treatment failure with tumor necrosis factor alpha (TNF-α) inhibitors for immune-mediated diseases such as psoriasis [30]. Furthermore, a recent retrospective analysis found that both short- and long-term efficacy is negatively affected by higher BMI, particularly for anti-interleukin (IL) therapeutics such as ixekizumab, secukinumab, and ustekinumab [31].
Data for the effect of body weight on efficacy of anti–IL-17 and anti–IL-23p19 agents for the treatment of psoriasis are limited to subgroup analyses of clinical trial populations. Patients with psoriasis who failed to respond to treatment with secukinumab were more frequently obese (BMI ≥ 30) relative to responders (mean weight, 103.9 kg for nonresponders [< PASI 50] vs 81.2 kg for PASI 100 responders); a clinical trial evaluating higher doses of secukinumab in obese patients has been completed with results forthcoming [19, 32]. Response rates following treatment were modestly lower in guselkumab-treated patients weighing ≥ 100 kg vs < 100 kg at week 24 and tildrakizumab-treated patients weighing > 90 kg vs ≤ 90 kg at week 12 [33, 34]. In a follow-up analysis of the tildrakizumab results, the difference in PASI score improvement from baseline between patients in the lowest weight decile vs patients in the highest weight decile narrowed by week 28, and efficacy in all weight deciles was maintained at week 52, suggesting the effect of body weight on efficacy may be transient [35]. Subgroup analysis of the risankizumab clinical trials showed comparable efficacy across weight categories of ≤ 100 kg and > 100 kg, weight quartiles, and BMI categories of < 25, 25– < 30, and ≥ 30 kg/m2 [36]. As the studies on the effect of MetS on psoriasis treatment efficacy were not controlled for the effects of weight and obesity, comparison between patients with MetS and “healthy obese” patients is needed to determine whether MetS is truly an independent risk factor for treatment failure.
Safety Concerns for Psoriasis Treatment with Respect to Metabolic Syndrome
Because MetS is associated with increased risk for CVD, nonalcoholic fatty liver disease (NAFLD), and other adverse outcomes, psoriasis treatment safety is of particular concern in patients with concomitant MetS [2, 3, 6]. Despite the prevalence of MetS in patients with psoriasis, relatively few studies have prospectively evaluated the effect of MetS on biologic treatment safety. In the study to explore the effect of secukinumab compared to placebo on fat tissue and skin (ObePsO-S)(NCT03055494), secukinumab treatment reduced systemic inflammation markers relative to patients receiving the placebo, which is suggestive of safety in obese patients and those with MetS [37]. In the tildrakizumab subgroup analyses, adverse events—including those of special concern in patients with MetS, such as major adverse cardiovascular events (MACE), infections, malignancies, and new-onset or worsening diabetes mellitus—were similar among patients with and without MetS through up to 3 years of treatment [16, 17], but clinical trial data stratified by MetS status are lacking for other agents.
In the absence of data specifically in patients with MetS, safety of biologics in patients with psoriasis and MetS may be inferred from the effects of treatment on safety issues related to MetS including cardiovascular safety, potential for exacerbation of MetS, and complications such as NAFLD, infections in patients with diabetes, and polypharmacy due to treatment for multiple comorbidities. Available data in these areas suggest that biologics may have safety advantages over conventional systemic treatment for psoriasis in patients with MetS, as discussed in detail below.
Cardiovascular Events
Because CVD is the chief risk of MetS, cardiovascular safety of psoriasis treatment is particularly important in patients with concomitant MetS. Systemic treatment with biologic agents is largely neutral with respect to cardiovascular safety, with the possible exception of the anti–IL-12/23p40 antibodies [38]. A 2011 meta-analysis of 22 placebo-controlled clinical trials revealed no significant difference in the frequency of MACE with TNF-α inhibitors and anti–IL-12/23p40 antibodies compared to placebo; MACE occurred in 10/3179 patients treated with ustekinumab or briakinumab vs 0/1474 placebo-treated patients (Mantel-Haenszel risk difference, 0.012 events/person-year; 95% CI − 0.001 to 0.026; P = 0.12) and in 1/3858 patients treated with TNF-α inhibitors vs 1/1812 placebo-treated patients (Mantel-Haenszel risk difference, − 0.0005 events/person-year; 95% CI − 0.010 to 0.009; P = 0.94) [39]. A 2013 meta-analysis revealed a statistically significant increase in MACE risk in patients with psoriasis treated with ustekinumab or briakinumab vs placebo (OR = 4.23; 95% CI 1.07–16.75; P = 0.04), but not when ustekinumab-treated patients were analyzed separately (OR = 3.96; 95% CI 0.51–30.41; P = 0.19) [40]. Briakinumab was ultimately withdrawn from development for psoriasis treatment [41]. Integrated safety data from the ustekinumab development program suggested no effect of ustekinumab treatment on serious cardiovascular events, and MACE risk was similar in patients treated with ustekinumab relative to those receiving TNF-α inhibitors in a 2019 cohort study (adjusted hazard ratio [HR], 1.10; 95% CI 0.80–1.52) [42, 43]. Further, in the Vascular Inflammation in Psoriasis—Ustekinumab (VIP-U) trial, patients treated with ustekinumab had reduced aortic vascular inflammation at week 12 compared to baseline (− 6.58%; 95% CI − 13.64 to 0.47%) compared with placebo-treated patients (12.07%; 95% CI 3.26–20.88%) [44].
Other biologics used in psoriasis treatment have no specific cardiovascular safety concerns. A 2017 meta-analysis of randomized controlled trials of TNF-α inhibitors (adalimumab, etanercept, and infliximab), anti–IL-17A agents (secukinumab and ixekizumab), and ustekinumab detected no association of MACE with treatment using any of these biologic agents during the clinical trial periods [45]. There was no overall differential risk in serious cardiovascular events or MACE in a large observational cohort study including 60,028 patients with psoriasis or psoriatic arthritis treated with ustekinumab or TNF-α inhibitors [42]. Clinical trial data for several anti–IL-17 and anti–IL-23p19 antibodies suggested no cardiovascular safety signals [46,47,48,49,50]. In a post hoc analysis of pooled phase 3 and 4 data in patients with psoriasis, secukinumab treatment reduced the inflammatory biomarkers' high-sensitivity C-reactive protein and neutrophil–lymphocyte ratio with no effect on traditional cardiovascular risk parameters compared with placebo [51]. Postmarketing data for the IL-17A antibody secukinumab also contained no cardiovascular safety signals; the exposure-adjusted incidence rate of MACE per 100 patient-years over the entire treatment period remained low for each indication (psoriasis, psoriatic arthritis, and ankylosing spondylitis) [52]. In analyses of the US Truven Health Analytics MarketScan Database, patients with psoriasis treated with TNF-α inhibitors had significantly lower risk for MACE compared with patients treated with methotrexate (adjusted HR, 0.55; 95% CI 0.45–0.67; P < 0.0001) or phototherapy (adjusted HR, 0.77; 95% CI 0.60–0.99; P = 0.046) [53, 54].
Conventional psoriasis therapeutics like methotrexate and cyclosporine, although older and more established treatment options, may pose increased cardiovascular risks compared with biologics [38, 53, 55]. Patients using methotrexate had higher frequency of cardiovascular events in the US Truven Health Analytics MarketScan Database (Kaplan-Meier rates, 1.5% for the TNF inhibitors cohort vs 4.1% for the methotrexate cohort, P < 0.001) [53]. In a recent randomized, placebo-controlled, double-blind clinical trial, low-dose methotrexate treatment did not reduce inflammatory cytokine levels or decrease the risk for cardiovascular events in patients with atherosclerosis but also did not exacerbate or increase the number of cardiovascular events compared with placebo [56]. Similarly, in a nationwide Danish cohort study in patients with severe psoriasis, the incidence of cardiovascular events per 1000 patient-years was 6.28, 6.08, 18.95, and 14.63 among patients receiving methotrexate, cyclosporine, retinoid, and other therapies (including topical medications, phototherapy, and climate therapy), respectively, compared with 4.16 in patients treated with biologics [38].
Exacerbation of MetS
Body Weight
Another area of concern is the potential for psoriasis treatment to worsen components of MetS, which would be expected to indirectly increase risk for related conditions. Association between treatment with TNF-α inhibitors and weight gain was apparent in several observational studies [28, 57,58,59,60], and a 2020 meta-analysis confirmed that psoriasis patients treated with TNF-α inhibitors had a significant increase in body weight compared with patients receiving conventional systemic therapies (mean difference, 1.4 kg; 95% CI 0.88–1.93 kg) [61]. The same meta-analysis detected no significant increase in weight or BMI in psoriasis patients treated with anti–IL-12/23p40 or anti–IL-17 antibodies [61]. Conversely, in the PSOLAR registry, treatment with TNF-α inhibitors, ustekinumab, or methotrexate was associated with weight loss after 6 and 12 months of use; however, the study was not designed to test weight, and > 50% of patients were excluded from the analysis because weight was not measured within prespecified windows [62]. Another single, small study documented that a subset of patients treated with ustekinumab had an increase in mean weight [57]. Clinical trial data analyses for both secukinumab and tildrakizumab presented no clinically significant change from baseline body weight [17, 63]. Overall, no evidence yet suggests that change in body weight or body composition during biologic treatment significantly affects cardiometabolic risk in patients with psoriasis.
Cholesterol
The Janus-associated kinase (JAK) inhibitors tofacitinib, upadacitinib, baricitinib, and filgotinib are associated with increases in both HDL and low-density lipoprotein cholesterol levels. Because all of the fractions of cholesterol are affected, in general the “atherogenic index” does not change, and the alterations are therefore not generally considered clinically significant [64,65,66,67,68]. In 2021, the US Food and Drug Administration required an update of the package insert for tofacitinib and other JAK inhibitors to carry a warning of risk for serious heart-related events, blood clots, cancer, and death based on data from a clinical safety trial in high-risk patients treated with tofacitinib compared with TNF blockers [69]. The conventional systemic psoriasis therapies methotrexate, cyclosporine, and acitretin are also associated with an increased risk of hypertriglyceridemia or increased lipid levels; however, the implications of these changes are not known [55, 70, 71]. In contrast, biologics used in psoriasis treatment have minimal effects on serum lipid profile. In one retrospective study, treatment with adalimumab, etanercept, and ustekinumab was associated with a 9% increase in serum triglyceride levels in 139 patients diagnosed with psoriasis [72]. Safety analyses of the secukinumab and ixekizumab clinical trials revealed no clinically relevant effects on lipid profiles [73, 74], and analysis of serum lipid profiles in patients with or without MetS treated with tildrakizumab showed very limited changes from baseline [75].
Hyperglycemia/Insulin Resistance
Insulin resistance may be the underlying source of MetS [2]. The majority of studies show no changes in glucose metabolism or insulin sensitivity during biologic treatment of psoriasis. A 2015 meta-analysis of seven studies of patients with rheumatoid arthritis and other smaller studies in patients with psoriasis or MetS suggested that biologic treatment with TNF-α inhibitors may decrease fasting glucose and/or improve insulin resistance [76,77,78]. However, there were no significant changes in fasting glucose levels or insulin sensitivity following 12 weeks of treatment with adalimumab and infliximab [79]. In the VIP studies, there was no change in baseline markers for glucose metabolism following treatment with adalimumab, ustekinumab, or secukinumab [44, 80, 81]. Clinical trial data assessing cardiometabolic laboratory parameters following secukinumab and ixekizumab treatment showed no significant changes from baseline fasting glucose levels or insulin sensitivity [63, 73, 74]. Tildrakizumab use was not associated with consistent changes from baseline glucose levels, and favorable trends were observed in some patients [75]. Comparable data are not yet available for risankizumab and guselkumab. In contrast, patients with psoriasis treated with cyclosporine for 16 weeks had an increased risk of developing diabetes compared with all other treatment regimens (acitretin, efalizumab, and etanercept) in a large observational study [82]. Any effects of biologic treatment of psoriasis on glucose levels or insulin sensitivity seem unlikely to affect cardiovascular risk.
Liver Function
NAFLD is often found in patients with MetS and is significantly more prevalent in patients with vs without psoriasis, occurring in 65.6% of patients with psoriasis vs 35.0% of controls matched for age, sex, and BMI (P < 0.01) in one study and in 21.2% of patients with psoriasis vs 7.8% of controls (P < 0.04) in another [55, 83, 84]. Additionally, in a two-stage, cross-sectional study, patients diagnosed with psoriasis had higher prevalence of NAFLD and patients diagnosed with both NAFLD and psoriasis had increased prevalence of subclinical atherosclerosis compared with controls [85]. Biologic therapies for psoriasis appear to be relatively safe with respect to liver disease. In 89 patients with moderate to severe plaque psoriasis, MetS, and NAFLD, treatment with etanercept (but not psoralen/ultraviolet light A therapy) was associated with reduction from baseline in aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio—a marker for hepatic damage—at week 24 [86]. In an observational study of 44 patients with psoriasis treated with ustekinumab in Spain, 6 patients developed Grade 1 elevated hepatic transaminases during 52 weeks of treatment, of whom 5 had liver toxicity associated with other psoriasis treatments; no signs or symptoms of liver disease or severe adverse liver effects were detected [87]. Pooled analysis of phase 3 secukinumab data found no increase in AST and ALT levels during 52 weeks of treatment, although increases were noted in the patients in the etanercept active-control arm after week 16 [63]. Lastly, tildrakizumab treatment was not associated with liver-related adverse events or elevations in transaminase levels relative to treatment with placebo through week 12 or etanercept through week 28 or with liver-related safety signals through 1 year of exposure [88].
In contrast, many conventional systemic agents can be harmful to liver function in patients with psoriasis [55]. Patients with NAFLD or chronic liver disease are at increased risk of hepatotoxicity and hepatic fibrosis with methotrexate use [89]. In a 2007 study of patients with psoriasis treated with methotrexate, patients who were overweight or had type 2 diabetes mellitus were at a higher risk of developing severe liver fibrosis during methotrexate treatment (even at lower doses) compared with patients without such risk factors [90]. Incidence of liver disease was higher in patients with psoriasis compared with the general population and even higher among patients receiving systemic therapy—most frequently, methotrexate (adjusted HR for patients treated with systemic therapy = 1.97; 95% CI 1.63–2.38)—compared with those not treated with systemic agents (adjusted HR = 1.37; 95% CI 1.29–1.45) [83]. Biologics may thus be preferable to conventional systemic agents in patients with MetS due to the elevated risk for liver damage in these patients.
Other Safety Concerns for Patients with MetS
A number of biologic treatments for psoriasis are associated with increased risk for infections. This may be of particular concern in patients with concomitant MetS, as diabetes mellitus is a significant risk factor for serious infections among hospitalized patients with psoriasis (OR, 1.14; 95% CI 1.13–1.15) [91]. The TNF-α inhibitors all carry “black box” warnings in the US for risk of serious infections; the labeling for ustekinumab and the anti–IL-17 antibodies note that “serious infections have occurred” [24, 25, 74, 92,93,94,95,96]. Prescribing information for the anti–IL-23p19 antibodies states only that these “may increase the risk of infection,” which may make them preferable in patients with diabetes mellitus [97,98,99].
Finally, patients with multiple comorbidities associated with MetS typically need to use multiple medications [100]. Conventional treatments for psoriasis need to be used carefully when polypharmacy is a concern because of potential for drug interactions and organ impairment; however, polypharmacy is less of a concern for biologic use [101].
Conclusions
Many factors, including medical history and comorbidities, must be considered when selecting the optimal therapy for patients with psoriasis [102, 103]. Although MetS is a frequent comorbidity that may reduce treatment efficacy, few specific data are available on psoriasis treatment in patients with concomitant MetS. There is a need for larger studies controlled for obesity and body weight to determine the effect of MetS on treatment efficacy. The majority of biologic treatments appear relatively safe for patients with psoriasis and MetS, with no harmful effects on cardiovascular health or other safety concerns.
References
Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–62.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5.
Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654–62.
National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2(2): e000062.
Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–8.
Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41.
Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2013;133(10):2340–6.
Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69(12):2114–7.
Wu JJ, Kavanaugh A, Lebwohl MG, Gniadecki R, Merola JF. Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2022;36(6):797–806.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–209.
Dregan A, Chowienczyk P, Molokhia M. Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders. Heart. 2017;103(23):1867–73.
Talamonti M, Galluzzo M, Bernardini N, Caldarola G, Persechino S, Cantoresi F, et al. Psoriasis Area and Severity Index response in moderate-severe psoriatic patients switched to adalimumab: results from the OPPSA study. J Eur Acad Dermatol Venereol. 2018;32(10):1737–44.
Enos CW, Ramos V, McLean RR, Lin T, Foster N, Dube B, Van Voorhees AS. Comorbid obesity and history of diabetes are independently associated with poorer treatment response to biologics at 6-months: a prospective analysis in CorEvitas’ Psoriasis Registry. J Am Acad Dermatol. 2021;86:68–76 (In press).
Wang HN, Huang YH. Changes in metabolic parameters in psoriatic patients treated with secukinumab. Ther Adv Chronic Dis. 2020;11:2040622320944777.
Lebwohl MG, Leonardi CL, Mehta NN, Gottlieb AB, Mendelsohn AM, Parno J, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84(2):398–407.
Lebwohl MG, Leonardi CL, Mehta NN, Gottlieb AB, Mendelsohn AM, Parno J, et al. Tildrakizumab efficacy and safety are not altered by metabolic syndrome status in patients with psoriasis: post hoc analysis of 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2020;82(2):519–22.
Fernandez AP, Dauden E, Lebwohl MG, Menter MA, Leonardi C, Gooderham M, Gebauer K, Tada Y, Lacour JP, Bianchi L, Egeberg A, Pau-Charles I, Mendelsohn AM, Rozzo S, Mehta NN. Tildrakizumab efficacy and safety in patients with psoriasis and concomittant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. JEADV. 2022. https://doi.org/10.1111/jdv.18167 (accepted).
Pinter A, Gerdes S, Papavassilis C, Reinhardt M. Characterization of responder groups to secukinumab treatment in moderate to severe plaque psoriasis. J Dermatolog Treat. 2019;31:769–75.
Jacobi A, Rustenbach SJ, Augustin M. Comorbidity as a predictor for drug survival of biologic therapy in patients with psoriasis. Int J Dermatol. 2016;55(3):296–302.
Feldman SR, Zhang J, Martinez DJ, Lopez-Gonzalez L, Marchlewicz EH, Shrady G, et al. Real-world treatment patterns and healthcare costs of biologics and apremilast among patients with moderate-to-severe plaque psoriasis by metabolic condition status. J Dermatolog Treat. 2021;32(2):203–11.
Shalom G, Cohen AD, Ziv M, Eran CB, Feldhamer I, Freud T, et al. Biologic drug survival in Israeli psoriasis patients. J Am Acad Dermatol. 2017;76(4):662-9.e1.
Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–8.
STELARA® (ustekinumab) injection, for subcutaneous or intravenous use. Full prescribing information. Janssen Biotech, Inc., Horsham, 2020.
STELARA (ustekinumab). Summary of product characteristics. Janssen Biologics B.V., Leiden, 2020.
Lebwohl M, Yeilding N, Szapary P, Wang Y, Li S, Zhu Y, et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63(4):571–9.
Giunta A, Babino G, Ruzzetti M, Manetta S, Chimenti S, Esposito M. Influence of body mass index and weight on etanercept efficacy in patients with psoriasis: a retrospective study. J Int Med Res. 2016;44(1 suppl):72–5.
Esposito M, Mazzotta A, Saraceno R, Schipani C, Chimenti S. Influence and variation of the body mass index in patients treated with etanercept for plaque-type psoriasis. Int J Immunopathol Pharmacol. 2009;22(1):219–25.
Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol. 2010;63(3):448–56.
Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS ONE. 2018;13(5): e0195123.
Pirro F, Caldarola G, Chiricozzi A, Burlando M, Mariani M, Parodi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41(10):917–25.
Efficacy and safety of 2 secukinumab regimens in 90 kg or more wt group with moderate/severe chronic plaque psoriasis (AIN457A2324). https://www.clinicaltrials.gov/ct2/show/NCT03504852. Accessed 4 Mar 2020.
Gordon KB, Blauvelt A, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2018;178(1):132–9.
Poulin Y, Ramon M, Rosoph L, Weisman J, Mendelsohn AM, Parno J, et al. Efficacy of tildrakizumab by patient demographic and disease characteristics across a phase 2b and 2 phase 3 trials in patients with moderate-to-severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2020;34:1500–9.
Leonardi C, Menter A, Draelos Z, Heim J, Parno J, Mendelsohn A, et al. Impact of body weight on efficacy of tildrakizumab in moderate to severe plaque psoriasis. J Am Acad Dermatol. 2019;81(4 Suppl 1):AB76.
Strober B, Menter A, Leonardi C, Gordon K, Lambert J, Puig L, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34:2830–8.
Krueger J, Pariser DL, P, Bagel J, Armstrong A, Muscianisi E, Kianifard F, Sarkar R, Steadman J, Blauvelt A. Secukinumab treatment normalizes inflammatory markers and immune response and leads to clinical improvement in patients with psoriasis: findings from the primary analysis of the OBEPSO-S study. 24th World Congress of Dermatology. 2019.
Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld LE, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128–34.
Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306(8):864–71.
Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27(5):622–7.
Grogan K. Abbott withdraws briakinumab applications in USA, Europe PharmaTimes online [April 19, 2021]. Available from: http://www.pharmatimes.com/news/abbott_withdraws_briakinumab_applications_in_usa,_europe_979765.
Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol. 2019;155(6):700–7.
Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, et al. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol. 2011;164(4):862–72.
Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial). J Invest Dermatol. 2020;140(1):85-93.e2.
Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017;176(4):890–901.
Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–86.
Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82(2):352–9.
Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–61.
Blauvelt A, Reich K, Papp KA, Kimball AB, Gooderham M, Tyring SK, et al. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol. 2018;179(3):615–22.
Merola JF, McInnes IB, Deodhar AA, Dey AK, Adamstein NH, Quebe-Fehling E, et al. Effect of secukinumab on traditional cardiovascular risk factors and inflammatory biomarkers: post hoc analyses of pooled data across three indications. Rheumatol Ther. 2022;9:935–55.
Deodhar A, Mease PJ, McInnes IB, Baraliakos X, Reich K, Blauvelt A, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111.
Wu JJ, Guerin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76(1):81–90.
Wu JJ, Joshi AA, Reddy SP, Batech M, Egeberg A, Ahlehoff O, et al. Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol. 2018;32(8):1320–6.
Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–113.
Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–62.
Owczarczyk-Saczonek A, Placek W, Rybak-d’Obyrn J, Wygonowska E. Influence of ustekinumab on body weight of patients with psoriasis: an initial report. Postepy Dermatol Alergol. 2014;31(1):29–31.
Gisondi P, Cotena C, Tessari G, Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol. 2008;22(3):341–4.
Saraceno R, Schipani C, Mazzotta A, Esposito M, Di Renzo L, De Lorenzo A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57(4):290–5.
Tan E, Baker C, Foley P. Weight gain and tumour necrosis factor-alpha inhibitors in patients with psoriasis. Australas J Dermatol. 2013;54(4):259–63.
Wu MY, Yu CL, Yang SJ, Chi CC. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta-analysis. J Am Acad Dermatol. 2020;82(1):101–9.
Shear NH, Alhusayen R, Fernandez-Obregon A, Kimball AB, Menter A, Wu JJ, et al. Observations from our evaluation of bodyweight changes after initiation of a biologic therapy in the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2017;31(12):e544–7.
Gerdes S, Pinter A, Papavassilis C, Reinhardt M. Effects of secukinumab on metabolic and liver parameters in plaque psoriasis patients. J Eur Acad Dermatol Venereol. 2020;34(3):533–41.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507.
Kivitz AJ, Cohen S, Keystone E, van Vollenhoven RF, Haraoui B, Kaine J, et al. A pooled analysis of the safety of tofacitinib as monotherapy or in combination with background conventional synthetic disease-modifying antirheumatic drugs in a Phase 3 rheumatoid arthritis population. Semin Arthritis Rheum. 2018;48(3):406–15.
Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12):2867–77.
Kremer JM, Genovese MC, Keystone E, Taylor PC, Zuckerman SH, Ruotolo G, et al. Effects of baricitinib on lipid, apolipoprotein, and lipoprotein particle profiles in a phase IIb study of patients with active rheumatoid arthritis. Arthritis Rheumatol. 2017;69(5):943–52.
Vanhoutte F, Mazur M, Voloshyn O, Stanislavchuk M, Van der Aa A, Namour F, et al. Efficacy, safety, pharmacokinetics, and pharmacodynamics of filgotinib, a selective JAK-1 inhibitor, after short-term treatment of rheumatoid arthritis: results of two randomized phase IIa trials. Arthritis Rheumatol. 2017;69(10):1949–59.
FDA. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions 2021 [Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.
Owczarczyk-Saczonek A, Drozdowski M, Maciejewska-Radomska A, Choszcz D, Placek W. The effect of subcutaneous methotrexate on markers of metabolic syndrome in psoriatic patients - preliminary report. Postepy Dermatol Alergol. 2018;35(1):53–9.
Chan BC, Reid N, Armour K, Scott RS, George PM, Maurice PD. Hypertriglyceridaemia with acitretin use: a proposal for its management in the context of overall cardiovascular risk. Br J Dermatol. 2014;171(3):665–7.
Hoffmann JH, Knoop C, Enk AH, Hadaschik EN. Routine laboratory parameter dynamics and laboratory adverse events in psoriasis patients on long-term treatment with adalimumab, etanercept, and ustekinumab. Acta Derm Venereol. 2017;97(6):705–10.
Egeberg A, Wu JJ, Korman N, Solomon JA, Goldblum O, Zhao F, et al. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J Am Acad Dermatol. 2018;79(1):104-9.e8.
von Stebut E, Reich K, Thaci D, Koenig W, Pinter A, Korber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054–62.
Menter MA, Mehta NN, Lebwohl MG, Gottlieb AB, Mendelsohn AM, Rozzo SJ, et al. The effect of tildrakizumab on cardiometabolic risk factors in psoriasis by metabolic syndrome status: post hoc analysis of two phase 3 trials (reSURFACE 1 and reSURFACE 2). J Drugs Dermatol. 2020;19(8):703–8.
Burska AN, Sakthiswary R, Sattar N. Effects of tumour necrosis factor antagonists on insulin sensitivity/resistance in rheumatoid arthritis: a systematic review and meta-analysis. PLoS ONE. 2015;10(6): e0128889.
Al-Mutairi N, Shabaan D. Effects of tumor necrosis factor alpha inhibitors extend beyond psoriasis: insulin sensitivity in psoriasis patients with type 2 diabetes mellitus. Cutis. 2016;97(3):235–41.
Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146–50.
Kofoed K, Clemmensen A, Mikkelsen UR, Simonsen L, Andersen O, Gniadecki R. Effects of anti-tumor necrosis factor therapy on body composition and insulin sensitivity in patients with psoriasis. Arch Dermatol. 2012;148(9):1089–91.
Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6): e007394.
Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S). J Invest Dermatol. 2020;140:1784-93.e2.
Gisondi P, Cazzaniga S, Chimenti S, Giannetti A, Maccarone M, Picardo M, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27(1):e30-41.
Ogdie A, Grewal SK, Noe MH, Shin DB, Takeshita J, Chiesa Fuxench ZC, et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J Invest Dermatol. 2018;138(4):760–7.
Awosika O, Eleryan MG, Rengifo-Pardo M, Doherty L, Martin LW, Ehrlich A. A case-control study to evaluate the prevalence of nonalcoholic fatty liver disease among patients with moderate-to-severe psoriasis. J Clin Aesthet Dermatol. 2018;11(6):33–7.
Gonzalez-Cantero A, Teklu M, Sorokin AV, Prussick R, González-Cantero J, Martin-Rodriguez JL, et al. Subclinical liver disease is associated with subclinical atherosclerosis in psoriasis: results from two observational studies. J Invest Dermatol. 2021;142:88–96.
Campanati A, Ganzetti G, Di Sario A, Damiani A, Sandroni L, Rosa L, et al. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48(7):839–46.
Llamas-Velasco M, Concha-Garzon MJ, Garcia-Diez A, Dauden E. Liver injury in psoriasis patients receiving ustekinumab: a retrospective study of 44 patients treated in the clinical practice setting. Actas Dermosifiliogr. 2015;106(6):470–6.
West DT, Mendelsohn AM, Rozzo SJ, Girolomoni G. No increased risk of liver dysfunction from tildrakizumab treatment: post hoc analysis of the tildrakizumab psoriasis clinical program. J Am Acad Dermatol. 2019;81(4 Suppl 1):AB439.
Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate hepatotoxicity and the impact of nonalcoholic fatty liver disease. Am J Med Sci. 2017;354(2):172–81.
Rosenberg P, Urwitz H, Johannesson A, Ros AM, Lindholm J, Kinnman N, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46(6):1111–8.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States. J Am Acad Dermatol. 2016;75(2):287–96.
Egeberg A, Ottosen MB, Gniadecki R, Broesby-Olsen S, Dam TN, Bryld LE, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–19.
CIMZIA® (certolizumab pegol) for injection, for subcutaneous use. Full prescribing information. UCB, Inc., Smyrna, GA, USA. 2019.
SILIQ™ (brodalumab) injection, for subcutaneous use. Full prescribing information. Valeant Pharmaceuticals North America, Bridgewater, NJ, USA. 2018.
HUMIRA® (adalimumab) injection, for subcutaneous use. Full prescribing information. AbbVie Inc., North Chicago, IL, USA. 2020.
COSENTYX® (secukinumab) injection, for subcutaneous use. Full prescribing information. Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. 2020.
ILUMYA™ (tildrakizumab-asmn) injection, for subcutaneous use. Full prescribing information. Sun Pharmaceutical Industries, Inc., Cranbury, NJ, USA. 2018.
SKYRIZI® (risankizumab-rzaa) injection, for subcutaneous use. Full prescribing information. AbbVie Inc., North Chicago, IL, USA. 2020.
TREMFYA® (guselkumab) injection, for subcutaneous use. Full prescribing information. Janssen Biotech, Inc., Horsham, PA, USA. 2019.
Patel AY, Shah P, Flaherty JH. Number of medications is associated with outcomes in the elderly patient with metabolic syndrome. J Geriatr Cardiol. 2012;9(3):213–9.
Di Caprio R, Caiazzo G, Cacciapuoti S, Fabbrocini G, Scala E, Balato A. Safety concerns with current treatments for psoriasis in the elderly. Expert Opin Drug Saf. 2020;19(4):523–31.
Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53.
Thatiparthi A, Martin A, Liu J, Egeberg A, Wu JJ. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22(4):425–42.
Gordon K, Korman N, Frankel E, Wang H, Jahreis A, Zitnik R, et al. Efficacy of etanercept in an integrated multistudy database of patients with psoriasis. J Am Acad Dermatol. 2006;54(3 Suppl 2):S101–11.
Hsu S, Green LJ, Lebwohl MG, Wu JJ, Blauvelt A, Jacobson AA. Comparable efficacy and safety of brodalumab in obese and nonobese patients with psoriasis: analysis of two randomized controlled trials. Br J Dermatol. 2020;182(4):880–8.
Reich K, Puig L, Mallbris L, Zhang L, Osuntokun O, Leonardi C. The effect of bodyweight on the efficacy and safety of ixekizumab: results from an integrated database of three randomised, controlled Phase 3 studies of patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(7):1196–207.
de Groot M, Appelman M, Spuls PI, de Rie MA, Bos JD. Initial experience with routine administration of etanercept in psoriasis. Br J Dermatol. 2006;155(4):808–14.
Vilarrasa E, Notario J, Bordas X, Lopez-Ferrer A, Gich IJ, Puig L. ORBIT (Outcome and retention rate of biologic treatments for psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74(6):1066–72.
Menter A, Papp KA, Gooderham M, Pariser DM, Augustin M, Kerdel FA, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30(7):1148–58.
Acknowledgements
Funding
Funding for Dermatology and Therapy’s Rapid Service Fee was provided by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance was provided by Hilary Durbano, PhD, of AlphaBioCom, LLC, King of Prussia, PA, USA, and funded by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors contributed to the conception and design of this review. All authors contributed to writing the first draft and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Joseph F. Merola is a consultant for AbbVie; Amgen; Biogen; Bristol Myers Squibb; Dermavant; Eli Lilly; Janssen; LEO Pharma; Novartis; Pfizer; Regeneron; Sanofi; Sun Pharmaceutical Industries, Inc.; and UCB. Arthur Kavanaugh has conducted clinical research sponsored by and/or consulted for AbbVie, Amgen, Celgene, Eli Lilly, Novartis, and Pfizer. Mark G. Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie; Amgen; Arcutis; Avotres; Boehringer Ingelheim; Dermavant Sciences; Eli Lilly; Incyte; Janssen Research & Development, LLC; Ortho Dermatologics; Regeneron; and UCB, Inc.; is a consultant for Aditum Bio; Almirall; AltruBio Inc.; AnaptysBio; Arcutis; Aristea Therapeutics; Arrive Technologies; Avotres Therapeutics; BiomX; Boehringer Ingelheim; Bristol Myers Squibb; Cara Therapeutics; Castle Biosciences; Corrona; Dermavant Sciences; Dr. Reddy’s Laboratories; Evelo Biosciences; Evommune, Inc.; Facilitation of International Dermatology Education; Forte Biosciences; Foundation for Research and Education in Dermatology; Helsinn Therapeutics; Hexima Ltd.; LEO Pharma; Meiji Seika Pharma; Mindera; Pfizer; Seanergy; and Verrica. Robert Gniadecki has served on advisory boards and/or received lecture honoraria from AbbVie; Amgen; Celgene; Eli Lilly; Janssen Research & Development, LLC; LEO Pharma; Mallinckrodt; Merck; and Novartis. Jashin J. Wu is or has been an investigator for AbbVie, Amgen, Eli Lilly, Janssen, Novartis; a consultant for AbbVie; Almirall; Amgen; Arcutis; Aristea Therapeutics; Bausch Health; Boehringer Ingelheim; Bristol Myers Squibb; Dermavant; Dr. Reddy's Laboratories; Eli Lilly; Galderma; Janssen; LEO Pharma; Mindera Novartis; Regeneron; Sanofi Genzyme; Solius; Sun Pharmaceutical Industries, Inc.; UCB; and Zerigo Health; and a speaker for AbbVie; Amgen; Bausch Health; Novartis; Regeneron; Sanofi Genzyme; Sun Pharmaceutical Industries, Inc.; and UCB.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Merola, J.F., Kavanaugh, A., Lebwohl, M.G. et al. Clinical Efficacy and Safety of Psoriasis Treatments in Patients with Concomitant Metabolic Syndrome: A Narrative Review. Dermatol Ther (Heidelb) 12, 2201–2216 (2022). https://doi.org/10.1007/s13555-022-00790-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00790-2